Abstract
Purpose:
To investigate the effects of pycnogenol on peritoneal adhesions and additionally to investigate the immunohistochemical effects of free oxygen radicals and reactive lymph nodes detected in the adhesive tissue that was sampled surrounding the cecum on intra-abdominal adhesions.
Methods:
Twenty-seven Wistar Albino rats were divided into three groups. In group 1 (sham), laparotomy was performed and stitched up. In group 2 (control), after laparotomy was performed, punctate hemorrhage was induced by cecal abrasion in the cecum and each rat was intraperitoneally administered 2 cc of saline. In group 3 (experimental), after laparotomy was performed, punctate hemorrhage was induced by cecal abrasion in the cecum and each rat was intraperitoneally administered a sterile Pycnogenol derivative. The rats in all groups were re-laparotomized on postoperative day 7; samples were obtained from the peritoneal tissue surrounding the cecum, and the rats were sacrificed.
Results:
In group 3, there was a statistically significant difference in terms of inflammation, lymph node size, and free oxygen radicals; these parameters tended to increase. In terms of fibrosis evaluated using H&E and MT, there was no significant difference between groups 2 and 3.
Conclusions:
No positive outcomes indicating that pycnogenol reduces intra-abdominal adhesions were obtained. However, it caused severe inflammation in the tissue. Moreover, a significant increase in lymph node size was detected secondary to inflammation. Additionally, in immunohistochemical analyses conducted to detect oxidative stress, pycnogenol increased the production of free oxygen radicals in the tissue.
Key words:
Pycnogenol; Lymphadenopathy; Free Radicals; Rats.
Introduction
Today, intra-abdominal adhesions occurring in the postoperative period have become more common due to the increasing number of abdominal operations performed as a result of development in anesthesia and surgical techniques. Peritoneal adhesions are one of the most significant problems to be overcome via surgical procedures11 Durmus AS, Yildiz H, Yaman I, Simsek H. Efficacy of vitamin E and selenium for the prevention of intraabdominal adhesions in rats: uterine horn models. Clinics. 2011;66:1247-251. doi: 10.1590/S1807-59322011000700021.
https://doi.org/10.1590/S1807-5932201100...
,22 Sahbaz A, Aynioglu O, Isik H, Gun BD, Cengil O, Erol O. Pycnogenol prevents peritoneal adhesions. Arch Gynecol Obstet. 2015;292:1279. doi: 10.1007/s00404-015-3764-4.
https://doi.org/10.1007/s00404-015-3764-...
. Researchers have conducted studies for preventing complications that may result in morbidity, particularly intestinal obstruction and infertility33 Brochhausen C, Schmitt VH, Planck CN, Rajab TK, Hollemann D, Tapprich C, Krämer B, Wallwiener C, Hierlemann H, Zehbe R, Planck H, Kirkpatrick CJ. Current strategies and future perspectives for intraperitoneal adhesion prevention. J Gastrointest Surg. 2012;16:1256-74. doi: 10.1007/s11605-011-1819-9.
https://doi.org/10.1007/s11605-011-1819-...
. These complications are serious and may require reoperation. Reoperations may cause high morbidity and mortality rates. The etiopathogenesis of peritoneal adhesions is not precisely known; however, inflammation, ischemia, and the presence of oxidative stress may be prevalent its etiopathogenesis22 Sahbaz A, Aynioglu O, Isik H, Gun BD, Cengil O, Erol O. Pycnogenol prevents peritoneal adhesions. Arch Gynecol Obstet. 2015;292:1279. doi: 10.1007/s00404-015-3764-4.
https://doi.org/10.1007/s00404-015-3764-...
,44 Fletcher NM, Abuanzeh S, Saed MG, Diamond MP, AbuSoud HM, Saed GM. Nicotinamide adenine dinucleotide phosphate oxidase expression is differentially regulated to favor a prooxidant state that contributes to postoperative adhesion development. Reprod Sci. 2014;21:1050-9. doi: 10.1177/1933719114522524.
https://doi.org/10.1177/1933719114522524...
5 Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17:4545-53. doi: 10.3748/wjg.v17.i41.4545.
https://doi.org/10.3748/wjg.v17.i41.4545...
-66 Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260-73. doi: 10.1159/000050149.
https://doi.org/10.1159/000050149...
. Many types of steroids and non-steroidal anti-inflammatory, fibrinolytic, and antioxidant agents as well as different techniques have been used to reduce or prevent the formation of postoperative peritoneal adhesions22 Sahbaz A, Aynioglu O, Isik H, Gun BD, Cengil O, Erol O. Pycnogenol prevents peritoneal adhesions. Arch Gynecol Obstet. 2015;292:1279. doi: 10.1007/s00404-015-3764-4.
https://doi.org/10.1007/s00404-015-3764-...
,55 Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17:4545-53. doi: 10.3748/wjg.v17.i41.4545.
https://doi.org/10.3748/wjg.v17.i41.4545...
