Abstract
Purpose:
To evaluate whether their combination was more effective than either alone in decreasing renal damage due to ischemia/reperfusion (I/R) injury in rats.
Methods:
Thirty-two Wistar rats were assigned to four groups. Following right nephrectomy, their left kidneys were subjected to warm ischemia (IR), cold ischemia (TH+IR), intraperitoneal injection of 10 mg/kg melatonin (MEL+IR), or injection of 10 mg/kg melatonin followed by cold ischemia (MEL+TH+IR). Eight randomly assigned right kidneys constituted the control group. After 240 min of reperfusion, left nephrectomy was performed for histopathological evaluation, lipid peroxidation, and measurement of antioxidant enzyme activity. Serum was collected to measure urea and creatinine concentrations.
Results:
Histopathological damage induced by ischemia and reperfusion was more attenuated in the MEL+TH+IR group than in the MEL+IR and TH+IR groups (p<0.037). Superoxide dismutase activity was significantly higher (p<0.029) and creatinine (p<0.001) and urea (p<0.001) concentrations were significantly lower in the MEL+TH+IR group than in the MEL+IR and TH+IR groups.
Conclusion:
The combination of melatonin (MEL) and topical hypothermia (TH) better protects against renal I/R injury than does MEL or TH alone.
Key words:
Melatonin; Hypothermia; Reperfusion Injury; Oxidative Stress; Kidney; Rats.
Introduction
Renal ischemia, the most common cause of acute kidney injury, occurs in many clinical settings, such as renal transplantation, partial nephrectomy, and cardiovascular surgery11 Paller MS. Acute renal failure: controversies, clinical trials, and future directions. Semin Nephrol. 1998;18(5):482-9. PMID: 9754600. and accounts for high mortality rates in intensive care units (ICUs)22 Rodriguez F, Bonacasa B, Fenoy FJ, Salom MG. Reactive oxygen and nitrogen species in the renal ischemia/reperfusion injury. Curr Pharm Des. 2013;19(15):2776-94. PMID: 23092323.. Renal ischemia-reperfusion (I/R) injury increases antibody production, which can be harmful to renal allografts, and may explain the pathophysiology underlying the association between delayed graft function and long-term allograft failure33 Fuquay R, Renner B, Kulik L, McCullough JW, Amura C, Strassheim D, Pelanda R, Torres R, Thurman JM. Renal ischemia-reperfusion injury amplifies the humoral immune response. J Am Soc Nephrol. 2013;24(7):1063-72. PMID: 23641055.. Blood flow during the reperfusion phase of I/R injury can produce oxygen free radicals, which promote lipid peroxidation and can cause tissue injury. Lipid membrane peroxidation and oxidative damage to proteins and DNA can result in apoptosis and cell death44 Kosieradzki M, Rowinski W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008;40(10):3279-88. PMID:19100373.. I/R injury may result from the downregulation of the antioxidants, such as catalase, superoxide dismutase, and glutathione peroxidase55 Singh I, Gulati S, Orak JK, Singh AK. Expression of antioxidant enzymes in rat kidney during ischemia-reperfusion injury. Mol Cell Biochem. 1993;125(2):97-104. PMID: 8283974.. Thus, antioxidants and radical scavengers, which inhibit this pathway, can protect from I/R injury66 Greenwald RA. Superoxide dismutase and catalase as therapeutic agents for human diseases. A critical review. Free Radic Biol Med. 1990;8(2):201-9. PMID: 2185145..
Topical hypothermia (TH) has also been shown to protect against renal I/R injury and has been used in kidney preservation, especially during kidney transplantation. A reduction in the core temperature of the kidney to below 4°C reduces the metabolism and enzymatic activity of the majority of kidney cells to 5-8%77 Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235-47. PMID: 7598460.. Improvement in the preserving solutions that target harmful pathways during cold storage leads to better preservation of the organ quality for a longer period of time88 McAnulty JF, Reid TW, Waller KR, Murphy CJ. Successful six-day kidney preservation using trophic factor supplemented media and simple cold storage. Am J Transplant. 2002;2(8):712-8. PMID: 12243492.. Despite these advantages, hypothermia also has undesirable side effects, including cell swelling, acidosis, altered enzyme activity, calcium accumulation, and production of reactive oxygen species (ROS)99 Bartels-Stringer M, Kramers C, Wetzels JF, Russel FG, Groot H, Rauen U. Hypothermia causes a marked injury to rat proximal tubular cells that is aggravated by all currently used preservation solutions. Cryobiology. 2003;47(1):82-91. PMID: 12963415..
