Abstract
Purpose:
To evaluate the bone structure of the mandibular condyle through an animal model, after ovariectomy.
Methods:
Thirty-six female Wistar rats were divided into 2 groups. The OVX group was submitted to bilateral ovariectomy, the SHAM group underwent the same surgical treatment, but without removal of the ovaries. After 90, 105 and 135 days after surgery, six animals from each group were submitted to euthanasia and the part containing the condyle was removed.
Results:
The microscopic analysis shows an increase in marrow spaces over time in the OVX group. The morphometric study shows reduction in the amount of bone tissue in the OVX group 135 days period in comparison with for the initial period (90 days) (p <0.05, ANOVA, Tukey).
Conclusion:
The estrogen deficiency also affects the bone structure of the condyle.
Key words:
Mandibular Condyle; Osteoporosis; Ovariectomy; Rats
Introduction
Osteoporosis is becoming a public health problem due to the high number of affected people. Osteoporosis is estimated to affect 200 million women worldwide - approximately one-tenth of women aged 60, one-fifth of women aged 70, two-fifths of women aged 80 and two-thirds of women aged 9011 Kanis JA, Group WHOS. WHO technical report. Univ Sheffield, UK; 2007. Available from: http://apps.who.int/iris/bitstream/10665/42841/1/WHO_TRS_921.pdf.. The main risk factor is estrogen deficiency; thus, this disease is more common in women. Osteoporosis is characterized by the substantial reduction of bone mass and development of more porous and thin bones, extremely fragile, compromising bone strength and subjecting them to fracture22 Rachner TD, Khosla S, Hofb LC. Osteoporosis?: now and the future. Lancet. 2011;377(9773):1276-87. doi: 10.1016/S0140-6736(10)62349-5.
https://doi.org/10.1016/S0140-6736(10)62...
,33 Soares CD, Carvalho MGF de, Carvalho RA de, Trindade SRP, Rêgo ACM do, Araújo-Filho I, Marques MM. Chenopodium ambrosioides L. extract prevents bone loss. Acta Cir Bras. 2015;30(12):812-8. doi: 10.1590/S0102-865020150120000004.
https://doi.org/10.1590/S0102-8650201501...
.
A deficiency of the hormone results in a high rate of bone remodeling, in which the resorption formation exceeds formation, thus resulting in a body bone mass loss44 Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 2014;3. doi: 10.1038/bonekey.2013.215.
https://doi.org/10.1038/bonekey.2013.215...
,55 Almeida M, Laurent MR, Dubois V, Claessens F, O'Brien CA, Bouillon R, Vanderschueren D, Manolagas SC. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. 2017;97(1):135-87.doi: 10.1152/physrev.00033.2015.
https://doi.org/10.1152/physrev.00033.20...
. The osteoprotegerin (OPG) is a nuclear factor kappa-B receptor ligand (RANKL) activator, which is necessary for cytokine regulation of bone formation and bone resorption. The RANKL/ratio OPG is the key element that regulates the balance between bone formation and bone resorption, and the lack of estrogen leads to an imbalance in this proportion. The OPG is a RANKL inhibitor; it binds with high affinity to RANKL and prevents its interaction with RANK on the osteoclast. Therefore, OPG downregulates osteoclast formation and prevents osteoporosis66 Kini U, Nandeesh BN. Physiology of bone formation, remodeling, and metabolism. In: Radionuclide and hybrid bone imaging. Springer; 2012. p.29-57. doi: 10.1007/978-3-642-02400-9_2.
https://doi.org/10.1007/978-3-642-02400-...
,77 Siddiqui JA, Partridge NC. Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology. 2016;31(3):233-45. doi: 10.1152/physiol.00061.2014.
https://doi.org/10.1152/physiol.00061.20...
. Both estrogens and the TGF-beta increase the production of OPG, consequently decreasing the differentiation and activation of osteoclasts and inducing apoptosis66 Kini U, Nandeesh BN. Physiology of bone formation, remodeling, and metabolism. In: Radionuclide and hybrid bone imaging. Springer; 2012. p.29-57. doi: 10.1007/978-3-642-02400-9_2.
https://doi.org/10.1007/978-3-642-02400-...
. Thus, the high rate of bone resorption observed when the suppression of this hormone occurs can be explained.
Osteoporosis can affect the trabecular bone of the whole skeleton. Although the most common fractures in osteoporosis occur in the spine, wrist, and hip, sites where trabecular bone predominates are susceptible to suffer, due to the porosity and changes in the structure or micro-architecture88 Hans D, Goertzen AL, Krieg M, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26(11):2762-9. doi: 10.1002/jbmr.499.
https://doi.org/10.1002/jbmr.499...
,99 Kado DM, Miller Martinez D, Lui L, Cawthon P, Katzman WB, Hillier TA, Fink HA, Ensrud KE. Hyperkyphosis, kyphosis progression, and risk of non spine fractures in older community dwelling women: The Study of Osteoporotic Fractures (SOF). J Bone Miner Res. 2014;29(10):2210-6. doi: 10.1002/jbmr.2251.
https://doi.org/10.1002/jbmr.2251...