,77 Kamel Remah M. Prevention of postoperative peritoneal adhesions. Eur J Obstet Gynecol Reprod Biol. 2010;150:111-8. doi: 10.1016/j.ejogrb.2010.02.003.
https://doi.org/10.1016/j.ejogrb.2010.02...
. Additionally, conditions such as inflammation and infection that are secondary to intra-abdominal operations may cause adhesions, whereas they may be responsible for the etiopathogenesis of lymphadenopathy that may develop secondary to inflammation22 Sahbaz A, Aynioglu O, Isik H, Gun BD, Cengil O, Erol O. Pycnogenol prevents peritoneal adhesions. Arch Gynecol Obstet. 2015;292:1279. doi: 10.1007/s00404-015-3764-4.
https://doi.org/10.1007/s00404-015-3764-...
,77 Kamel Remah M. Prevention of postoperative peritoneal adhesions. Eur J Obstet Gynecol Reprod Biol. 2010;150:111-8. doi: 10.1016/j.ejogrb.2010.02.003.
https://doi.org/10.1016/j.ejogrb.2010.02...
. In the present study, we aimed to investigate the effects of Pycnogenol, also known as Pinus pinaster bark extract, on peritoneal adhesions; the antioxidant, antimicrobial, and anti-inflammatory effects of Pycnogenol have been reported in the literature in terms of preventing and reducing intra-abdominal adhesions that cause serious complications88 Peng YJ, Lee CH, Wang CC, Salter DM, Lee HS. Pycnogenol attenuates the inflammatory and nitrosative stress on joint inflammation induced by urate crystals. Free Radic Biol Med. 2012;52:765-74. doi: 10.1016/j.freeradbiomed.2011.12.003.
https://doi.org/10.1016/j.freeradbiomed....
9 Rohdewald PA. Review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002;40:158-68. PMID: 11996210.
10 Petrassi C, Mastromarino A, Spartera C. PYCNOGENOL in chronic venous insufficiency. Phytomedicine. 2000;7:383-8. doi: 10.1016/S0944-7113(00)80059-8.
https://doi.org/10.1016/S0944-7113(00)80...
11 Torras MA, Faura CA, Schönlau F, Rohdewald P. Antimicrobial activity of pycnogenol. Phytother Res. 2005;19:647-48. doi: 10.1002/ptr.1662.
https://doi.org/10.1002/ptr.1662...
-1212 Iravani S, Zolfaghari B. Pharmaceutical and nutraceutical effects of Pinus pinaster bark extract. Res Pharm Sci. 2011;6:1-11. PMID: 22049273.. Additionally, we aimed to investigate the immunohistochemical effects of free oxygen radicals and reactive lymph nodes detected in the adhesive tissue that was sampled surrounding the cecum on intra-abdominal adhesions.
Methods
Experimental protocol
This study was performed in the laboratory of Canakkale Onsekiz Mart University Health Sciences Research and Application Center, Turkey, with the approval of the university’s Laboratory Animals Ethics Committee (No: 2017/27348).
All rats obtained from the Laboratory Animal Research Center of Canakkale Onsekiz Mart University, were used in this study and were housed in special metal cages at room temperature under stable environmental conditions (21 ± 2°C, 12-h light/dark cycle). The rats were given access to normal water and standard food, with no restriction. Before the experimental procedure, all rats were weighed with an analytical balance and body weights (BW) were recorded. Wistar albino rats weighing 200-240 g were divided into three groups (in each group, n = 9). All rats were anesthetized with intramuscular (IM) ketamine (Ketalar 500 mg, 90 mg/kg body weight [BW]; Pfizer) and xylazine (Kepro Xylazine 20, 10 mg/kg BW; Biopharm Veterinary Drugs).
Group 1 (sham group): Rats were laparotomized and stitched up under anesthesia. On postoperative day 7, the rats were relaparotomized under anesthesia; the peritoneal tissue surrounding the cecum was sampled, and the rats were sacrificed.
Group 2 (control group: physiological saline solution): After rats were laparotomized under anesthesia, punctate hemorrhage was induced by cecal abrasion in the cecum (Figure 1); each rat was intraperitoneally administered 2 cc of saline solution. On postoperative day 7, the rats were relaparotomized under anesthesia; the peritoneal tissue surrounding the cecum was sampled, and the rats were sacrificed.
Group 3 (experimental group: Pycnogenol): After rats were laparotomized under anesthesia, punctate hemorrhage was induced by cecal abrasion in the cecum; each rat was intraperitoneally administered a sterile Pycnogenol derivative diluted with 10 mg/kg saline. On postoperative day 7, the rats were relaparotomized under anesthesia; the peritoneal tissue surrounding the cecum was sampled, and the rats were sacrificed.