Melatonin (N-acetyl-5-methoxytryptamine) is an important product of the pineal gland that acts as a regulator of sleep, immune function, and circadian rhythm. Furthermore, as an electron donor, melatonin (MEL) is a potent scavenger of ROS1010 Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7(6):444-58. PMID: 11060493.,1111 Reiter RJ, Tan DX, Terron MP, Flores LJ, Czarnocki Z. Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim Pol. 2007;54(1):1-9. PMID: 17351668.. MEL has been shown to have important antioxidant and anti-inflammatory effects in situations related to oxidative stress and inflammation1212 Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27(2):189-200. PMID: 16217132.
13 Mayo JC, Sainz RM, Tan DX, Hardeland R, Leon J, Rodriguez C, Reiter RJ. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J Neuroimmunol. 2005;165(1-2):139-49. PMID: 15975667.-1414 Reiter RJ, Tan DX, Maldonado MD. Melatonin as an antioxidant: physiology versus pharmacology. J Pineal Res. 2005;39(2):215-6. PMID: 16098101.. MEL has been found to reduce I/R injury, activate antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT), and reduce lipid peroxidation and apoptosis1010 Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7(6):444-58. PMID: 11060493.,1515 Reiter RJ, Guerrero JM, Garcia JJ, Acuna-Castroviejo D. Reactive oxygen intermediates, molecular damage, and aging. Relation to melatonin. Ann N Y Acad Sci. 1998;854:410-24. PMID: 9928448..
The finding that both TH and MEL reduce I/R injury suggested that the two together may have synergistic effects. Thus, in this study, we evaluated whether TH and MEL alone or in combination, had greater benefit in terms of improving histopathological parameters, oxidative stress damage, antioxidant enzyme activity, and biochemical parameters after I/R in rat kidneys.
Methods
The study was approved by the Animals Research Ethics Committee of Hospital de Clínicas de Porto Alegre (HCPA) (case 15-0472) and was performed in accordance with the International Guiding Principles for Biomedical Research Involving Animals, published by the Council for International Organizations of Medical Sciences (CIOMS), as well as with the Brazilian law on the scientific use of animals (Law 11794/2008).
Animals and experimental design
Adult male Wistar rats, weighing 276-406 g (age, 8-10 weeks old), were housed in the Animal Experimentation Unit of Hospital de Clínicas de Porto Alegre in groups of four in separate cages at room temperature (22 ± 2°C) with a 12-hour light/dark cycle and free access to water and rat chow. The rats were randomly allocated to four experimental groups of eight rats each. Following right nephrectomy, their left kidneys were subjected to warm ischemia for 40 min and reperfusion for 4 h (IR group); cold ischemia for 40 min and reperfusion for 4 h (TH+IR group); intraperitoneal injection of 10 mg/kg MEL 10 min prior to warm ischemia for 40 min and reperfusion for 4 h (MEL+IR group); or intraperitoneal injection of 10 mg/kg MEL 10 min prior to cold ischemia for 40 min and reperfusion for 4 h (MEL+TH+IR group). Eight randomly assigned right kidneys constituted the control group.
The surgical procedure began with the induction of inhalation general anesthesia with an isoflurane vaporizer at 3-5%, administrated through a campanula. Blood was collected from the retro-orbital plexus. The animals were placed on a warm surgical table in the supine position, and their rear paw reflexes were tested to ensure that adequate anesthesia was achieved. Anesthesia was maintained with 1 L/min oxygen and 2-3% isoflurane. A common electronic rectal thermometer (Termomed 1.0, Incoterm, Porto Alegre, Brazil) was used to assess systemic temperature. If needed, a heating lamp was used for maintaining the systemic temperature between 35.5°C and 37.5°C. Before making an abdominal incision, bupivacaine 0.5% was injected into the abdominal wall to ensure pain control during and after the procedure. MEL (Sigma, St. Louis, MO, USA) was dissolved in absolute ethanol and diluted in saline to yield an ethanol concentration of 1%. Rats in the MEL+IR and MEL+TH+IR groups were intraperitoneally administered MEL (10 mg/kg) 10 min prior to ischemia. A longitudinal median incision was made on the abdomen and surgical retractors were applied. Right nephrectomy was performed. Half of the right kidney was immersed in 10% formalin and the other half frozen and stored at -80°C. Ischemia was induced in the left kidneys by clamping the renal pedicle with an atraumatic microvascular surgery clip (Medicon, Tuttlingen, Germany). Cortical left kidney temperature was assessed with an intra-parenchymal probe connected to a specific thermometer (BAT 12, IITC Life Science, W. Hills, CA, USA). The left kidneys in the TH, TH+IR and MEL+TH+IR groups were flushed with ice-cold saline solution to achieve a target temperature of 4°C. To avoid systemic hypothermia, a suction system was installed to remove the cold saline solution, and the left kidney was isolated from the rest of the abdominal cavity using a device made of polystyrene and latex. After 40 min of TH or warm ischemia, the clamp was removed from the renal pedicle and the abdominal wall was closed. The animals were moved to a new warm cage with water, but without food. After 4h of reperfusion, they were moved again to the surgical table and anesthetized by inhalation to induce general anesthesia. The abdominal wall sutures were removed and left nephrectomy was performed. Blood samples were collected through heart puncture. Cardiectomy was performed to ensure animal death under anesthesia.
