Abstracts
PURPOSE: To analyze the ventricular enlargement and myelination of the corpus callosum in adult dogs after four and eight weeks of kaolin-induction of hydrocephalus. METHODS: 36 dogs were randomly divided into 3 groups: 1 - without hydrocephalus, 2 - kaolin-induction of hydrocephalus until the fourth week, and 3 - kaolin-induction of hydrocephalus until the eighth week. Ventricular ratios and volumes were calculated using magnetic resonance images, and myelination of the corpus callosum were histologically evaluated using solocromo-cianin stain. RESULTS: Radiological hydrocephalus was observed in 93.75% and overall mortality was 38.4%. Ventricular volumes and ratios were higher in groups 2 and 3 compared to group 1 and similar when measures in the fourth and eighth weeks were compared in the group 3. Indices of luminescence in the knee and in the splenium of the corpus callosum were higher in group 2 than in group 1 indicating that there was loss of myelin in group 2, and similar in groups 1 and 3, showing a tendency to remyelination after 8 weeks. CONCLUSION: The corpus callosum of dogs with kaolin-induced hydrocephalus responds with demyelination of the knee and splenium by the fourth week with a tendency to remyelination by the eighth week.
Hydrocephalus; Corpus Callosum; Myelin Sheath; Dogs
OBJETIVO: Analisar a dilatação ventricular e a mielinização do corpo caloso em cães adultos após quatro e oito semanas da indução de hidrocefalia por caulin. MÉTODOS: 36 cães foram aleatoriamente divididos em 3 grupos: 1- sem hidrocefalia, 2- quatro semanas de hidrocefalia induzida por caulin, 3- oito semanas de hidrocefalia induzida por caulin. As razões e volumes ventriculares foram calculados utilizando imagens de ressonância magnética, e, a mielinização do corpo caloso por estudo histológico (coloração com solocromo- cianina). RESULTADOS: Hidrocefalia foi observada radiologicamente em 93,75% e a mortalidade global foi de 38,4%. Os volumes e as razões ventriculares foram maiores nos grupos 2 e 3 em relação ao grupo 1 e semelhantes nas quarta e oitava semanas no grupo 3. Índices de luminescência no joelho e no esplênio do corpo caloso foram maiores no grupo 2 em relação ao grupo 1, indicando que houve perda de mielina no grupo 2, e semelhantes nos grupos 1 e 3, mostrando uma tendência à remielinização em torno de 8 semanas. CONCLUSÃO: O corpo caloso de cães com hidrocefalia induzida por caulin responde com desmielinização no joelho e esplênio em torno de quatro semanas com tendência à remielinização em torno da oitava semana.
Hidrocefalia; Corpo Caloso; Bainha de Mielina; Cães
3 - ORIGINAL ARTICLE MODELS, BIOLOGICAL
Changes caused by hidrocephalus, induced by kaolin, in the corpus callosum of adult dogs1 1 Research performed at Division of Neurosurgery, Department of Surgery and Anatomy, Faculty of Medicine of Ribeirao Preto, University of Sao Paulo (FMRP-USP), Sao Paulo, Brazil.
Alterações causadas pela hidrocefalia, induzida por caulim, no corpo caloso de cães adultos
Edvaldo José Rodrigues CardosoI; João José LachatII; Luiza Silva LopesIII; Antonio Carlos dos SantosIV; Benedicto Oscar ColliV
IPhD, Division of Neurosurgery, Department of Surgery and Anatomy, FMRP-USP, Ribeirao Preto-SP, Brazil. Responsible for manuscript writing, responsible for intellectual and scientific content of the study, responsible for English language, macroscopic and histopathological examinations, supervised all phases of the study
IIPhD, Assistant Professor, Head of Division of Anatomy, Department of Surgery and Anatomy, FMRP-USP, Ribeirao Preto, Sao Paulo, Brazil. Designed the protocol, involved with technical procedures, critical revision, helped with technical procedures
IIIPhD, Assistant Professor, Division of Anatomy, Department of Surgery and Anatomy, FMRP-USP, Ribeirao Preto, Sao Paulo, Brazil. Designed the protocol, involved with technical procedures, critical revision, helped with technical procedures
IVPhD, Assistant Professor, Division of Radiology, Department of Clinic Medicine, FMRP-USP, Ribeirao Preto, Sao Paulo, Brazil. Designed the protocol, involved with technical procedures, critical revision, helped with technical procedures
VPhD, Chairman and Head of the Division of Neurosurgery, Department of Surgery and Anatomy, FMRP-USP, Ribeirao Preto, Sao Paulo, Brazil. Supervised all phases of the study, designed the protocol, involved with technical procedures, critical revision, helped with technical procedures, provided guidelines for the surgical interventions, statistical analysis
Correspondence 1 Research performed at Division of Neurosurgery, Department of Surgery and Anatomy, Faculty of Medicine of Ribeirao Preto, University of Sao Paulo (FMRP-USP), Sao Paulo, Brazil.