. The mechanisms that occur in ovariectomized rats are the same as occur in humans, mimicking the post-menopausal condition. Thus this animal model is suitable for studies related to post-menopausal osteoporosis and has been used in many studies1010 Fuegl A, Tangl S, Keibl C, Watzek G, Redl H, Gruber R. The impact of ovariectomy and hyperglycemia on graft consolidation in rat calvaria. Clin Oral Implants Res. 2011;22(5):524-9. doi: 10.1111/j.1600-0501.2010.02048.x.
https://doi.org/10.1111/j.1600-0501.2010...
,1111 Kosugi K, Yonezu H, Kawashima S, Honda K, Arai Y, Shibahara T. A longitudinal study of the effect of experimental osteoporosis on bone trabecular structure in the rat mandibular condyle. Cranio. 2013;31(2):140-50. doi: 10.1179/crn.2013.022.
https://doi.org/10.1179/crn.2013.022...
. Preliminary studies using this animal model indicate cortical and spinal cord bone loss and that this is due to estrogenic suppression1212 Sakakura Y, Shide N, Tsuruga E, Irie K, Yajima T. Effects of running exercise on the mandible and tibia of ovariectomized rats. J Bone Miner Metab. 2001;19(3):159-67. PMID: 11368301.. Via X-ray analysis in the region of the mandible it was found that in the internal alveolar septum bone of the SHAM group (non-ovariectomized), the trabeculae bone form network structures linked to each other. In the OVX group (ovariectomized), the trabeculae bone form isolated islands in the medullary bone, showing a separation approximately 4 times higher in comparison to that found in the SHAM group1313 Tanaka M, Ejiri S, Nakajima M, Kohno S, Ozawa H. Changes of cancellous bone mass in rat mandibular condyle following ovariectomy. Bone. 1999;25(3):339-47. PMID: 10495138.. Other studies using computed tomography showed that postmenopausal osteoporosis can change the entire skeleton, including the structure of the mandibular condyle and that there is a correlation in bone mineral density between the mandibular condyle and the spine. It was also shown that the bone volume in OVX rat condyle is significantly lower than in SHAM rats1414 Tanaka M, Toyooka E, Kohno S, Ozawa H, Ejiri S. Long-term changes in trabecular structure of aged rat alveolar bone after ovariectomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2003;95(4):495-502. doi: 10.1067/moe.2003.135.
https://doi.org/10.1067/moe.2003.135...
.
The prevention of any problems that may affect basic functions of the stomatognathic components constitutes one of the most important task of the dental surgeon. The close relationship between the anatomy and function of the element present in the masticatory activity should provide the individual with satisfactory conditions to consume all foods, with total absence of restrictions, pain or unwanted noise. Whereas the jaw bone is articulated with the skull, but independent of it, it is necessary that its functions are in harmony with the jaw in order to satisfactorily produce the masticatory function. Just as the bony parts of the body may change and thus damage certain areas of the human body, there are also pathological factors that may affect the anatomical structures that affect the functional activity of the masticatory set1515 van der Bilt A. Human oral function: a review. Braz J Oral Sci. 2015;1(1):7-18. doi: 10.20396/bjos.v1i1.8640964.
https://doi.org/10.20396/bjos.v1i1.86409...
. Osteoporosis predominates among these factors, even if this pathology more frequently acts in the long bones, such as the femur and in the bones of the spine. Alveolar spongy bone should also be very sensitive to osteoporosis because of their anatomy, and the temporomandibular joint (TMJ) appears quite fragile when the disease is present. Patients systemically compromised by Osteoporosis often suffer deterioration of periodontal diseases and the possibility of bone fractures, since the loss or decrease in alveolar bone is observed in these patients. Consequently, there are more amount of dental open spaces with consequent accumulation of plaque, making it difficult to biofilm control and therefore prone to aggravation of the symptoms of periodontal disease and infectious processes, which in turn accelerates the horizontal bone loss and vertical. So, bone loss, dental changes linked to position of teeth in the dental arches and lack of prosthesis adaptation due to decreased bone mass, contributes to changes in the condyles, since the occlusal relationship of teeth are altered and therefore the stomatognathic system and the function is compromised, generating specific pathologies of the temporomandibular joint and condylar complex region1616 Jonasson G, Rythén M. Alveolar bone loss in osteoporosis: a loaded and cellular affair? Clin Cosmet Investig Dent. 2016;8:95. doi: 10.2147/CCIDE.S92774.
https://doi.org/10.2147/CCIDE.S92774...
17 Lindh C, Horner K, Jonasson G, Olsson P, Rohlin M, Jacobs R, Karayianni K, van der Stelt P, Adams J, Marjanovic E, Pavitt S, Devlin H. The use of visual assessment of dental radiographs for identifying women at risk of having osteoporosis: the OSTEODENT project. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2008;106(2):285-93. doi: 10.1016/j.tripleo.2007.09.008.
https://doi.org/10.1016/j.tripleo.2007.0...