Histopathological changes
Half of each kidney was fixed in 10% formalin, embedded in paraffin, sliced, and stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) stain. The sections were examined under light microscope (Olympus bx41, Tokyo, Japan) at a magnification of 400× in a blinded manner by an experienced renal pathologist. The samples were graded histopathologically, as described previously1616 Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35(3):198-204. PMID: 6340272.. Briefly, grade 1 indicated mitoses and necrosis of individual cells; grade 2 indicated necrosis of all cells in adjacent proximal convoluted tubules, with survival of surrounding tubules; grade 3 indicated necrosis confined to the distal third of the proximal convoluted tubule with a band of necrosis extending across the inner cortex; and grade 4 indicated necrosis affecting all three segments of the proximal convoluted tubules. Finally, normal findings were designated grade 0 (Figure 1).
Histopathological evaluation of rat kidneys showing the degrees of acute tubular necrosis described by Jablonski et al.1616 Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35(3):198-204. PMID: 6340272. (examined under a light microscope; Olympus bx41, Tokyo, Japan): Photomicrographs of the renal cortex: (A) showing no abnormality, grade 0 (HE ×400); (B) showing necrosis of individual cells and mitotic cells in Grade 1 acute tubular necrosis (HE ×400); (C) showing necrosis of a group of proximal convoluted tubules in Grade 2 acute tubular necrosis (HE ×400); (D) showing necrosis of the distal third of the proximal convoluted tubules in Grade 3 acute tubular necrosis (HE ×400); and (E) showing necrosis affecting all three segments of the proximal convoluted tubules.
Kidney homogenate preparation
Kidneys were weighed and homogenized for 40 sec in an Ultra-Turrax homogenizer (IKA Works Inc., Wilmington, DE, USA) at 4°C in the presence of 1.15% KCl (9 ml per g of tissue) and phenyl-methyl-sulfonyl fluoride (PMSF) at a concentration of 100 mM in isopropanol (10 μl/ml KCl). The homogenate was centrifuged for 10 min at 3,000 rpm in a refrigerated centrifuge (SORVALL Super T21; Kendro Laboratory Products, Weaverville, NC, USA), the precipitate was discarded, and the supernatant was frozen at -80°C for subsequent biochemical analysis.
Protein
Protein concentrations were determined using the Bradford method with bovine albumin as a standard, followed by spectrophotometry at 595 nm.
Oxidative stress damage
Malondialdehyde (MDA) levels were measured using thiobarbituric acid reactive substances (TBARS).
Antioxidant enzyme activity
Superoxide dismutase (SOD) activity was determined by assessing the inhibition of superoxide-dependent adrenaline auto-oxidation, spectrophotometrically at 480 nm. The results were expressed as USOD/min/mg protein. Catalase (CAT) activity was calculated based on the decomposition of hydrogen peroxide, as shown spectrophotometrically at 240 nm, with the results expressed as pmol/mg protein.
Biochemical functional parameter analysis
Serum concentrations of urea and creatinine (sCr) at baseline and at the end of the experiment, from the blood samples collected from the retro-orbital plexus and heart, respectively, were estimated using a Roche Cobas 8000 c702 automatic biochemistry apparatus. Biochemical functional parameters were analyzed only with regard to experimental groups because urea and sCr were estimated from peripheral blood.
Statistical analysis
Data were presented as frequency and percentage or as mean ± SD. Associations between variables were assessed using χ22 Rodriguez F, Bonacasa B, Fenoy FJ, Salom MG. Reactive oxygen and nitrogen species in the renal ischemia/reperfusion injury. Curr Pharm Des. 2013;19(15):2776-94. PMID: 23092323. tests. Continuous variables were compared by one-ANOVA, followed by Turkey’s test for post hoc comparisons. Statistical significance was set at p<0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences (PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc).