ABSTRACT
PURPOSE: To analyze the ventricular enlargement and myelination of the corpus callosum in adult dogs after four and eight weeks of kaolin-induction of hydrocephalus.
METHODS: 36 dogs were randomly divided into 3 groups: 1 - without hydrocephalus, 2 - kaolin-induction of hydrocephalus until the fourth week, and 3 - kaolin-induction of hydrocephalus until the eighth week. Ventricular ratios and volumes were calculated using magnetic resonance images, and myelination of the corpus callosum were histologically evaluated using solocromo-cianin stain.
RESULTS: Radiological hydrocephalus was observed in 93.75% and overall mortality was 38.4%. Ventricular volumes and ratios were higher in groups 2 and 3 compared to group 1 and similar when measures in the fourth and eighth weeks were compared in the group 3. Indices of luminescence in the knee and in the splenium of the corpus callosum were higher in group 2 than in group 1 indicating that there was loss of myelin in group 2, and similar in groups 1 and 3, showing a tendency to remyelination after 8 weeks.
CONCLUSION: The corpus callosum of dogs with kaolin-induced hydrocephalus responds with demyelination of the knee and splenium by the fourth week with a tendency to remyelination by the eighth week.
Key words: Hydrocephalus. Corpus Callosum. Myelin Sheath. Dogs.
RESUMO
OBJETIVO: Analisar a dilatação ventricular e a mielinização do corpo caloso em cães adultos após quatro e oito semanas da indução de hidrocefalia por caulin.
MÉTODOS: 36 cães foram aleatoriamente divididos em 3 grupos: 1- sem hidrocefalia, 2- quatro semanas de hidrocefalia induzida por caulin, 3- oito semanas de hidrocefalia induzida por caulin. As razões e volumes ventriculares foram calculados utilizando imagens de ressonância magnética, e, a mielinização do corpo caloso por estudo histológico (coloração com solocromo- cianina).
RESULTADOS: Hidrocefalia foi observada radiologicamente em 93,75% e a mortalidade global foi de 38,4%. Os volumes e as razões ventriculares foram maiores nos grupos 2 e 3 em relação ao grupo 1 e semelhantes nas quarta e oitava semanas no grupo 3. Índices de luminescência no joelho e no esplênio do corpo caloso foram maiores no grupo 2 em relação ao grupo 1, indicando que houve perda de mielina no grupo 2, e semelhantes nos grupos 1 e 3, mostrando uma tendência à remielinização em torno de 8 semanas.
CONCLUSÃO: O corpo caloso de cães com hidrocefalia induzida por caulin responde com desmielinização no joelho e esplênio em torno de quatro semanas com tendência à remielinização em torno da oitava semana.
Descritores: Hidrocefalia. Corpo Caloso. Bainha de Mielina. Cães.
Introduction
Hydrocephalus is defined as an excessive accumulation of cerebrospinal fluid within the cranium which may be associated with the dilation of cerebral ventricles, intracranial hypertension; headache; lethargy; urinary incontinence and ataxia1.
Hydrocephalus is known since antiquity and Hipócrates2 in 5th century BC was the first to describe the clinical aspects and to make post-mortem ventricular punctures in hydrocephalus. HILTON3, in the mid-nineteenth century, described ventricular dilation as an autopsy finding, reviving the interest in this subject abandoned since Hipócrates.2 Burr and Mccarthy4 were the first to attempt to produce experimental hydrocephalus. They unsuccessfully injected several irritant solutions (glycerin, urine, acids, adrenal extracts) to produce hydrocephalus. Further, several possible ways to trigger chronic hydrocephalus from the obstruction of the cerebral aqueduct by cotton injection5, blood6, bacteries7, kaolin-aluminum silicate8, and others were described.