18 Lohana M, Suragimath G, Abbayya K, Varma S, Zope S, Kale V. A study to assess and correlate osteoporosis and periodontitis in selected population of Maharashtra. J Clin Diagnosis Res. 2015;9(6):ZC46-50. doi: 10.7860/JCDR/2015/13725.6116.
https://doi.org/10.7860/JCDR/2015/13725....
-1919 Sachelarie L, Farcas DM, Dartu L, Vasiliu M, Daraba O, Nazarie S, Mocanu C, Burlui V. Comparative study of diseases of the stomatognathic system and specific parameters of osteoporosis. Osteoporos Int. 2016;27(2):845-8. doi: 10.1007/s00198-015-3251-6.
https://doi.org/10.1007/s00198-015-3251-...
. Therefore, to improve knowledge on the relationship of postmenopausal osteoporosis with the temporomandibular joints (TMJ) this study evaluated the bone mineralization of the condyle after ovariectomy, using an animal model.
Methods
This study was approved by the Ethics Committee on Animal Use at Universidade de Marilia (CEUA-UNIMAR, protocol nº 60) and followed the norms set forth by the National Institute of Health (NIH)2020 Garber JC. Guide for the care and use of laboratory animals. Available from http://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf
http://grants.nih.gov/grants/olaw/guide-...
.
Thirty six adult, 3 months older female Wistar rats (Rattus norvegicus) were used. During the experimental period they were kept in plastic containers and received water and food ad libitum under controlled environmental temperature and a light cycle of 12 hours. The animals were randomly divided into 2 experimental groups of 18 animals each, as described below:
-
OVX - underwent bilateral ovariectomy;
-
SHAM OVX - underwent the same treatment without surgical removal of the ovaries.
Surgical procedures
Before the surgery, the sexual maturity of the animals was verified through the vaginal secretion analysis through the procedure described in Marcondes2121 Marcondes FK, Bianchi FJ, Tanno P. Determination of the estrous cycle phases of rats?: some helpful considerations. Braz J Biol. 2002;62(4A):609-14. PMID: 12659010.. Briefly, the Vaginal secretion was collected with a plastic pipette filled with 10 µL of normal saline (NaCl 0.9%) by inserting the tip into the rat vagina, but not deeply. Vaginal fluid was placed on glass slides. The animals were followed daily for 7 consecutive days, where the regularity of the Estrous cycle was noted, by the proportion of the 3 cell types: epithelial, cornified and leukocytes.
For the surgical procedures, the animals were submitted to deep sedation with pre-anesthetic intramuscular (IM) administration of a muscle relaxant, xylazine hydrochloride (Rompum®-Bayer, São Paulo, Brazil) 10mg/kg followed by intramuscular administration of the general anesthetic ketamine hydrochloride (Dopalen® - Vetbrands) 90mg/kg body weight.
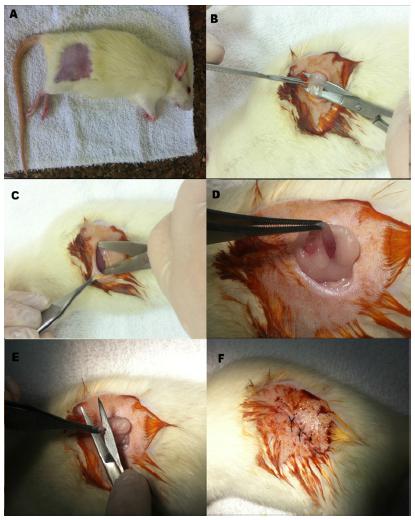
After the onset of anesthesia, shaving and disinfecting procedures were performed with Chlorhexidine solution (2% Riohex) in the target area (Figure 1A).
Ovariectomy surgery (A) Animal after sedation and shaving, ready for surgery. (B) After first incision, exposing the subcutaneous tissue, (C) tissue separation, (D) ovary and uterine tube, (E) ovary removal, (F) after suturing.
A bilateral ovariectomy was performed with incisions on both sides, as illustrated in Figure 1B and C, followed by exposure of the ovaries (Figure 1D) and their surgical removal (Figure 1E). Afterwards, suturing of the inner plane with absorbable sutures (Vicryl 4-0®, Johnson & Johnson) and the skin surface with silk thread (silk 4-0®, Johnson & Johnson) was conducted (Figure 1F).
The SHAM group (fictitious ovariectomy) underwent the same surgical procedure, with exposition of the ovaries and returning them, intact, to the original position.
After surgery, the animals received antibiotic Enrofloxacin (Flotril® 2.5% - Schering-Plough) at a dose of (10.0mg/kg), subcutaneously once a day for 7 days and Sodium Dipyrone (25 mg/kg) for analgesia, daily (2 times) for 3 days.