Results
The baseline characteristics of rats in the four study groups and the control group, including weight, temperature, and serum creatinine and urea concentrations, were similar (data not shown).
Histopathological evaluation revealed significant differences among the groups studied (Figure 2). The degree of I/R injury was greater in the IR, TH+IR and MEL+IR groups than in the control group. There are rats in the IR and TH+IR groups presented with grade 4 injury, whereas none of the rats in the MEL+IR group presented with a grade 4 injury, and one had a grade 0 injury. The MEL+TH+IR group showed the lowest degree of injury, with none of the rats having a grade 3 or 4 injury and one having grade 0, similar to the control group of kidneys not subjected to I/R.
Differences in histopathological grade between the groups. IR, ischemia and reperfusion; TH+IR, topical hypothermia + ischemia and reperfusion; MEL+IR, melatonin + ischemia and reperfusion; MEL+TH+IR, melatonin + topical hypothermia + ischemia and reperfusion. Chi-squared test (p<0.037).
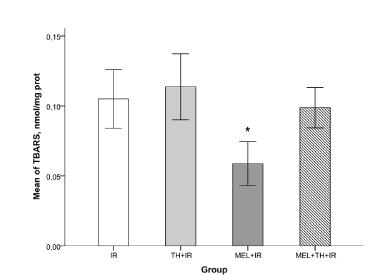
Oxidative stress damage was measured by TBARS tissue assay, which assesses the degree of lipid peroxidation. TBARS values were highest in the IR and TH+IR groups, but were lower in rats administered MEL. The TBARS values were significantly lower in the MEL+IR than in all of the other groups (p<0.05; Figure 3).
Means of TBARS. IR, ischemia and reperfusion; TH+IR, topical hypothermia + ischemia and reperfusion; MEL+IR, melatonin + ischemia and reperfusion; MEL+TH+IR, melatonin + topical hypothermia + ischemia and reperfusion; TBARS, thiobarbituric acid reactive species. *MEL+IR vs IR, p<0.002; TH+IR, p<0.001; MEL+TH+IR, p<0.008. Note: For comparing continuous variables the one-ANOVA test was used. Turkey’s test was applied for post-hoc comparisons.
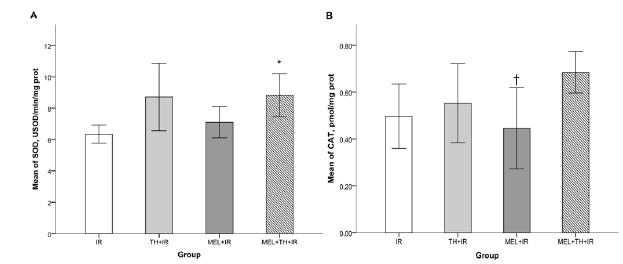
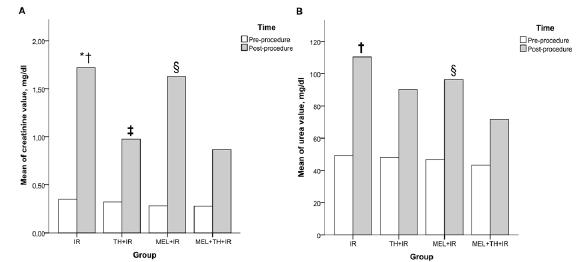
SOD activity was lower in the IR than in the other groups, and was significantly higher in the MEL+TH+IR group than in the IR group (p<0.029), CAT release was higher in the MEL+TH+IR group than in the other groups, but the difference was not statistically significant (p>0.05). Assessment of functional parameters at the end of the experimental protocol showed that serum creatinine and urea concentrations were highest in the IR group. The concentrations of both were significantly lower in the MEL+TH+IR group than in the IR and MEL+IR groups (p<0.05). Differences from baseline were also lowest in the MEL+TH+IR group. The TH+IR group showed significant differences in creatinine concentrations compared with the IR and MEL+IR groups (Figures 4 and 5).
Means of SOD and Catalase. IR, ischemia and reperfusion; TH+IR, topical hypothermia + ischemia and reperfusion; MEL+IR, melatonin + ischemia and reperfusion; MEL+TH+IR, melatonin + topical hypothermia + ischemia and reperfusion; SOD, superoxide dismutase. A, SOD; B, catalase. *IR vs. MEL+TH+IR (p<0.029). †No statistically significant differences. Note: For comparing continuous variables, a one-ANOVA test was used. Turkey’s test was applied for post-hoc comparisons.