The first structure that suffers because of the increase in CSF pressure and ventricular expansion is the ependyma, followed by the white matter9,10. Axonal degeneration and loss of myelin are observed in chronic hydrocephalus in the corpus callosum. The corpus callosum, that is the major commissural connection between both cerebral hemispheres, may be completely destroyed in extreme degrees of ventricular dilatation11.
There is a great controversy in pathophysiology, evolution, and consequences of treatment of hydrocefallus12. There is no consensus regarding the ideal animal for the experimental production of hydrocephalus, neither for the ideal weight or age of the animal. There is also no consensus regarding the ideal material for the induction of hydrocephalus3. This study intends to contribute to the study of experimental kaolin-induced hydrocephalus in dogs, analyzing the effectiveness of the method, the temporal progression of the event accompanied by magnetic resonance imaging (MRI) and the correlation of ventricular enlargement and myelinization changes of the corpus callosum.
Methods
Thirty-six healthy adult mongrel dogs (Canis familiaris) of both sexes, weighing between 7 and 18 kg, provided by the Central Biotery of the Campus of Ribeirão Preto of the University of São Paulo were used. All dogs underwent screening prior to be housed for exclusion of diseases that could interfere with the final result. After an observation period of 21 days the animals were sequentially selected for inclusion in the study groups. Throughout the experiment the animals were kept according to the standards recommended by the Brazilian College of Animal Experimentation, an affiliate of the International Council for Laboratory Animal Science, always seeking to maintain an ethical procedure in all stages of the research.
Experimental hydrocephalus was induced using in a transdermal injection (with a 40/12 mm disposable needle), of hydrated aluminum silicate / kaolin (30 g in 20 ml 0.9% saline in a dose of 50mg/kg) in the cistern magna8. The dogs were divided into three groups. Group 1 (control) - 10 default anesthetized dogs, submitted to simulation of hydrocephalus by the puncture of the cisterna magna and injection of saline, and MRI examination of the brain and upper cervical cord before, 4 and 8 weeks after the kaolin injection; Group 2 (hydrocephalus) - 16 animals treated with standard sedation and anesthesia, followed by the induction of hydrocephalus through kaolin injection in the cisterna magna, and MRI examination of the brain and upper cervical cord before and 4 weeks after the kaolin injection; Group 3 - (hydrocephalus) - 10 animals submitted to anesthesia, induction of hydrocephalus in a manner similar to group 2 and MRI examination of the brain and upper cervical cord before and 4, and 8 weeks after the kaolin injection. The animals were sacrificed after their last MRI examination. In total 26 dogs underwent the induction of hydrocephalus.
MRI were performed using an 1.5 Tesla (Magneton Vision, Siemens®, Erlangen, Germany) machine and the images were stored on a CD with DICOM® system and subsequently analyzed in computers with the software display, developed by the "Brain Image Centre", Montreal Neurological Institute, McGill University, Montreal - Quebec, Canada. The software was run on the Linux® platform for measurement of ventricular volume, brain and ventricular measures using a coronal section through the optic chiasm (right ventricle). The volume was calculated by counting the voxels, which have 1mm3 each.
After anesthesia, the internal carotid artery of the animals were dissected for continuous infusion of saline, and the right femoral artery were exposed for blood for depletion till occurrence of death. Following, cerebral perfusion was performed with injection of 500 ml of buffered 10% formaldehyde directly injected into the carotid arteries. The brains were removed from the skull through a craniectomy and suspended and fixed in 10% buffered formaldehyde using a cotton yarn number 0 attached to arterial circle (circle of Willis), for 15 days.
After midline section of the brain, 3 mm fragments from the knee, body and splenium of the corpus callosum from the left hemisphere were removed for morphological analysis. The right hemisphere was preserved. The fragments of the corpus callosum were dehydrated and included in paraffin blocks and sectioned in serial sections of 4 micrometers which were mounted on slides and deparaffinized in xylene and rehydrated with the reverse order of alcoholic solutions. Next, the sections were stained using the technique of solocromo-cyanine (myelin sheath blue, cell nuclei in blue-red) for visualization of myelination and measurement of its luminescence. The slides of the three studied regions of the corpus callosum were photographed in a Axioskop® 2 plus (Carl Zeiss) photomicrography system equipped with a AxioCam® HRC (Carl Zeiss) digital camera, for determination of the myelination index, using a Satellite® INT computer and the software Axiovision® (Carl Zeiss). The intensity of myelin is expressed by the inverse ratio of luminescence (the higher the index, the lower the degree of myelination). The measurements of color were made respectively in five fields of each blade. Final numbers were considered as the mean counts and measurements made at each site of the corpus callosum.
