Abstract
Purpose:
To analyze the preoperative serum matrix metalloproteinase-9 (MMP-9) levels and prognosis of patients with hilar cholangiocarcinoma (HC) undergoing radical resection.
Methods:
Preoperative serum MMP-9 levels in patients with HC undergoing radical resection were detected by enzyme-linked immunosorbent assay (ELISA). The ROC curve assay was used to analyze the preoperative serum MMP-9 level to determine the most valuable cut-off point. The relationship between MMP-9 and clinicopathological features of HC patients was analyzed. Kaplan-Meier method was used to analyze the prognostic factors, and COX regression model was used to analyze the independent risk factors affecting prognosis.
Results:
Preoperative serum MMP-9 levels were significantly elevated in the death patients compared with the survival patients. The most valuable cut-off point for preoperative serum MMP-9 for prognosis was 201.93 ng/mL. Preoperative serum MMP-9 was associated with Bismuth-Corlette classification) and lymph node metastasis. Kaplan-Meier analysis showed that MMP-9, Bismuth-Corlette classification, Lymph node metastasis, Portal vein invasion, Hepatic artery invasion, Liver invasion, Incised margin, and Preoperative biliary drainage were related to prognosis. Cox regression model confirmed that hepatic artery invasion, liver invasion, incised margin, and MMP-9 have the potential to independence predicate prognosis in HC patients.
Conclusion:
Preoperative serum MMP-9 has high predictive value for prognosis and is an independent influencing factor for the prognosis of patients with hilar cholangiocarcinoma.
Key words:
Klatskin Tumor; Matrix Metalloproteinase 9; Postoperative Period; Prognosis
Introduction
Hilar cholangiocarcinoma (HC), also known as Klatskin tumor, is a malignant tumor that grows in the confluence of the left and right hepatic ducts and the common hepatic duct. In cholangiocarcinoma, HC is more common that is accounting for about 58%-66%11 Soares KC, Kamel I, Cosgrove DP, Herman JM, Pawlik T M. Hilar cholangiocarcinoma: diagnosis, treatment options, and management. Hepatobiliary Surg Nutr. 2014;3(1):18-34. doi: 10.3978/j.issn.2304-3881.2014.02.05.
https://doi.org/10.3978/j.issn.2304-3881...
. HC has a high mortality rate in the early stages and is considered to be one of the most deadly malignant tumors22 Sudan D, DeRoover A, Chinnakotla S, Fox I, Shaw Jr B, McCashland T, Sorrell M, Tempero M, Langnas A. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2(8):774-9. PMID: 12243499.. However, the cause of HC is not yet clear. Liver resection and liver transplantation in HC patients are currently the only radical surgery33 Matsuyama R, Mori R, Ota Y, Homma Y, Kumamoto T, Takeda K, Morioka D, Maegawa J, Endo I. Significance of vascular resection and reconstruction in surgery for hilar cholangiocarcinoma: with special reference to hepatic arterial resection and reconstruction. Ann Surg Oncol. 2016;23(Suppl 4):475-84. doi: 10.1245/s10434-016-5381-2.
https://doi.org/10.1245/s10434-016-5381-...
, however, only a small number of patients still have the opportunity to undergo radical surgery after diagnosis, and the postoperative prognosis is poor. Other adjuvant therapies such as chemotherapy and radiotherapy have not achieved satisfactory results in improving the prognosis of patients44 Lee SG, Song GW, Hwang S, Ha TY, Moon DB, Jung DH, Kim KH, Ahn CS, Kim MH, Lee SK, Sung KB, Ko GY, Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci. 2010;17(4):476-89. doi: 10.1007/s00534-009-0204-5.
https://doi.org/10.1007/s00534-009-0204-...
. Studying factors related to prognosis after surgical treatment and finding related markers for preventing HC recurrence or metastasis are of great significance for improving the long-term survival of HC patients.
Matrix metalloproteinase-9 (MMP-9), also known as gelatinase B or type IV collagenase B, is a zinc-dependent proteolytic enzyme involved in the degradation of many different components of the basement membrane and subsequent remodeling of extracellular matrix (ECM)55 Vafadari B, Salamian A, Kaczmarek L. MMP-9 in translation: from molecule to brain physiology, pathology, and therapy. J Neurochem. 2016;139 Suppl 2:91-114. doi: 10.1111/jnc.13415.
https://doi.org/10.1111/jnc.13415...
. MMP-9 not only plays a key role in the development of cancers, but also their characteristics of being secreted into the blood stream have inspired many researchers to evaluate the associations between circulating level of MMP-9 and clinicopathological characteristics of cancers66 Dragutinovic VV, Radonjic NV, Petronijevic ND, Tatic SB, Dimitrijevic IB, Radovanovic NS, Krivokapic ZV. Matrix metalloproteinase-2 (MMP-2) and -9 (MMP-9) in preoperative serum as independent prognostic markers in patients with colorectal cancer. Mol Cell Biochem. 2011;355(1-2):173-8. doi: 10.1007/s11010-011-0851-0.
https://doi.org/10.1007/s11010-011-0851-...