Means of creatinine and urea levels between the study groups before and after the procedure. IR, ischemia and reperfusion; TH+IR, topical hypothermia + ischemia and reperfusion; MEL+IR, melatonin + ischemia and reperfusion; MEL+TH+IR, melatonin + topical hypothermia + ischemia and reperfusion. A, creatinine; B, urea. *IR vs TH+IR (p<0.001); †IR vs. MEL+TH+IR (p<0.001). ‡TH+IR vs. MEL+IR (p=0.001). §MEL+IR vs MEL+TH+IR (p=0.001). Note: For comparing continuous variables the two-way ANOVA test was used. Turkey’s test was applied for post-hoc comparisons.
Discussion
In our study, I/R induced histopathological changes, including grades 3 and 4 injury1616 Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35(3):198-204. PMID: 6340272., in both the IR and TH+IR groups. Pre-treatment with MEL alone (MEL+IR) slightly attenuated these histopathological damages, with no kidney experiencing grade 4 injury, but the damage did not differ significantly from that in the control group. MEL has been shown to act as a cytoprotective agent in I/R-induced lesions, reversing the damage caused by I/R nephrotoxicity1717 Banaei S, Ahmadiasl N, Alihemmati A. Comparison of the protective effects of erythropoietin and melatonin on renal ischemia-reperfusion injury. Trauma Mon. 2016;21(3):e23005. PMID: 27921018.. The combination of MEL pre-treatment plus topical hypothermia at 4°C (MEL+TH+IR) markedly reduced the degree of histopathological damage induced by I/R. Although some of these kidneys had grade 1 and 2 injuries, none had grade 3 and 4 lesions, thereby exhibited no difference with the control group. Thus, combined treatment showed benefits, reducing the histopathological extent of tissue injury.
Several studies have evaluated the effects of MEL alone or in combination with other substances on I/R injury1818 Yilmaz M, Mogulkoc R, Baltaci AK. Effect of three-week zinc and melatonin supplementation on the oxidant-antioxidant system in experimental renal ischemia-reperfusion in rats. Acta Clin Croat. 2015;54(4):395-401. PMID: 27017711.
19 Sezgin G, Ozturk G, Guney S, Sinanoglu O, Tuncdemir M. Protective effect of melatonin and 1,25-dihydroxyvitamin D3 on renal ischemia-reperfusion injury in rats. Ren Fail. 2013;35(3):374-9. PMID: 23356461.-2020 Kurcer Z, Oguz E, Ozbilge H, Baba F, Aksoy N, Celik H, Cakir H, Gezen MR. Melatonin protects from ischemia/reperfusion-induced renal injury in rats: this effect is not mediated by proinflammatory cytokines. J Pineal Res. 2007;43(2):172-8. PMID: 17645695.. Indeed, one previous study evaluated whether pre-treatment with MEL reduced I/R injury2121 Sener G, Sehirli AO, Keyer-Uysal M, Arbak S, Ersoy Y, Yegen BC. The protective effect of melatonin on renal ischemia-reperfusion injury in the rat. J Pineal Res. 2002;32(2):120-6. PMID: 12071469.. In that study, which used an experimental IR model in rats, pre-treatment with 10 mg/kg MEL 10 minutes before ischemia attenuated histopathological changes and reduced biochemical indices. I/R-induced renal damage can also be reduced by topical hypothermia, which reduces cellular metabolism and oxidative stress, making it essential for organ viability during the period of ischemia77 Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235-47. PMID: 7598460..
I/R-induced renal injury is associated with lipid peroxidation, a mechanism that causes oxidative damage to the cell membrane, leading to the production of free radicals1010 Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7(6):444-58. PMID: 11060493., and TBARS is a good indicator of the degree of lipid peroxidation. We found that the level of TBARS was significantly higher in the IR group, indicative of increased lipid peroxidation due to high oxidative stress2222 Eschwege P, Paradis V, Conti M, Holstege A, Richet F, Deteve J, Menager P, Legrand A, Jardin A, Bedossa P, Benoit G. In situ detection of lipid peroxidation by-products as markers of renal ischemia injuries in rat kidneys. J Urol. 1999;162(2):553-7. PMID: 10411087.. We also found that TBARS was significantly lower in the MEL+IR group than in the other groups (p<0.001). This finding, that MEL reduces the production of TBARS, indicates that MEL decreases lipid peroxidation and cellular damage. This reduction in lipid peroxidation may be partly due to MEL scavenging the peroxynitrite (ONOO-) radicals and hydroxyl (OH) ions2323 Reiter RJ, Oh CS, Fujimori O. Melatonin Its intracellular and genomic actions. Trends Endocrinol Metab. 1996;7(1):22-7. PMID: 18406721.. Although TBARS also tended to be lower in the MEL+TH+IR group than in the IR and IR+TH groups, these differences were not significant.