After 90 days, the analysis of Estrous cycle was repeated and the predominance of Leukocytes was verified, which characterizes the Diestro phase of the animals, due to estrogen reduction2121 Marcondes FK, Bianchi FJ, Tanno P. Determination of the estrous cycle phases of rats?: some helpful considerations. Braz J Biol. 2002;62(4A):609-14. PMID: 12659010.. After 90, 105 and 135 days 6 animals from each group were euthanized with barbiturate overdose (150mg/kg via IP) and pieces of TMJ removed and placed in a 10% formalin for 48 hours for subsequent macroscopic, radiographic and microscopic analyses.
Digital radiography
Initially the pieces were wrapped in tissue paper to remove excess formalin. A dental X-ray equipment CRANEX- D Soredex (Finland) with the digital image receiver (CCD) with pixel size of 96 micrometers was employed. The acquisition parameters were:
Al Filter 2.7mm; potential 66 kVp, current 10 mA.
Microscopic analysis
The TMJ pieces were fixed in 10% formalin for 48 hours for subsequent macroscopic, radiographic and microscopic analyses. The samples were then decalcified using EDTA 18% (ethylenediaminetetraacetic acid) and embedded in paraffin. The microscopic sections were obtained in the longitudinal direction of condyle with six micrometers thickness and stained using Hematoxylin/Eosin (HE).
The slides were observed under a Nikon Eclipse 80i light microscope coupled to an image analyzer (Image Pro Plus® 5.1).
The histomorphometry was performed using the ImageJ program2222 Rasband WS, ImageJ US. Bethesda, Md, USA. ImageJ; 1997. https://imagej.nih.gov/ij/ through a grid superimposed on the image, to count the tissue areas present in the region of interest. The values were used to calculated each tissue percentage according to equation 1:
Statistical analysis
Quantitative results of the percentage of bone tissue obtained from histomorphometry were statistically analyzed by the Shapiro-Wilk test to verify the normality of data. Subsequently, ANOVA test followed by Tukey test was performed to compare the results of the groups. The significance level of 5% was considered. The results were considered statically different when p<0.05.
Results
After removal, no differences were observed macroscopically between the OVX and SHAM groups.
Figure 2 shows radiographic images of the TMJ of samples collected 90 days post-surgery. The red arrow shows the condyle area in which indications of demineralization can be noted.
Digital Radiography of the condyle region (arrow) 90 days after the ovariectomy. (A) SHAM group (B) OVX group.
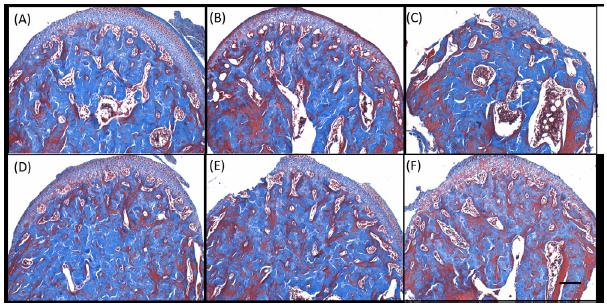
Figure 3 is the photomicrograph at x4 magnification of a longitudinal section of mandibular condyle, stained with Picrosirius red, taken with polarized light. This image shows the standard region used for bone tissue quantification and the main microscopic structures. Figure 4 shows the photomicrographs at x10 magnification Masson Trichrome stained of both groups in the analyzed periods, where the increase of the medullary space in the OVX group can be seen. Figure 5 shows Picrosirius red stained sections with x40 magnification, showing the architecture of the analyzed specimens. The condylar cartilage can be subdivided into four layers that can be easily distinguished in these images. The fibrous layer consists basically of fibroblasts and collagen type I in the process of organization. They are observed in all the analyzed specimens (OVX and SHAM).
Photomicrography of longitudinal section of mandibular condyle, stained with Picrosirius red. The dashed square represents the standardized area used for histomorphometry The main structures present in the image are articular layer (#) and medullary space(*). (A) OVX group 135 days and (B) SHAM group 135 days.
Photomicrographs of region at x10 magnification Masson Trichrome stained of both groups. (A-C) OVX group and (D-F) SHAM group after (A,D) 90 days, (B,E) 105 days and (C,F) 135 days post-surgery.
Picrosirius stained sections at 40X magnification of both groups: (A-C) OVX group and (D-F) SHAM group after (A,D) 90 days, (B,E) 105 days and (C,F) 135 days post-surgery. Type II collagen (#), elongated cells (*), low mineralization areas (white arrows).
The proliferative layer is composed of mesenchymal or undifferentiated cells. There is a greater concentration in the peripheral areas in the SHAM group specimens. These cells have the great capacity to differentiate in fibroblasts that form the fibrous layer, or in chondroblasts that is, the cartilaginous cells found in hyaline cartilage. They are observed in all specimens, but the OVX group presents smaller areas of mineralization. The mature layer is composed mainly of chondroblasts that synthesize collagen type II in the cartilaginous matrix (#).
It is observed that in the OVX groups, there was a decrease in the cartilage thickness, especially characterized by chondrocytes layers with low activity and areas of low mineralization (arrows) in comparison to the SHAM group specimens. The mineralized area presented larger volume in the analyzed specimens.