Statistical analysis was performed using the nonparametric Mann-Whitney-U and Kruskal-Wallis (analysis of variance - ANOVA) tests, with Dunn's multiple comparisons post test, to compare variables detected at different times in the same group or variables between different groups. An error probability not exceeding 5% for two-tailed tests was considered significant. The tests were performed using the GraphPad PRISM software (version 2.0, GraphPad Software Inc. San Diego, CA).
Results
In total twenty-six dogs underwent induction of hydrocephalus. Twelve dogs died during the experiment, 2 in group one (control) and 10 in hydrocephalus groups (2 and 3) with an overall mortality of 38.4% among animals from hydrocephalus groups). Hydrocephalus was observed radiographically in 15 of 16 (93.75%) dogs that survived 4 weeks. One death observed in the control group (group 1) was due to an accident during the puncture of the cisterna magna and another that died between the fourth and eighth weeks had the cause not identified. Of the 10 deaths observed in animals of experimental groups (groups 2 and 3), 7 occurred before the fourth week after induction of hydrocephalus.
Ventricular ratios obtained by MRI in three groups of animals are presented in Table 1.
There was no significant difference between the ventricular ratios obtained immediately before and after 4 and 8 weeks in animals of group 1 (simulation of induction of hydrocephalus), p = 0, 9637, three groups, Kruskal-Wallis - ANOVA.
A significant increase in ventricular ratios was observed at 4 weeks after induction of hydrocephalus in the animals of group 2 (4 weeks hydrocephalus) in relation to that obtained immediately before induction (p = 0, 0286, Mann- Whitney).
There was a significant difference between the ventricular ratios obtained before and after 4 and 8 weeks of hydrocephalus induction (p <0.0010, three groups, Kruskal-Wallis - ANOVA) in the animals of group 3 (4 and 8 weeks hydrocephalus), with It was observed significant difference between the ratios obtained before and at 4 and before and at 8 weeks after induction of hydrocephalus (p <0.01 for both), and no significant difference between the ratios obtained at 4 and 8 weeks (p> 0.05, Dunn's multiple comparison post test).
The ventricular volumes obtained by MRI in three groups of animals are presented in Table 2.
There was no significant difference between the ventricular volumes obtained immediately prior to the simulation of hydrocephalus induction (group 1) or hydrocephalus induction (groups 2 and 3) (p = 0,2133, three groups [Kruskal- Wallis - ANOVA]). There was a significant difference among the left ventricular volumes obtained after 4 weeks in animals of three groups (p = 0, 0010, three groups [Kruskal-Wallis - ANOVA]). The Dunn's multiple comparison post test showed significant difference between the volumes obtained in animals in groups 1 and 2 (p <0.001) and groups 1 and 3 (p <0.01) and showed no significant difference between groups 2 and 3 (p> 0.05).
There was no significant difference between the ventricular volumes obtained immediately before and after 4 weeks of the simulation of hydrocephalus induction in group 1 (p = 0, 3154, Mann-Whitney).
A significant increase in the ventricular volume was observed at 4 weeks after induction of hydrocephalus in relation to that obtained immediately before induction (p = 0, 020, Mann- Whitney) in animal of group 2 (4 weeks hydrocephalus).
There was a significant difference between left ventricular volumes obtained before and after 4 and 8 weeks of induction of hydrocephalus (p <0.0002, three groups [Kruskal-Wallis - ANOVA]) in animals of group 3 (8 weeks hydrocephalus). The Dunn's multiple comparison post test showed significant difference between the ratios obtained before and 4 weeks after induction of hydrocephalus (p <0.01), and before and 8 weeks after induction (p <0.001 ), and showed no significant difference between the ratios obtained from 4 and 8 weeks after hydrocephalus induction (p> 0.05).