I/R causes accumulation of free radicals and the reduction of antioxidant enzymes, which have deleterious effects on the cell membrane, DNA, and protein. SOD and CAT activities decrease markedly during renal ischemia, with the main factor being the time of exposure to ischemic insult2424 Li Z, Nickkholgh A, Yi X, Bruns H, Gross ML, Hoffmann K, Mohr E, Zorn M, Buchler MW, Schemmer P. Melatonin protects kidney grafts from ischemia/reperfusion injury through inhibition of NF-kB and apoptosis after experimental kidney transplantation. J Pineal Res. 2009;46(4):365-72. PMID: 19552759.,2525 Conti M, Eschwege P, Ahmed M, Paradis V, Droupy S, Loric S, Bedossa P, Charpentier B, Legrand A, Benoit G. Antioxidant enzymatic activities and renal warm ischemia: correlation with the duration of ischemia. Transplant Proc. 2000;32(8):2740-1. PMID: 11134780.. In agreement with findings showing that MEL activates SOD1010 Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7(6):444-58. PMID: 11060493., we found that SOD activity was significantly higher in the MEL+TH+IR group than in the IR group (p< 0.029). In contrast, neither TH nor MEL alone significantly increased SOD activity. These findings suggest that the combination of MEL and TH stimulated protective antioxidant enzymes, corroborating our histopathological findings.
We also found that IR significantly increased creatinine and urea concentrations, showing that I/R had deleterious effects on renal function, even acutely. Creatinine concentrations were lower in the MEL+TH+IR than in the IR and MEL+IR groups, showing that combined therapy additionally protects renal function (p<0.001). Moreover, cold ischemia alone in the TH+IR group showed better protection of renal function when compared with the IR and MEL+IR groups (p<0.001). As expected, post-ischemia urea concentrations were higher in the IR group, showing that I/R damaged renal function. Although urea concentrations were lower in the TH+IR and MEL+IR groups than in the IR group, the differences were not statistically significant. In contrast, urea concentrations were significantly lower in the MEL+TH+IR group than in the other groups (P<0.001), likely due to the synergistic protective effect of both interventions. The benefits of cold ischemia at 4°C include reduction of cellular metabolism2626 Santos EB, Koff WJ, Grezzana Filho Tde J, De Rossi SD, Treis L, Bona SR, Pegas KL, Katz B, Meyer FS, Marroni NA, Corso CO. Oxidative stress evaluation of ischemia and reperfusion in kidneys under various degrees of hypothermia in rats. Acta Cir Bras. 2013;28(8):568-73. PMID: 23896835.,2727 Ribeiro GB, Santos EBD, Bona SR, Schaefer PG, Garcez TA, Rabolini EB, Smaniotto GP, Marroni NP, Corso CO. The effects of local ischemic preconditioning and topical hypothermia in renal ischemia/reperfusion injury in rats. Acta Cir Bras. 2017;32(10):816-26. PMID: 29160368. and in the production of xanthine oxidase77 Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235-47. PMID: 7598460., whereas the benefits of MEL include the activation of antioxidant enzymes and the protection of oxidative phosphorylation. Thus, together, cold ischemia and MEL reduce creatinine and urea concentrations, both parameters of renal function.
To our knowledge, our study is the first to assess the effects of both MEL and TH on IR-induced renal injury. This experimental model was found to be adequate, as IR-induced histopathological changes, altered factors related to oxidative stress, and worsened renal function. All of these parameters were attenuated by MEL and TH, especially by their combination. This combination showed benefits in the main outcome studied, histopathology, as well as altering the activity of the antioxidant enzyme SOD, and the functional parameters creatinine and urea.
Although MEL has been studied in several contexts, its role in organ transplantation has not yet been established. Experimental studies have shown, however, that MEL may have potential beneficial effects on organ transplantation2828 Esteban-Zubero E, Garcia-Gil FA, Lopez-Pingarron L, Alatorre-Jimenez MA, Inigo-Gil P, Tan DX, Garcia JJ, Reiter RJ. Potential benefits of melatonin in organ transplantation: a review. J Endocrinol. 2016;229(3):R129-46. PMID: 27068700.. MEL has a wide therapeutic window and few important side effects2929 Andersen LP, Gogenur I, Rosenberg J, Reiter RJ. The Safety of melatonin in humans. Clin Drug Investig. 2016;36(3):169-75. PMID: 26692007., suggesting that further studies are warranted.