It is possible to observe a proliferative layer forming parallel fibers of connective tissue in SHAM and OVX groups, with resting cartilage area. This is characterized by less activity of the chondrocytes near the articular surface and are observed in the OVX group, which present elongated cells and smaller areas of mineralization (*).
The ImageJ software was employed to measure the bone tissue area. Figure 6 shows the bone tissue percentage in the analysis periods. There are statistically significant differences between results. The bone tissue percentage in the OVX-group at 135 days (78.8 ± 4.8) is lower than the SHAM-90day (89 ± 3.5) group and SHAM-105day (86.3 ± 2.1) (p<0.05, ANOVA, Tukey), demonstrating that bone loss due to hormonal suppression. Moreover, this loss is gradual, since the amount of bone in the OVX-135day group (78.8 ± 4.8) is less than the OVX-90day group (85.8 ± 1.7) (p<0.05, ANOVA, Tukey).
Histomorphometry of bone tissue of SHAM and OVX groups after 90, 105 and 135 days post-surgery. The letters a, b and c indicate groups with statistical differences (p<0.05, ANOVA, Tukey).
Discussion
Osteoporosis is a common disease in the elderly especially in postmenopausal women. It is characterized by low bone mass and deterioration of bone micro architecture with increased bone fragility2323 Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276-87. doi: 10.1016/S0140-6736(10)62349-5.
https://doi.org/10.1016/S0140-6736(10)62...
. It has been proposed that the mechanisms that occur in ovariectomized rats are the same as occur in humans, simulating postmenopausal condition2424 Thompson DD, Simmons HA, Pirie CM, Ke HZ. FDA Guidelines and animal models for osteoporosis. Bone. 1995;17(4):S125-33. PMID: 857990.. Thus, it should constitute an appropriate model for studies related to post-menopausal osteoporosis. The experimental design used in this work sought similarity to what occurs in the practice. After ovariectomy, there was a 90-day interval for the collection of the pieces and the reduction of estrogen was confirmed by the predominance of leukocytes in the vaginal secretion, which characterizes the diestrous phase, as opposed to the estrus phase, corresponding to the ovulation stage.
Few studies discuss the changes that occur in the condyle region and is very important in dental practice. Thus, this work was used this animal model, focused on this region. Although the effects of ovariectomy and consequent estrogen deficiency is a widely studied subject, the recent study of Nicolielo2525 Nicolielo LFP, Jacobs R, Albdour EA, Hoste X, Abeloos J, Politis C, Swennen G. Is oestrogen associated with mandibular condylar resorption? A systematic review. Int J Oral Maxillofac Surg. 2017;46(11):1394-402. doi: 10.1016/j.ijom.2017.06.012.
https://doi.org/10.1016/j.ijom.2017.06.0...
, which consists of a systematic review from 1993 to 2017, in order to determine whether estrogen should be considered a risk factor for condylar resorption, suggests that further studies need to be conducted to conclude about mandibular condylar resorption. In this sense, this study contributes with quantitative microscopic analysis of the bone structure, conducted in an animal model.
Osteoporosis has a major impact on alveolar bone. Studies have shown that alveolar bone loss is one of the most likely causes of tooth loss or mobility. Low bone density in the jaw triggered by osteoporosis can also lead to other dental problems1616 Jonasson G, Rythén M. Alveolar bone loss in osteoporosis: a loaded and cellular affair? Clin Cosmet Investig Dent. 2016;8:95. doi: 10.2147/CCIDE.S92774.
https://doi.org/10.2147/CCIDE.S92774...
,1717 Lindh C, Horner K, Jonasson G, Olsson P, Rohlin M, Jacobs R, Karayianni K, van der Stelt P, Adams J, Marjanovic E, Pavitt S, Devlin H. The use of visual assessment of dental radiographs for identifying women at risk of having osteoporosis: the OSTEODENT project. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2008;106(2):285-93. doi: 10.1016/j.tripleo.2007.09.008.
https://doi.org/10.1016/j.tripleo.2007.0...
. For example, women who suffer from osteoporosis are more likely to experience difficulties connected with ill-fitting dentures1717 Lindh C, Horner K, Jonasson G, Olsson P, Rohlin M, Jacobs R, Karayianni K, van der Stelt P, Adams J, Marjanovic E, Pavitt S, Devlin H. The use of visual assessment of dental radiographs for identifying women at risk of having osteoporosis: the OSTEODENT project. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2008;106(2):285-93. doi: 10.1016/j.tripleo.2007.09.008.
https://doi.org/10.1016/j.tripleo.2007.0...
,1818 Lohana M, Suragimath G, Abbayya K, Varma S, Zope S, Kale V. A study to assess and correlate osteoporosis and periodontitis in selected population of Maharashtra. J Clin Diagnosis Res. 2015;9(6):ZC46-50. doi: 10.7860/JCDR/2015/13725.6116.
https://doi.org/10.7860/JCDR/2015/13725....