Histological examination
All specimens were fixed with 10% buffered formaldehyde. Specimens embedded in paraffin blocks were stained with hematoxylin and eosin (H&E) by taking 4-5-μm-thick sections. In addition to Hematoksilen & Eozin (H&E), specimens were histochemically stained with Masson’s Trichrome (MT) by taking 3-4-μm-thick sections. In addition, immunohistochemical staining was performed using Glutathione S-transferase (GSTP-1), Glutathione Reductase (Glut Red), Superoxide dismutase (SOD1) and Catalase (CAT) with DAKO kits in a DAKO automatic staining device by taking 3-μm-thick sections from paraffin blocks.
Histopathologically, the following parameters were evaluated (Chart 1):
-
- Fibrosis
-
- Inflammation
-
- Number of lymph nodes
-
- Size of the largest lymph node
The effects of fibrosis were evaluated as follows:
No effect: 0; Minor effect: 1; Moderate effect: 2; Intense effect: 3.
GSTP-1, SOD-1, CAT, and Glut Red staining were evaluated as follows:
0: no staining; 1: weak positivity; 2: moderate positivity; 3: strong positivity;
Histochemical fibrosis assessments performed using MT were evaluated as follows:
0: no staining; 1: weak positivity; 2: moderate positivity; 3: strong positivity.
Statistical analysis
The data were analyzed by the IBM SPSS 20 program. The normal distribution of the variables was investigated with visual (histogram and probability plots) and analytical methods taking account of sample size (Kolmogorow Smirnow test). As all variables did not have normal distribution, the Kruskal-Wallis test was used for comparisons between groups. Descriptive statistics are given as arithmetic mean ± standard deviation and median (quartiles). A P < 0.05 was accepted as statistically significant. For pairwise comparisons, the One-Way ANOVA test was used. Differences among groups and changes in course of time for number of lymph nodes and total scores variants were analyzed with two way variance analysis. Bonferroni test was used for the differences among groups. Statistical significance level was accepted as P < 0.05.
Results
From statistical analyses, the results of specimens in groups 1, 2, and 3 were not normally distributed; therefore, they were compared using the Kruskal-Wallis test.
-Inflammation (Figures 2 and 4), fibrosis, lymph node size (Figure 3) and histochemical fibrosis assessment using MT (Figure 5) parameters were evaluated by the Mann-Whitney U test in pair-group analysis. The asymptomatic sign value between groups 1, 2, and 3 for each parameter was P 0.001 (P < 0.05); and there was a statistical difference between all groups.
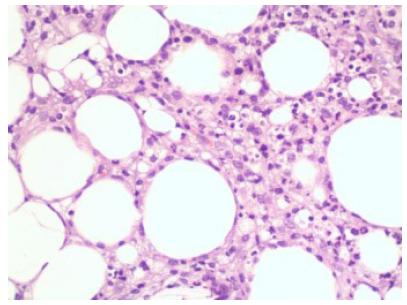
Foreign body reaction against Pycnogenol accompanied by severe inflammation and osteoclastic giant cells in mature adipose tissue (hematoxylin-eosin staining ×100).
Strong positivity (score 3) with Masson’s trichrome (Histochemistry Masson’s trichrome staining ×40).
-For fibrosis: (P < 0.05)
P-value between groups 1 and 2 was 0.001 and statistically significant.
P-value between groups 1 and 3 was 0.001 and statistically significant.
P-value between groups 2 and 3 was 0.730 and was not statistically significant.
-For inflammation: (P < 0.05)
P-value between groups 1 and 2 was 0.005 and statistically significant.
P-value between groups 1 and 3 was 0.001 and statistically significant.
P-value between groups 2 and 3 was 0.002 and statistically significant.
-For histochemical assessment performed with MT: (P < 0.05)
P-value between groups 1 and 2 was 0.001 and statistically significant.
P-value between groups 1 and 3 was 0.001 and statistically significant.
P-value between groups 2 and 3 was 0.996 and was not statistically significant.
-In terms of lymph node size between the groups: (P < 0.05)
P-value between groups 1 and 2 was 0.003 and statistically significant.
P-value between groups 1 and 3 was 0.001 and statistically significant.
P-value between groups 2 and 3 was 0.024 and statistically significant.
-The total immunohistochemical score and number of lymph nodes were normally distributed, and statistical evaluation between the three groups was done using one-way ANOVA.
-- In terms of the total immunohistochemical score:
The mean values were 2.33 in group 1, 7.44 in group 2, and 7.89 in group 3.
The P-value was 0.0001 (P < 0.005), and there was a significant difference.
In immunohistochemical analyses (SOD-1, Glut Red, CAT, GSTP-1) on free oxygen radicals, Pycnogenol statistically increased the production of free oxygen radicals in tissues (Figures 6 to 10).
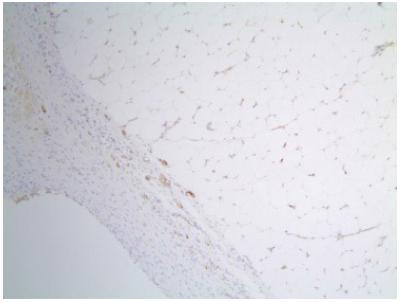
Strong staining (score 3) with SOD-1 in adipose tissue (immunohistochemistry SOD-1 staining ×400).