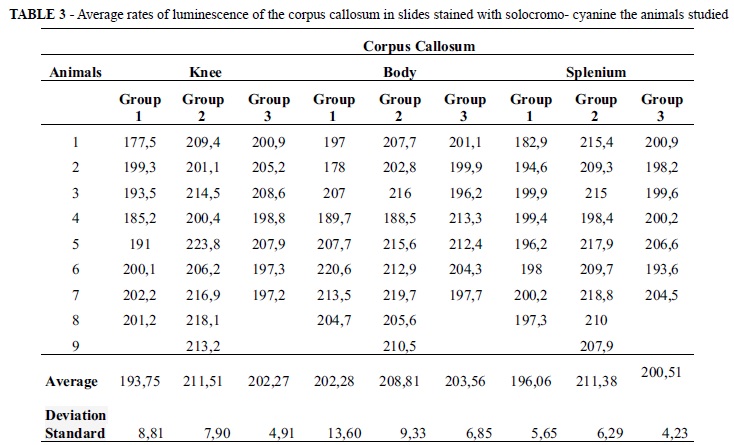
The average rates obtained by analysis of luminescence of solocromo - cyanine staining in the corpus callosum of animal studies are presented in Table 3.
There was significant difference between the rates of luminescence observed on the knee and the splenium of the corpus callosum among the animals of groups 1, 2 and 3 (respectively p = 0, 0024, three groups [and p = 0,0009, three groups [Kruskal-Wallis - Anova]). Dunn's multiple comparison post test showed higher levels of luminescence in group 2 compared with group 1 in the two regions of the corpus callosum (p <0.05 for both) indicating that there was loss of myelin in group 2 and showed no significant difference between the rates of luminescence obtained in groups 1 and 3, and groups 2 and 3 (p> 0.05 for both).
There was no significant difference between the levels of luminescence observed on the body of the corpus callosum between the animals in groups 1, 2 and 3 (p = 0, 3091, three groups [Kruskal-Wallis - Anova]).
Discussion
Although the existence of CSF and hydrocephalus has been known since Hippocrates, there are still major doubts regarding the pathophysiology of hydrocephalus12.
Despite several attempts at the experimental induction of hydrocephalus since the beginning of the past century8, there is no consensus about the best experimental animal model for studying this clinicopathological entity3.
The dog was proved to be a good experimental model for hydrocephalus because it is easy to manipulate and had a good response to the technique used for the induction of hydrocephalus, mainly after injection of kaolin in the cistern magna.
Kaolin is a chemically inert, cheap, easy to handle substance which causes an intense inflammatory response in the meninges, especially at the base of the brain, causing mechanical obstruction of CSF circulation, without injury to the brain parenchyma, when injected directly in the cisterna magna of the dogs13, through transcutaneous puncture. One or two days after the injection of kaolin in cisterna, ventricular dilation starts, on the first lateral ventricles dilating, followed by downwards progressively14.
In our study, injection of kaolin (dose of 50 mg / kg) in the cisterna magna, was efficient in the induction of hydrocephalus (93.7% of dogs developed radiologically hydrocephalus). Our success rate of kaolin-induced hydrocephalus was similar to those reported by Hochwald15, Defeo14, Darder8 and discordant from the results published by Ingraham,16 Bachs and Walker6. The reasons for which kaolin does not induce hydrocephalus in all of the dogs are not yet fully understood. Kaolin produces an intense inflammation restricted to the meninges and consequentional obstruction of the foramina of Luschka and Magendie beyond the obliteration of the basal cisterns and of the cisterna magna. Thus, it not only hinders the circulation, but also the reuptake of LCR16.
Puncture of magna cisterna by used tecnics8, was relatively easy and safe because the passage of the needle through the duramater was generally noticeable. Only one dog from the group 1 probably died due to bulbar injury during the cisterna magna puncture during the study.
Despite the high positivity rate of hydrocephalus in the remaining dogs, the mortality observed during the experiment was high (12 of 36 dogs in total - an index of 33.3%, and 10 dogs in 26 experimental groups 2 and 3 - 38.4 index %). Of the 10 deaths observed in animals of experimental groups (groups 2 and 3), 7 occurred before the fourth week after hydrocephalus induction, and coincident with the period of acute hydrocephalus8. Acute changes in CSF dynamics triggered by the induction of hydrocephalus associated with other factors such as prior nutritional status and due to inadequate compensatory CSF pathways functioning, may have been the cause of death of these animals.