.
Dervis et al.2626 Dervis E. Oral implications of osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2005;100(3):349-56. doi: 10.1016/j.tripleo.2005.04.010.
https://doi.org/10.1016/j.tripleo.2005.0...
discuss the association between osteoporosis and oral health and its effects such as periodontal diseases, tooth loss and other oral tissue change. Although the authors concluded that there is need for further studies, the paper shows that individuals with osteoporosis has increased risk for oral osteoporosis. Pavicin et al.2727 Pavicin IS, Sipina M, Badel T, Jukic T. The impact of osteoporosis on dental health in women older. Acta Stomatol Croat. 2013;47(4):329-35. doi: 10.15644/asc47/4/5.
https://doi.org/10.15644/asc47/4/5...
performed a cross-sectional study to determine the impact of osteoporosis on dental health in women older than 45 years. They conclude that dental status in menopausal and post-menopausal women is not related to BMD (bone mineral density) and further studies are necessary to establish the role of osteoporosis in oral health.
The results of this work show that the animal model used in this work and methodology were appropriate for the proposed study. Microscopic analysis (Figure 4) shows increased medullary spaces during the periods in the OVX group and in the SHAM group these changes are less apparent. This results is similar to that found by Tanaka et al.1313 Tanaka M, Ejiri S, Nakajima M, Kohno S, Ozawa H. Changes of cancellous bone mass in rat mandibular condyle following ovariectomy. Bone. 1999;25(3):339-47. PMID: 10495138.. Despite the osteoporosis induction method and the observation periods are different to this work the conclusions are similar. They also found changes the bone structure due to ovariectomy, inhibiting the bone gain in the OVX group. The SHAM group also presented higher bone volume than the OVX group, determined by histomorphometry. They also suggest that the mechanical loading can aid in maintaining mandibular bone volume in the OVX group. In long-term conditions, one year of estrogen deficiency, Tanaka et al.1414 Tanaka M, Toyooka E, Kohno S, Ozawa H, Ejiri S. Long-term changes in trabecular structure of aged rat alveolar bone after ovariectomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2003;95(4):495-502. doi: 10.1067/moe.2003.135.
https://doi.org/10.1067/moe.2003.135...
determine changes in the trabecular jaw bone structure. The thickness in the OVX group were significantly lower than those found in the SHAM group, and the trabecular separation was 4-fold higher in the OVX group than in the sham group. With a more approximated experimental design of this work, Hsu et al.2828 Hsu P-Y, Tsai M-T, Wang S-P, Chen Y-J, Wu J, Hsu J-T. Cortical bone morphological and trabecular bone microarchitectural changes in the mandible and femoral neck of ovariectomized rats. PLoS One. 2016;11(4):e0154367. doi: 10.1371/journal.pone.0154367.
https://doi.org/10.1371/journal.pone.015...
found results similar to this work, by microcomputed tomography, but in other regions that are mandible and femoral neck.
There are other studies evaluating the region of the mandible after ovariectomy which we can correlate with our present work. Cao et al.2929 Cao T, Shirota T, Ohno K, Michi KI. Mineralized bone loss in partially edentulous trabeculae of ovariectomized rabbit mandibles. J Periodontal Res. 2004;39(1):37-41. PMID: 14687226. through ovariectomized rabbits model, also found results with conclusion similar to our work using quantitative computed tomography showing loss in the mandible region caused by ovariectomy. Also determined by histomorphometry decrease in trabecular number and also in their separation, indicating mandibular bone loss in partially endentelous ovariectomized rabbit. Even though the method of analysis and the animal model are different that used in this work, these results corroborates with the results of our study.
Kosugi et al.1111 Kosugi K, Yonezu H, Kawashima S, Honda K, Arai Y, Shibahara T. A longitudinal study of the effect of experimental osteoporosis on bone trabecular structure in the rat mandibular condyle. Cranio. 2013;31(2):140-50. doi: 10.1179/crn.2013.022.
https://doi.org/10.1179/crn.2013.022...
performed a longitudinal study to determine the effect of experimental osteoporosis on bone trabecular structure in the mandibular condyle and also in the hip and knee joints using micro-CT. The osteoporosis was induced through injection of FK506 immunosuppressant drug for 5 weeks. Their results showed bone loss in the mandibular condyle, proximal femur and distal femur and the comparison demonstrated that the loss is similar. So, they conclude that osteoporosis affects not only the femur, but also the mandibular condyle. Although osteoporosis induction mechanism differs from our work, the result regarding to mandibular condyle is the same.
More recently Jiang et al.3030 Jiang L, Shen X, Wei L, Zhou Q, Gao Y. Effects of bisphosphonates on mandibular condyle of ovariectomized osteoporotic rats using micro-ct and histomorphometric analysis. J Oral Pathol Med. 2017 May;46(5):398-404. doi: 10.1111/jop.12499.
https://doi.org/10.1111/jop.12499...
studied the effects of bifosphonates on mandibular condyle of ovariectomized rats using micro-ct and histomorphometric analysis. The OVX group (untreated) and SHAM also belong to studied groups and Micro-CT analysis of condyle showed statistical differences in the measurements performed: bone volume/tissue volume (BV/TV,%), trabecular thickness (Tb.Th, lm), and trabecular separation (Tb.Sp, lm) between OVX and SHAM groups, demonstrating bone loss due to ovariectomy as well as in this work.