-In terms of number of lymph nodes:
The means values were 3.1111 in group 1, 3.6667 in group 2, and 1.4444 in group 3.
The P-value was 0.017 (P < 0.005), and there was no statistically significant difference.
In terms of the total immunohistochemical score and number of lymph nodes, post hoc pair-group analyses were evaluated using Bonferroni correction ***. (The mean difference was significant at the level of 0.15.) (*** In terms of the total immunohistochemical score, the P-value was 0.001 between groups 1 and 2, 0.0001 between groups 1 and 3, and 0.996 between groups 2 and 3. *** In terms of the number of lymph nodes, the P-value was 0.996 between groups 1 and 2, 0.101 between groups 1 and 3, and 0.018 between groups 2 and 3.). Tables 1 to 3 list all evaluated parameters.
Discussion
At present, peritoneal adhesions are still one of the most important complications to be overcome in surgical procedures. Bleeding, cauterization, ischemia, infections, and conditions that may damage the peritoneum may trigger adhesions55 Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17:4545-53. doi: 10.3748/wjg.v17.i41.4545.
https://doi.org/10.3748/wjg.v17.i41.4545...
6 Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260-73. doi: 10.1159/000050149.
https://doi.org/10.1159/000050149...
-77 Kamel Remah M. Prevention of postoperative peritoneal adhesions. Eur J Obstet Gynecol Reprod Biol. 2010;150:111-8. doi: 10.1016/j.ejogrb.2010.02.003.
https://doi.org/10.1016/j.ejogrb.2010.02...
. While numerous cells such as mesothelial cells, histiocytes, fibroblasts, and lymphocytes help in healing the injured area, they also play a role in the development of peritoneal adhesion22 Sahbaz A, Aynioglu O, Isik H, Gun BD, Cengil O, Erol O. Pycnogenol prevents peritoneal adhesions. Arch Gynecol Obstet. 2015;292:1279. doi: 10.1007/s00404-015-3764-4.
https://doi.org/10.1007/s00404-015-3764-...
3 Brochhausen C, Schmitt VH, Planck CN, Rajab TK, Hollemann D, Tapprich C, Krämer B, Wallwiener C, Hierlemann H, Zehbe R, Planck H, Kirkpatrick CJ. Current strategies and future perspectives for intraperitoneal adhesion prevention. J Gastrointest Surg. 2012;16:1256-74. doi: 10.1007/s11605-011-1819-9.
https://doi.org/10.1007/s11605-011-1819-...
4 Fletcher NM, Abuanzeh S, Saed MG, Diamond MP, AbuSoud HM, Saed GM. Nicotinamide adenine dinucleotide phosphate oxidase expression is differentially regulated to favor a prooxidant state that contributes to postoperative adhesion development. Reprod Sci. 2014;21:1050-9. doi: 10.1177/1933719114522524.
https://doi.org/10.1177/1933719114522524...
-55 Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17:4545-53. doi: 10.3748/wjg.v17.i41.4545.
https://doi.org/10.3748/wjg.v17.i41.4545...
. It has been demonstrated that adhesions are often the result of improper healing due to injured peritoneal tissues associated with oxidative stress22 Sahbaz A, Aynioglu O, Isik H, Gun BD, Cengil O, Erol O. Pycnogenol prevents peritoneal adhesions. Arch Gynecol Obstet. 2015;292:1279. doi: 10.1007/s00404-015-3764-4.
https://doi.org/10.1007/s00404-015-3764-...
. In our study, in addition to parameters such as fibrosis and inflammation, we performed GSTP-1, Glut Red, SOD-1, and CAT staining to immunohistochemically examine oxidative stress parameters in tissues. After postoperative days 5 and 7, these adhesive strips progressed to persistent fibrous adhesions22 Sahbaz A, Aynioglu O, Isik H, Gun BD, Cengil O, Erol O. Pycnogenol prevents peritoneal adhesions. Arch Gynecol Obstet. 2015;292:1279. doi: 10.1007/s00404-015-3764-4.
https://doi.org/10.1007/s00404-015-3764-...
,1313 Holmdahl L, Ivarsson ML. The role of cytokines, coagulation, and fibrinolysis in peritoneal tissue repair. Eur J Surg. 1999;165:1012-9. doi: 10.1080/110241599750007810
https://doi.org/10.1080/1102415997500078...
. It was also reported that hypoxia directly promotes the production of free oxygen radicals in tissues22 Sahbaz A, Aynioglu O, Isik H, Gun BD, Cengil O, Erol O. Pycnogenol prevents peritoneal adhesions. Arch Gynecol Obstet. 2015;292:1279. doi: 10.1007/s00404-015-3764-4.
https://doi.org/10.1007/s00404-015-3764-...