In addition to TH, current clinical strategies to reduce kidney damage by I/R include perfusion fluids, use of a hypothermic perfusion machine, administration of mannitol, and a reduced period of ischemia3030 Spaliviero M, Power NE, Murray KS, Sjoberg DD, Benfante NE, Bernstein ML, Wren J, Russo P, Coleman JA. Intravenous mannitol versus placebo during partial nephrectomy in patients with normal kidney function: a double-blind, clinically-integrated, randomized trial. Eur Urol. 2018;73(1):53-9. PMID: 28822586.. The use of MEL, if proven effective in animal models of I/R and in humans, may become a particularly helpful tool in preventing I/R-induced kidney damage.
Conclusion
The combination of melatonin (MEL) and topical hypothermia (TH) was more effective at attenuating ischemia-reperfusion induced injury than was either alone.
Acknowledgements
To the team of the Animal Experimentation Unit (UEA), Experimental Pathology Unit, Department of Clinical Pathology, Laboratory of Experimental Hepatology and Physiology, and Molecular and Protein Analysis Unit (UAMP) of Hospital de Clínicas de Porto Alegre (HCPA) for their assistance and technical support.
References
-
1Paller MS. Acute renal failure: controversies, clinical trials, and future directions. Semin Nephrol. 1998;18(5):482-9. PMID: 9754600.
-
2Rodriguez F, Bonacasa B, Fenoy FJ, Salom MG. Reactive oxygen and nitrogen species in the renal ischemia/reperfusion injury. Curr Pharm Des. 2013;19(15):2776-94. PMID: 23092323.
-
3Fuquay R, Renner B, Kulik L, McCullough JW, Amura C, Strassheim D, Pelanda R, Torres R, Thurman JM. Renal ischemia-reperfusion injury amplifies the humoral immune response. J Am Soc Nephrol. 2013;24(7):1063-72. PMID: 23641055.
-
4Kosieradzki M, Rowinski W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008;40(10):3279-88. PMID:19100373.
-
5Singh I, Gulati S, Orak JK, Singh AK. Expression of antioxidant enzymes in rat kidney during ischemia-reperfusion injury. Mol Cell Biochem. 1993;125(2):97-104. PMID: 8283974.
-
6Greenwald RA. Superoxide dismutase and catalase as therapeutic agents for human diseases. A critical review. Free Radic Biol Med. 1990;8(2):201-9. PMID: 2185145.
-
7Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235-47. PMID: 7598460.
-
8McAnulty JF, Reid TW, Waller KR, Murphy CJ. Successful six-day kidney preservation using trophic factor supplemented media and simple cold storage. Am J Transplant. 2002;2(8):712-8. PMID: 12243492.
-
9Bartels-Stringer M, Kramers C, Wetzels JF, Russel FG, Groot H, Rauen U. Hypothermia causes a marked injury to rat proximal tubular cells that is aggravated by all currently used preservation solutions. Cryobiology. 2003;47(1):82-91. PMID: 12963415.
-
10Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7(6):444-58. PMID: 11060493.
-
11Reiter RJ, Tan DX, Terron MP, Flores LJ, Czarnocki Z. Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim Pol. 2007;54(1):1-9. PMID: 17351668.
-
12Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27(2):189-200. PMID: 16217132.
-
13Mayo JC, Sainz RM, Tan DX, Hardeland R, Leon J, Rodriguez C, Reiter RJ. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J Neuroimmunol. 2005;165(1-2):139-49. PMID: 15975667.
-
14Reiter RJ, Tan DX, Maldonado MD. Melatonin as an antioxidant: physiology versus pharmacology. J Pineal Res. 2005;39(2):215-6. PMID: 16098101.
-
15Reiter RJ, Guerrero JM, Garcia JJ, Acuna-Castroviejo D. Reactive oxygen intermediates, molecular damage, and aging. Relation to melatonin. Ann N Y Acad Sci. 1998;854:410-24. PMID: 9928448.
-
16Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35(3):198-204. PMID: 6340272.
-
17Banaei S, Ahmadiasl N, Alihemmati A. Comparison of the protective effects of erythropoietin and melatonin on renal ischemia-reperfusion injury. Trauma Mon. 2016;21(3):e23005. PMID: 27921018.