In this work, the amount of bone tissue in the OVX group at 90-days is higher than at 135-days and bone tissue volume in the SHAM group at 90 and 105-days is higher than the OVX at 135-days, demonstrating the progression of bone loss in mandibular condyle in ovariectomized rats and the effect of hormonal suppression. These results corroborates with the literature, as described. These results corroborate several in the literature, as described. Although the experiments are not exactly the same, such as how to induce osteoporosis and the methods of analysis. Therefore, it is important to show that different methodologies can be employed for the same purpose, expanding the options provided for future research that can take advantage of these to increase knowledge about the effects of osteoporosis as well as the influence of existing treatments.
Conclusion
Estrogen deficiency changes the mandibular condyle mineralization. This result should be considered in the clinical practice of dentistry and by other professionals dealing with the craniofacial region.
Acknowledgements
To Maira Couto, Wilson Orcini and Jessica Bonete for the technical assistance.
References
-
1Kanis JA, Group WHOS. WHO technical report. Univ Sheffield, UK; 2007. Available from: http://apps.who.int/iris/bitstream/10665/42841/1/WHO_TRS_921.pdf.
-
2Rachner TD, Khosla S, Hofb LC. Osteoporosis?: now and the future. Lancet. 2011;377(9773):1276-87. doi: 10.1016/S0140-6736(10)62349-5.
» https://doi.org/10.1016/S0140-6736(10)62349-5 -
3Soares CD, Carvalho MGF de, Carvalho RA de, Trindade SRP, Rêgo ACM do, Araújo-Filho I, Marques MM. Chenopodium ambrosioides L. extract prevents bone loss. Acta Cir Bras. 2015;30(12):812-8. doi: 10.1590/S0102-865020150120000004.
» https://doi.org/10.1590/S0102-865020150120000004 -
4Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 2014;3. doi: 10.1038/bonekey.2013.215.
» https://doi.org/10.1038/bonekey.2013.215 -
5Almeida M, Laurent MR, Dubois V, Claessens F, O'Brien CA, Bouillon R, Vanderschueren D, Manolagas SC. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. 2017;97(1):135-87.doi: 10.1152/physrev.00033.2015.
» https://doi.org/10.1152/physrev.00033.2015 -
6Kini U, Nandeesh BN. Physiology of bone formation, remodeling, and metabolism. In: Radionuclide and hybrid bone imaging. Springer; 2012. p.29-57. doi: 10.1007/978-3-642-02400-9_2.
» https://doi.org/10.1007/978-3-642-02400-9_2 -
7Siddiqui JA, Partridge NC. Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology. 2016;31(3):233-45. doi: 10.1152/physiol.00061.2014.
» https://doi.org/10.1152/physiol.00061.2014 -
8Hans D, Goertzen AL, Krieg M, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26(11):2762-9. doi: 10.1002/jbmr.499.
» https://doi.org/10.1002/jbmr.499 -
9Kado DM, Miller Martinez D, Lui L, Cawthon P, Katzman WB, Hillier TA, Fink HA, Ensrud KE. Hyperkyphosis, kyphosis progression, and risk of non spine fractures in older community dwelling women: The Study of Osteoporotic Fractures (SOF). J Bone Miner Res. 2014;29(10):2210-6. doi: 10.1002/jbmr.2251.
» https://doi.org/10.1002/jbmr.2251 -
10Fuegl A, Tangl S, Keibl C, Watzek G, Redl H, Gruber R. The impact of ovariectomy and hyperglycemia on graft consolidation in rat calvaria. Clin Oral Implants Res. 2011;22(5):524-9. doi: 10.1111/j.1600-0501.2010.02048.x.
» https://doi.org/10.1111/j.1600-0501.2010.02048.x -
11Kosugi K, Yonezu H, Kawashima S, Honda K, Arai Y, Shibahara T. A longitudinal study of the effect of experimental osteoporosis on bone trabecular structure in the rat mandibular condyle. Cranio. 2013;31(2):140-50. doi: 10.1179/crn.2013.022.
» https://doi.org/10.1179/crn.2013.022 -
12Sakakura Y, Shide N, Tsuruga E, Irie K, Yajima T. Effects of running exercise on the mandible and tibia of ovariectomized rats. J Bone Miner Metab. 2001;19(3):159-67. PMID: 11368301.
-
13Tanaka M, Ejiri S, Nakajima M, Kohno S, Ozawa H. Changes of cancellous bone mass in rat mandibular condyle following ovariectomy. Bone. 1999;25(3):339-47. PMID: 10495138.