,1414 Awonuga AO, Belotte J, Abuanzeh S, Fletcher NM, Diamond MP, Saed GM. Advances in the pathogenesis of adhesion development: the role of oxidative stress. Reprod Sci. 2014;21:823-36. doi: 10.1177/1933719114522550.
https://doi.org/10.1177/1933719114522550...
. With the increase in the production of free oxygen radicals, the production of fibroblasts, tissue growth factor, vascular endothelial growth factor, and cytokines such as cyclooxygenase-2 that cause adhesions also increased1414 Awonuga AO, Belotte J, Abuanzeh S, Fletcher NM, Diamond MP, Saed GM. Advances in the pathogenesis of adhesion development: the role of oxidative stress. Reprod Sci. 2014;21:823-36. doi: 10.1177/1933719114522550.
https://doi.org/10.1177/1933719114522550...
,1515 Fletcher NM, Jiang ZL, Diamond MP, Abu-Soud HM, Saed GM. Hypoxia-generated superoxide induces the development of the adhesion phenotype. Free Radic Biol Med. 2008;45:530-6. doi: 10.1016/j.freeradbiomed.2008.05.002.
https://doi.org/10.1016/j.freeradbiomed....
. As a result, histopathologically, inflammation and fibrosis occur1616 Epstein JC, Wilson MS, Wilkosz S, Ireland G, O’Dwyer ST, Herrick SE. Human peritoneal adhesions show evidence of tissue remodeling and markers of angiogenesis. Dis Colon Rectum. 2006;49:1885-92. doi: 10.1007/s10350-006-0747-3.
https://doi.org/10.1007/s10350-006-0747-...
17 Herrick SE, Mutsaers SE, Ozua P, Sulaiman H, Omer A, Boulos P, Foster ML, Laurent GJ. Human peritoneal adhesions are highly cellular, innervated, and vascularized. J Pathol. 2000;192:67-72. doi:10.1002/1096-9896(2000)9999:9999<::AID-PATH678>3.0.CO;2-E.
https://doi.org/10.1002/1096-9896(2000)9...
-1818 Binnebösel M, Klink CD, Serno J, Jansen PL, von Trotha KT, Neumann UP, Junge K. Chronological evaluation of inflammatory mediators during peritoneal adhesion formation using a rat model. Langenbecks Arch Surg. 2011;396:371-8. doi: 10.1007/s00423-011-0740-8.
https://doi.org/10.1007/s00423-011-0740-...
. There are studies indicating that fibroblasts reduce antioxidant levels similar to free oxygen radicals1414 Awonuga AO, Belotte J, Abuanzeh S, Fletcher NM, Diamond MP, Saed GM. Advances in the pathogenesis of adhesion development: the role of oxidative stress. Reprod Sci. 2014;21:823-36. doi: 10.1177/1933719114522550.
https://doi.org/10.1177/1933719114522550...
15 Fletcher NM, Jiang ZL, Diamond MP, Abu-Soud HM, Saed GM. Hypoxia-generated superoxide induces the development of the adhesion phenotype. Free Radic Biol Med. 2008;45:530-6. doi: 10.1016/j.freeradbiomed.2008.05.002.
https://doi.org/10.1016/j.freeradbiomed....
16 Epstein JC, Wilson MS, Wilkosz S, Ireland G, O’Dwyer ST, Herrick SE. Human peritoneal adhesions show evidence of tissue remodeling and markers of angiogenesis. Dis Colon Rectum. 2006;49:1885-92. doi: 10.1007/s10350-006-0747-3.
https://doi.org/10.1007/s10350-006-0747-...
17 Herrick SE, Mutsaers SE, Ozua P, Sulaiman H, Omer A, Boulos P, Foster ML, Laurent GJ. Human peritoneal adhesions are highly cellular, innervated, and vascularized. J Pathol. 2000;192:67-72. doi:10.1002/1096-9896(2000)9999:9999<::AID-PATH678>3.0.CO;2-E.
https://doi.org/10.1002/1096-9896(2000)9...
-1818 Binnebösel M, Klink CD, Serno J, Jansen PL, von Trotha KT, Neumann UP, Junge K. Chronological evaluation of inflammatory mediators during peritoneal adhesion formation using a rat model. Langenbecks Arch Surg. 2011;396:371-8. doi: 10.1007/s00423-011-0740-8.
https://doi.org/10.1007/s00423-011-0740-...
. If the production of free oxygen radicals was reduced using antioxidants, adhesive fibroblasts transformed into normal fibroblasts and collagen production decreased1515 Fletcher NM, Jiang ZL, Diamond MP, Abu-Soud HM, Saed GM. Hypoxia-generated superoxide induces the development of the adhesion phenotype. Free Radic Biol Med. 2008;45:530-6. doi: 10.1016/j.freeradbiomed.2008.05.002.
https://doi.org/10.1016/j.freeradbiomed....