-
18Yilmaz M, Mogulkoc R, Baltaci AK. Effect of three-week zinc and melatonin supplementation on the oxidant-antioxidant system in experimental renal ischemia-reperfusion in rats. Acta Clin Croat. 2015;54(4):395-401. PMID: 27017711.
-
19Sezgin G, Ozturk G, Guney S, Sinanoglu O, Tuncdemir M. Protective effect of melatonin and 1,25-dihydroxyvitamin D3 on renal ischemia-reperfusion injury in rats. Ren Fail. 2013;35(3):374-9. PMID: 23356461.
-
20Kurcer Z, Oguz E, Ozbilge H, Baba F, Aksoy N, Celik H, Cakir H, Gezen MR. Melatonin protects from ischemia/reperfusion-induced renal injury in rats: this effect is not mediated by proinflammatory cytokines. J Pineal Res. 2007;43(2):172-8. PMID: 17645695.
-
21Sener G, Sehirli AO, Keyer-Uysal M, Arbak S, Ersoy Y, Yegen BC. The protective effect of melatonin on renal ischemia-reperfusion injury in the rat. J Pineal Res. 2002;32(2):120-6. PMID: 12071469.
-
22Eschwege P, Paradis V, Conti M, Holstege A, Richet F, Deteve J, Menager P, Legrand A, Jardin A, Bedossa P, Benoit G. In situ detection of lipid peroxidation by-products as markers of renal ischemia injuries in rat kidneys. J Urol. 1999;162(2):553-7. PMID: 10411087.
-
23Reiter RJ, Oh CS, Fujimori O. Melatonin Its intracellular and genomic actions. Trends Endocrinol Metab. 1996;7(1):22-7. PMID: 18406721.
-
24Li Z, Nickkholgh A, Yi X, Bruns H, Gross ML, Hoffmann K, Mohr E, Zorn M, Buchler MW, Schemmer P. Melatonin protects kidney grafts from ischemia/reperfusion injury through inhibition of NF-kB and apoptosis after experimental kidney transplantation. J Pineal Res. 2009;46(4):365-72. PMID: 19552759.
-
25Conti M, Eschwege P, Ahmed M, Paradis V, Droupy S, Loric S, Bedossa P, Charpentier B, Legrand A, Benoit G. Antioxidant enzymatic activities and renal warm ischemia: correlation with the duration of ischemia. Transplant Proc. 2000;32(8):2740-1. PMID: 11134780.
-
26Santos EB, Koff WJ, Grezzana Filho Tde J, De Rossi SD, Treis L, Bona SR, Pegas KL, Katz B, Meyer FS, Marroni NA, Corso CO. Oxidative stress evaluation of ischemia and reperfusion in kidneys under various degrees of hypothermia in rats. Acta Cir Bras. 2013;28(8):568-73. PMID: 23896835.
-
27Ribeiro GB, Santos EBD, Bona SR, Schaefer PG, Garcez TA, Rabolini EB, Smaniotto GP, Marroni NP, Corso CO. The effects of local ischemic preconditioning and topical hypothermia in renal ischemia/reperfusion injury in rats. Acta Cir Bras. 2017;32(10):816-26. PMID: 29160368.
-
28Esteban-Zubero E, Garcia-Gil FA, Lopez-Pingarron L, Alatorre-Jimenez MA, Inigo-Gil P, Tan DX, Garcia JJ, Reiter RJ. Potential benefits of melatonin in organ transplantation: a review. J Endocrinol. 2016;229(3):R129-46. PMID: 27068700.
-
29Andersen LP, Gogenur I, Rosenberg J, Reiter RJ. The Safety of melatonin in humans. Clin Drug Investig. 2016;36(3):169-75. PMID: 26692007.
-
30Spaliviero M, Power NE, Murray KS, Sjoberg DD, Benfante NE, Bernstein ML, Wren J, Russo P, Coleman JA. Intravenous mannitol versus placebo during partial nephrectomy in patients with normal kidney function: a double-blind, clinically-integrated, randomized trial. Eur Urol. 2018;73(1):53-9. PMID: 28822586.
-
Financial sources:
FIPE/HCPA
-
1
Research performed at Animal Experimentation Unit, Hospital de Clínicas de Porto Alegre (HCPA), Brazil. Part of Master degree thesis, Postgraduate Program in Medicine: Surgical Sciences. Tutor: Carlos Otávio Corso.
Publication Dates
-
Publication in this collection
Mar 2018
History
-
Received
27 Nov 2017 -
Reviewed
26 Jan 2018 -
Accepted
28 Feb 2018