-
14Tanaka M, Toyooka E, Kohno S, Ozawa H, Ejiri S. Long-term changes in trabecular structure of aged rat alveolar bone after ovariectomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2003;95(4):495-502. doi: 10.1067/moe.2003.135.
» https://doi.org/10.1067/moe.2003.135 -
15van der Bilt A. Human oral function: a review. Braz J Oral Sci. 2015;1(1):7-18. doi: 10.20396/bjos.v1i1.8640964.
» https://doi.org/10.20396/bjos.v1i1.8640964 -
16Jonasson G, Rythén M. Alveolar bone loss in osteoporosis: a loaded and cellular affair? Clin Cosmet Investig Dent. 2016;8:95. doi: 10.2147/CCIDE.S92774.
» https://doi.org/10.2147/CCIDE.S92774 -
17Lindh C, Horner K, Jonasson G, Olsson P, Rohlin M, Jacobs R, Karayianni K, van der Stelt P, Adams J, Marjanovic E, Pavitt S, Devlin H. The use of visual assessment of dental radiographs for identifying women at risk of having osteoporosis: the OSTEODENT project. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2008;106(2):285-93. doi: 10.1016/j.tripleo.2007.09.008.
» https://doi.org/10.1016/j.tripleo.2007.09.008 -
18Lohana M, Suragimath G, Abbayya K, Varma S, Zope S, Kale V. A study to assess and correlate osteoporosis and periodontitis in selected population of Maharashtra. J Clin Diagnosis Res. 2015;9(6):ZC46-50. doi: 10.7860/JCDR/2015/13725.6116.
» https://doi.org/10.7860/JCDR/2015/13725.6116 -
19Sachelarie L, Farcas DM, Dartu L, Vasiliu M, Daraba O, Nazarie S, Mocanu C, Burlui V. Comparative study of diseases of the stomatognathic system and specific parameters of osteoporosis. Osteoporos Int. 2016;27(2):845-8. doi: 10.1007/s00198-015-3251-6.
» https://doi.org/10.1007/s00198-015-3251-6 -
20Garber JC. Guide for the care and use of laboratory animals. Available from http://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf
» http://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf -
21Marcondes FK, Bianchi FJ, Tanno P. Determination of the estrous cycle phases of rats?: some helpful considerations. Braz J Biol. 2002;62(4A):609-14. PMID: 12659010.
-
22Rasband WS, ImageJ US. Bethesda, Md, USA. ImageJ; 1997. https://imagej.nih.gov/ij/
-
23Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276-87. doi: 10.1016/S0140-6736(10)62349-5.
» https://doi.org/10.1016/S0140-6736(10)62349-5 -
24Thompson DD, Simmons HA, Pirie CM, Ke HZ. FDA Guidelines and animal models for osteoporosis. Bone. 1995;17(4):S125-33. PMID: 857990.
-
25Nicolielo LFP, Jacobs R, Albdour EA, Hoste X, Abeloos J, Politis C, Swennen G. Is oestrogen associated with mandibular condylar resorption? A systematic review. Int J Oral Maxillofac Surg. 2017;46(11):1394-402. doi: 10.1016/j.ijom.2017.06.012.
» https://doi.org/10.1016/j.ijom.2017.06.012 -
26Dervis E. Oral implications of osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2005;100(3):349-56. doi: 10.1016/j.tripleo.2005.04.010.
» https://doi.org/10.1016/j.tripleo.2005.04.010 -
27Pavicin IS, Sipina M, Badel T, Jukic T. The impact of osteoporosis on dental health in women older. Acta Stomatol Croat. 2013;47(4):329-35. doi: 10.15644/asc47/4/5.
» https://doi.org/10.15644/asc47/4/5 -
28Hsu P-Y, Tsai M-T, Wang S-P, Chen Y-J, Wu J, Hsu J-T. Cortical bone morphological and trabecular bone microarchitectural changes in the mandible and femoral neck of ovariectomized rats. PLoS One. 2016;11(4):e0154367. doi: 10.1371/journal.pone.0154367.
» https://doi.org/10.1371/journal.pone.0154367 -
29Cao T, Shirota T, Ohno K, Michi KI. Mineralized bone loss in partially edentulous trabeculae of ovariectomized rabbit mandibles. J Periodontal Res. 2004;39(1):37-41. PMID: 14687226.
-
30Jiang L, Shen X, Wei L, Zhou Q, Gao Y. Effects of bisphosphonates on mandibular condyle of ovariectomized osteoporotic rats using micro-ct and histomorphometric analysis. J Oral Pathol Med. 2017 May;46(5):398-404. doi: 10.1111/jop.12499.
» https://doi.org/10.1111/jop.12499
-
Financial source:
none
-
1
Research performed at Research and Postgraduate Pro-Rectory, Universidade do Sagrado Coração (USC), Bauru-SP, Brazil.
Publication Dates
-
Publication in this collection
Oct 2017
History
-
Received
21 June 2017 -
Reviewed
24 Aug 2017 -
Accepted
28 Sept 2017