. There are several antioxidant agents, and many studies were conducted using them. Keleidari et al.1919 Keleidari B, Mahmoudieh M, Bahrami F, Mortazavi P, Aslani RS, Toliyat SA. The effect of vitamin A and vitamin C on postoperative adhesion formation: a rat model study. J Res Med Sci. 2014;19:28-32. PMID: 24672562. evaluated the effect of vitamin C on postoperative peritoneal adhesions and reported better wound healing and decreased adhesion formation in the experimental group than in the control group. The efficacy of vitamin E in the prevention of post-operative peritoneal adhesion has been reported11 Durmus AS, Yildiz H, Yaman I, Simsek H. Efficacy of vitamin E and selenium for the prevention of intraabdominal adhesions in rats: uterine horn models. Clinics. 2011;66:1247-251. doi: 10.1590/S1807-59322011000700021.
https://doi.org/10.1590/S1807-5932201100...
,2020 de la Portilla F, Ynfante I, Bejarano D, Conde J, Fernández A, Ortega JM, Carranza G. Prevention of peritoneal adhesions by intraperitoneal administration of vitamin E: an experimental study in rats. Dis Colon Rectum. 2004;47:2157-61. doi: 10.1007/s10350-004-0741-6.
https://doi.org/10.1007/s10350-004-0741-...
,2121 Yetkin G, Uludag M, Citgez B, Karakoc S, Polat N, Kabukcuoglu F. Prevention of peritoneal adhesions by intraperitoneal administration of vitamin E and human amniotic membrane. Int J Surg. 2009;7:561-5. doi: 10.1016/j.ijsu.2009.09.007.
https://doi.org/10.1016/j.ijsu.2009.09.0...
. Şahbaz et al.22 Sahbaz A, Aynioglu O, Isik H, Gun BD, Cengil O, Erol O. Pycnogenol prevents peritoneal adhesions. Arch Gynecol Obstet. 2015;292:1279. doi: 10.1007/s00404-015-3764-4.
https://doi.org/10.1007/s00404-015-3764-...
demonstrated that pycnogenol, which is an antioxidant, was effective for preventing peritoneal adhesion on postoperative day 10 owing to its anti-inflammatory and antioxidant effects. However, in contrast to the results of this study, we found that pycnogenol severely increased inflammation and resulted in foreign body reaction in some rats. In the present study, when we consider fibrosis using H & E and MT, there was a statistically significant increase in groups 2 and 3 compared to group 1. However, no significant difference was found between groups 2 and 3. Oxidative stress-induced cellular damage triggers adhesion. In our study, in immunohistochemical analyses performed on tissues taken on day 7 to detect oxidative stress using GSTP-1, Glut Red, SOD-1, and CAT, it was found that Pycnogenol statistically increased free oxygen radicals in the tissues. In a study conducted by Noda et al.2222 Noda Y, Anzai K, Mori A, Kohno M, Shinmei M, Packer L. Hydroxyl and superoxide anion radical scavenging activities of natural source antioxidants using the computerized JES-FR30 ESR spectrometer system. Biochem Mol Biol Int. 1997;42:35-44. PMID: 9192083., pycnogenol was reported to be one of the effective free radical scavengers. However, in our study, immunohistochemical analyses revealed that the production of free oxygen radicals increased in the tissue. We believe that the reason for this was that the intraperitoneal administration of pycnogenol was effective for increasing inflammation. In a study conducted on the efficacy of pycnogenol in wound healing in diabetic rats, it was reported that Pycnogenol is a feasible option to accelerate wound healing2323 Dogan E, Yanmaz L, Gedikli S, Ersoz U, Okumus Z. The effect of pycnogenol on wound healing in diabetic rats. Ostomy Wound Manage. 2017;63:41-7. PMID: 28448268.. In a study using an experimental sepsis model in rats, it was reported that the production of free oxygen radicals in rats treated with Pycnogenol was significantly reduced2424 Taner G, Aydın S, Bacanlı M, Sarıgöl Z, Sahin T, Başaran AA, Başaran N. Modulating effects of pycnogenol on oxidative stress and DNA damage induced by sepsis in rats. Phytother Res. 2014;28:1692-1700. doi: 10.1002/ptr.5184.
https://doi.org/10.1002/ptr.5184...
. In a study conducted in rats, it was reported that Pycnogenol facilitated the healing of colon anastomoses2525 Değer KC, Şeker A, Özer I, Bostancı EB, Dalgıç T, Akmansu M, Ekinci Ö, Erçin U, Bilgihan A, Akoğlu M. The effects of Pycnogenol® on colon anastomotic healing in rats given preoperative irradiation. Int J Surg. 2013;11:983-88. doi: 10.1016/j.ijsu.2013.06.004.
https://doi.org/10.1016/j.ijsu.2013.06.0...