On the MRI performed prior to hydrocephalus induction, the lateral ventricle of the dog is seen as an almost virtual frontal horn with a small ventricular dilation in the atrium. By the fourth week the ventricular cavities are better visualized and these findings were documented by measuring the ventricular volume and ventricular IRM reasons. Even the central cavity of the bulb and the higher portions of the cervical cord could be visualized (syringobulbia and syringomyelia).
The MRI examination proved to be very effective for monitoring the evolving hydrocephalus and the syringobulbia and syringomyelia in dogs, with kaolin-induced hydrocephalus in the cisterna magna. Syringomyelia and syringobulbia was not analyzed in this work.
Ventricular ratios and volumes in the MRI suffered a significant increase in the fourth week after hydrocephalus induction in animals at groups 2 and 3 compared to animals of group 1. In animals of group 3, who had further evaluation after 8 weeks, the volumes and ventricular ratios remained stable compared to the measures acquired in the fourth week. From the consistent evolution of reason and ventricular volume measured by MRI and due to the facility for performing its measurement, allowed to infer that, the ventricular ratio can be used alone to monitor the evolution of experimental hydrocephalus. Nevertheless, these data disagree with McAllister17, who studied hydrocephalus in adult dogs induced by the injection of cyanoacrylate glue prior to the fourth ventricle and observed a large evolutionarily ventricular volume increase compared with the ventricular ratio index by Evans (obtained by measuring the coronal section and the distance between the lateral portions of the lateral ventricles divided by the distance over the side of the brain parenchyma, at the level of the foramen of Monro).
Darder8 reported experimental kaolin-induced hydrocephalus in dogs occurring in two distinct phases: an initial hypertensive one and one after the fourth or fifth week of induction, in which there is a clearing of the clinical picture. Subjectively, this difference was also noted in our study, as opposed to the increase in ventricular volume. These data suggest that the clinical hydrocephalus may be more related to pressure disturbances that the ventricular volume change in adult dogs with kaolin-induced hydrocephalus.
As expected, there was no significant difference between reasons and ventricular volumes measured in MRI scans immediately prior to the simulation / induction of hydrocephalus in experimental and control groups.
The methodology used for the sacrifice the animals, calvariectomy and laminectomy and removal in one piece of the brain and cervical spine in one piece proved to be simple, reproducible and effective, with good preservation of brain specimens.
In this study, the average rates obtained by the analysis of luminescence of solocromo-cyanine staining showed a significant loss in the amount of myelin in the knee and in the splenium of the corpus callosum, but showed no significant difference in the body of the corpus callosum, by 4 weeks after induction of hydrocephalus. One explanation for this may be the larger expansion of the frontal and occipital horns of the ventricles in comparison to the body of the corpus callosum. The evolutionary analysis of the compromise of myelin showed that the levels of luminescence measurements performed 8 weeks after hydrocephalus induction had been reduced compared to measurements made in the fourth week, returning to values similar to the measurements made before hydrocephalus induction. This suggests an attempt of to remyelination. These data are in agreement with the work of Del Bigio18 and Lopes19.
Further studies are needed to better understand the pathophysiology of hydrocephalus lesions produced in the corpus callosum and other structures of the brain parenchyma. With the collected material, studies should be made by Immunohistochemical and silver impregnation evolution studies and the differentiation of oligodendrocytes and astrocytes of the corpus callosum, and the subcortical white substance of the cervical cord, will be performed using the material already obtained in this study trying better to clarify the presented the questions.
Conclusions
Injection of kaolin in the magna cisterna was effective for inducing hydrocephalus in adult dogs. Measure of the ventricular ratios and volumes using the magnetic resonance imaging proved to be a good method for monitoring the experimental hydrocephalus in adult dogs. With the concordant results of ventricular ratio and volume measured by MRI, and the facility for its performance allowed, to infer that the measurement of the ratio alone can be used for monitoring the evolution of experimental hydrocephalus. The corpus callosum of dogs did not respond homogeneously to the kaolin-induced hydrocephalus. There was a loss of myelin in the knee and in the splenium of the corpus callosum by four weeks after hydrocephalus induction and partial remyelination by the eighth week.