. In the present study, in addition to these histopathological findings, many reactive lymphadenopathies were detected in various sizes in almost all groups. Reactive lymphadenopathies usually occur secondary to infections, inflammation, or malignancy. Lymph nodes have functions such as microorganism filtration and antibody production. Lymph nodes grow as in the presence of microorganisms, malignant cells, or antigenic reaction, they result in the proliferation of lymphocytes or macrophage hyperplasia2626 Ferrer R. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58:1313-20. PMID: 9803196.. In acute inflammation, lymph nodes grow due to cellular infiltration and edema. Similarly, in chronic inflammation, lymph nodes also grow; however, mononuclear cell infiltration is present2626 Ferrer R. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58:1313-20. PMID: 9803196.. In our study, we believe that the presence of acute inflammation, which later becomes chronic, plays a role in the etiology of lymphoid hyperplasia, which was statistically significant, particularly in group 3. The foreign body reaction against Pycnogenol sporadically triggers inflammation and lymphoid hyperplasia. This is one of the positive results we obtained, and we plan to conduct studies to reveal the causes of this result. In the present study, we did not find a significant difference between the groups in terms of lymph node numbers.
Conclusions
Pycnogenol did not result in a reduction in intra-abdominal adhesions; rather, it led to severe inflammation in the tissue. Moreover, we found a significant increase in lymph node size secondary to inflammation. Additionally, in the immunohistochemical analyses, we found that Pycnogenol statistically increased the production of free oxygen radicals in the tissue. Although Pycnogenol was reported to have a positive effect on peritoneal adhesions in the literature, in the present study, we found inflammation in the early phase and a slight increase in the production of free oxygen radicals and the size of lymph nodes. We believe that this effect is due to the fact that we terminated the experimental model early (day 7). Further studies are needed to evaluate the effects of Pycnogenol in the prevention of adhesion.
References
-
1Durmus AS, Yildiz H, Yaman I, Simsek H. Efficacy of vitamin E and selenium for the prevention of intraabdominal adhesions in rats: uterine horn models. Clinics. 2011;66:1247-251. doi: 10.1590/S1807-59322011000700021.
» https://doi.org/10.1590/S1807-59322011000700021 -
2Sahbaz A, Aynioglu O, Isik H, Gun BD, Cengil O, Erol O. Pycnogenol prevents peritoneal adhesions. Arch Gynecol Obstet. 2015;292:1279. doi: 10.1007/s00404-015-3764-4.
» https://doi.org/10.1007/s00404-015-3764-4 -
3Brochhausen C, Schmitt VH, Planck CN, Rajab TK, Hollemann D, Tapprich C, Krämer B, Wallwiener C, Hierlemann H, Zehbe R, Planck H, Kirkpatrick CJ. Current strategies and future perspectives for intraperitoneal adhesion prevention. J Gastrointest Surg. 2012;16:1256-74. doi: 10.1007/s11605-011-1819-9.
» https://doi.org/10.1007/s11605-011-1819-9 -
4Fletcher NM, Abuanzeh S, Saed MG, Diamond MP, AbuSoud HM, Saed GM. Nicotinamide adenine dinucleotide phosphate oxidase expression is differentially regulated to favor a prooxidant state that contributes to postoperative adhesion development. Reprod Sci. 2014;21:1050-9. doi: 10.1177/1933719114522524.
» https://doi.org/10.1177/1933719114522524 -
5Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17:4545-53. doi: 10.3748/wjg.v17.i41.4545.
» https://doi.org/10.3748/wjg.v17.i41.4545 -
6Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260-73. doi: 10.1159/000050149.
» https://doi.org/10.1159/000050149 -
7Kamel Remah M. Prevention of postoperative peritoneal adhesions. Eur J Obstet Gynecol Reprod Biol. 2010;150:111-8. doi: 10.1016/j.ejogrb.2010.02.003.
» https://doi.org/10.1016/j.ejogrb.2010.02.003 -
8Peng YJ, Lee CH, Wang CC, Salter DM, Lee HS. Pycnogenol attenuates the inflammatory and nitrosative stress on joint inflammation induced by urate crystals. Free Radic Biol Med. 2012;52:765-74. doi: 10.1016/j.freeradbiomed.2011.12.003.
» https://doi.org/10.1016/j.freeradbiomed.2011.12.003 -
9Rohdewald PA. Review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002;40:158-68. PMID: 11996210.
-
10Petrassi C, Mastromarino A, Spartera C. PYCNOGENOL in chronic venous insufficiency. Phytomedicine. 2000;7:383-8. doi: 10.1016/S0944-7113(00)80059-8.
» https://doi.org/10.1016/S0944-7113(00)80059-8 -
11Torras MA, Faura CA, Schönlau F, Rohdewald P. Antimicrobial activity of pycnogenol. Phytother Res. 2005;19:647-48. doi: 10.1002/ptr.1662.
» https://doi.org/10.1002/ptr.1662 -
12Iravani S, Zolfaghari B. Pharmaceutical and nutraceutical effects of Pinus pinaster bark extract. Res Pharm Sci. 2011;6:1-11. PMID: 22049273.
-
13Holmdahl L, Ivarsson ML. The role of cytokines, coagulation, and fibrinolysis in peritoneal tissue repair. Eur J Surg. 1999;165:1012-9. doi: 10.1080/110241599750007810
» https://doi.org/10.1080/110241599750007810 -
14Awonuga AO, Belotte J, Abuanzeh S, Fletcher NM, Diamond MP, Saed GM. Advances in the pathogenesis of adhesion development: the role of oxidative stress. Reprod Sci. 2014;21:823-36. doi: 10.1177/1933719114522550.
» https://doi.org/10.1177/1933719114522550 -
15Fletcher NM, Jiang ZL, Diamond MP, Abu-Soud HM, Saed GM. Hypoxia-generated superoxide induces the development of the adhesion phenotype. Free Radic Biol Med. 2008;45:530-6. doi: 10.1016/j.freeradbiomed.2008.05.002.
» https://doi.org/10.1016/j.freeradbiomed.2008.05.002 -
16Epstein JC, Wilson MS, Wilkosz S, Ireland G, O’Dwyer ST, Herrick SE. Human peritoneal adhesions show evidence of tissue remodeling and markers of angiogenesis. Dis Colon Rectum. 2006;49:1885-92. doi: 10.1007/s10350-006-0747-3.
» https://doi.org/10.1007/s10350-006-0747-3 -
17Herrick SE, Mutsaers SE, Ozua P, Sulaiman H, Omer A, Boulos P, Foster ML, Laurent GJ. Human peritoneal adhesions are highly cellular, innervated, and vascularized. J Pathol. 2000;192:67-72. doi:10.1002/1096-9896(2000)9999:9999<::AID-PATH678>3.0.CO;2-E.
» https://doi.org/10.1002/1096-9896(2000)9999:9999<::AID-PATH678>3.0.CO;2-E -
18Binnebösel M, Klink CD, Serno J, Jansen PL, von Trotha KT, Neumann UP, Junge K. Chronological evaluation of inflammatory mediators during peritoneal adhesion formation using a rat model. Langenbecks Arch Surg. 2011;396:371-8. doi: 10.1007/s00423-011-0740-8.
» https://doi.org/10.1007/s00423-011-0740-8 -
19Keleidari B, Mahmoudieh M, Bahrami F, Mortazavi P, Aslani RS, Toliyat SA. The effect of vitamin A and vitamin C on postoperative adhesion formation: a rat model study. J Res Med Sci. 2014;19:28-32. PMID: 24672562.
-
20de la Portilla F, Ynfante I, Bejarano D, Conde J, Fernández A, Ortega JM, Carranza G. Prevention of peritoneal adhesions by intraperitoneal administration of vitamin E: an experimental study in rats. Dis Colon Rectum. 2004;47:2157-61. doi: 10.1007/s10350-004-0741-6.
» https://doi.org/10.1007/s10350-004-0741-6 -
21Yetkin G, Uludag M, Citgez B, Karakoc S, Polat N, Kabukcuoglu F. Prevention of peritoneal adhesions by intraperitoneal administration of vitamin E and human amniotic membrane. Int J Surg. 2009;7:561-5. doi: 10.1016/j.ijsu.2009.09.007.
» https://doi.org/10.1016/j.ijsu.2009.09.007 -
22Noda Y, Anzai K, Mori A, Kohno M, Shinmei M, Packer L. Hydroxyl and superoxide anion radical scavenging activities of natural source antioxidants using the computerized JES-FR30 ESR spectrometer system. Biochem Mol Biol Int. 1997;42:35-44. PMID: 9192083.
-
23Dogan E, Yanmaz L, Gedikli S, Ersoz U, Okumus Z. The effect of pycnogenol on wound healing in diabetic rats. Ostomy Wound Manage. 2017;63:41-7. PMID: 28448268.
-
24Taner G, Aydın S, Bacanlı M, Sarıgöl Z, Sahin T, Başaran AA, Başaran N. Modulating effects of pycnogenol on oxidative stress and DNA damage induced by sepsis in rats. Phytother Res. 2014;28:1692-1700. doi: 10.1002/ptr.5184.
» https://doi.org/10.1002/ptr.5184 -
25Değer KC, Şeker A, Özer I, Bostancı EB, Dalgıç T, Akmansu M, Ekinci Ö, Erçin U, Bilgihan A, Akoğlu M. The effects of Pycnogenol® on colon anastomotic healing in rats given preoperative irradiation. Int J Surg. 2013;11:983-88. doi: 10.1016/j.ijsu.2013.06.004.
» https://doi.org/10.1016/j.ijsu.2013.06.004 -
26Ferrer R. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58:1313-20. PMID: 9803196.
-
Financial source:
none
-
1
Research performed at Laboratory of Canakkale Onsekiz Mart University Health Sciences Research and Application Center, Turkey.
Publication Dates
-
Publication in this collection
Feb 2018
History
-
Received
16 Oct 2017 -
Reviewed
18 Dec 2017 -
Accepted
19 Jan 2018