Correspondence:
Edvaldo José Rodrigues Cardoso
Faculdade de Medicina de Ribeirão Preto
Departamento de Cirurgia e Anatomia
Divisão de Neurocirurgia
Av. Bandeirantes, 3900
Campus Universitário Monte Alegre
14048 - 900 Ribeirão Preto - SP Brasil
Conflict of interest: none
Financial source: FAPESP
Presented at the XII National Congress on Experimental Surgery of the Brazilian Society for Development of Research in Surgery-SOBRADPEC, 2011 October 26-29 Ribeirao Preto-SP, Brazil.
-
1Disponível em: http://www.ncbi.nlm.nih.gov/mesh/68006849
» link - 2. Jason IL, Walter DJ. History of hydrocephalus and its treatments. Neurosurg Focus. 2001;15;11:E1
- 3. Hochwald GM. Animals models of hydrocephalus: recents developments. Proc Soc Exp Biol Med. 1985;178:1-11.
- 4. Burr CW, McCarthy DJ. Acute internal hydrocephalus. A clinical and pathological Study. J Exp Med. 1900;5:195-8.
- 5. Dandy WE, Blackfan KD. An experimental and clinical study of internal hydrocephalus. J Am Med Assoc. 1913;61:2216-7.
- 6. Bachs A, Walker AE. Experimental hydrocephalus. J Neuropathol Exp Neurol. 1953;12:283-2.
- 7. Flexner RS. Experimental cerebrospinal meningitis in monkeys. J Exp Med. 1907;9:142-67.
- 8. Darder JG, Barbera AJ, Nicolas MC, Segura D, Broseta J, Salorio JCB. Sequential morphological and functional changes in kaolin induced hydrocephalus. J Neurosurg. 1984;61:918-24.
- 9. Del Bigio MR, Bruni E, Fewer HD. Human neonatal hydrocephalus. An electron microscopic study of the periventricular tissue. J Neurosurg. 1985;63:56-63.
- 10. Kiefer M, Eymann R, Von Tiling S, Müller A, Steudel W. I, Booz K. H. The ependyma in chronic hydrocephalus. Child's Nerv Syst. 1998;14:263- 70.
- 11. Mabe H, Suzuki K, Nagai H. Cerebral blood flow after ventriculoperitoneal shunt in children with hydrocephalus. Childs Nerv Syst. 1990;6:388-91.
- 12. Bergsneider M, Egnor MR, Johnston M, Kranz D, Madsen N JR, McAllister JP 2nd , Stewart C, Walker ML, Williams MA. What we don't (but should) know about hydrocephalus. J Neurosurg. 2006;104:157-9.
- 13. Hochwald GM, Lux WE, Sahar A, Ransohoff J. Experimental hydrocephalus: changes in CSF dynamics as a function of time. Arch Neurol. 1972;26:120-9.
- 14. Defeo DR, Myers P, Foltz EL, Everett B, Ramshaw B. Histological examination of kaolin-induced hydrocephalus. Its implications in the therapy of animals with experimentally induced hydrocephalus. J Neurosurg. 1979;50:70-4.
- 15. Hochwald GM, Epstein F, Malhan E, Ransohoff J. The relationship of compensated to decompensated hydrocephalus in the cat. J Neurosurg. 1973;39:694-7.
- 16. Ingraham FD, Matson DD, Alexander JR, Woods RP. Studies in the treatment of experimental Hydrocephalus. J Neurop Exp Neurol. 1948;7/2:123-43.
- 17. McAllister JP 2nd, Chovan P, Steiner CP, Johnson MJ, Aysman I, Wood AS, Tkach JA, Hahn JF, Luciano MG. Differential ventricular expansion in hydrocephalus. Eur J Pediatr Surg. 1998;8:39-42.
- 18. Del Bigio MR, Wilson MJ, Enno T. Chronic hydrocephalus in rats and humans: white matter loss and behavior changes. Ann Neurol. 2003;53:337-46.
- 19. Lopes LS, Machado HR, Lachat JJ. Study of corpus callosum in experimental hydrocephalic wistar rats. Acta Cir Bras. 2003;18 (Suppl. 5):10-4.
Publication Dates
-
Publication in this collection
24 Oct 2011 -
Date of issue
2011