. Clinical studies have shown that high expression of MMP-9 is associated with clinical pathological features of cancer such as lymph node metastasis and tumor differentiation77 Burlaka AP, Ganusevich II, Gafurov MR, Lukin SM, Sidorik EP. Stomach cancer: interconnection between the Redox state, activity of MMP-2, MMP-9 and stage of tumor growth. Cancer Microenviron. 2016;9(1):27-32. doi: 10.1007/s12307-016-0182-5.
https://doi.org/10.1007/s12307-016-0182-...
. Elevated serum MMP-9 often suggests a poor prognosis88 Schveigert D, Cicenas S, Bruzas S, Samalavicius NE, Gudleviciene Z, Didziapetriene J. The value of MMP-9 for breast and non-small cell lung cancer patients' survival. Adv Med Sci. 2013;58(1):73-82. doi: 10.2478/v10039-012-0066-y.
https://doi.org/10.2478/v10039-012-0066-...
. However, there are few studies on the relationship between MMP-9 and the histopathological features and prognosis of HC. There is currently no correlation report on the preoperative serum MMP-9 level predicting the prognosis of HC.
In this study, patients with HC radical surgery were enrolled. Enzyme-linked immunosorbent assay (ELISA) was used to analyze the most valuable cut-off point of preoperative serum MMP-9 for the prognosis of HC radical resection, and to explore the influencing factors of prognosis of HC radical mastectomy. We hope to provide appropriate targets for prognosis monitoring after HC.
Methods
This study was approved by the Ethics Committee of the Hunan Provincial People’s Hospital.
Two hundred and forty-one patients with HC who were admitted to Hunan Provincial People’s Hospital from March 2010 to March 2013 were included in the study. All patients underwent radical surgery and were diagnosed by pathology after surgery. Patients with perioperative death were excluded. Pathological results were excluded from mixed liver cancer, as well as patients who were lost to follow-up. The causes of death in follow-up cases were surgical factors (non-other cases, such as heart disease). Finally, 181 eligible cases were included, including 103 males and 78 females, aged 35-78 years. All patients were informed of the relevant conditions of the study and the required indicators to be recorded before surgery. Patients were signed informed consent and followed up regularly. The deadline for follow-up is June 30, 2018. The overall survival (OS) time is from the date of surgery to the date of death or the date of follow-up. All experiments were performed at Department of Hepatobiliary Surgery, Hunan Provincial People’s Hospital, China. Patient enrollment and follow-up are shown in Figure 1.
Detection of serum MMP-9 content
Three milliliters of venous peripheral blood of HC patients was collected before radical surgery, and centrifuged to obtain serum, which was then stored at -80°C. The concentration of MMP-9 was measured by ELISA. ELISA kit for MMP-9 WAS purchased from R&D Systems Inc. Assays were performed according to the manufacturer’s instructions.
Statistical analyses
Analysis was performed using SPSS 17.0 software (SPSS Inc, Chicago, IL). Measurement data conforming to the normal distribution were expressed as mean ± standard deviation(mean ± SD), Student t test was used for comparing the average of two groups. The predictive efficacy of serum MMP-9 on prognosis was assessed using the receiver operating characteristic curve (ROC).The most prognostic value of serum MMP-9 was calculated using the most approximate index. Count data was expressed in terms of number of cases (n), using χ 2 test or Fisher exact probability method to analyze. The survival curve was drawn using the Kaplan-Meier method. Differences in survival curves were detected by Log-rank method. Cox regression model was established, and the significance level of the variables retained by the model: the entry criterion was p<0.05. At the α=0.05 test level, the Backward LR method was used to screen out the independent risk factors affecting the prognosis of HC patients. The degree of influence of risk factors is expressed as the hazard ratio (HR). If p< 0.05 then the difference was considered statistically significant.
Results
Follow-up
To the follow-up deadline, 120 of the 181 patients died, with a minimum survival time of 3 months, a maximum of 59 months, and a median survival of 46 months. The overall survival curve of the patient is shown in Figure 2. The 1-, 3-, and 5-year survival rates were 88.4% (160/181), 73.5% (133/181), and 33.7% (61/181), respectively.
The relationship between preoperative serum MMP-9 level and prognosis of HC patients
The preoperative serum MMP-9 concentration in 181 patients with HC was (216.7±72.1) ng/mL. Preoperative serum MMP-9 levels were significantly elevated in the death group compared with the survival group (155.50±41.79 vs. 251.57±62.02, p<0.001). To determine the most valuable MMP-9 level cut-off point for prognosis, we applied the ROC curve method for analysis. We found that the area under the ROC curve of serum MMP-9 levels predicting patient mortality was 0.896 (Fig. 3), confirming that preoperative serum MMP-9 has a higher value in predicting postoperative prognosis in HC patients. When the MMP-9 level was 201.93 ng/mL, the Youden index was the largest. The sensitivity of MMP-9≥201.93 ng/mL for predicting death in HC patients was 80.33%, and the specificity was 86.89%. The result hinted that preoperative serum MMP-9 levels may be potential markers for predicting the prognosis of HC patients.
Preoperative serum MMP-9 predicts ROC curve analysis of postoperative death in HC patients.
Relationship between serum MMP-9 level and clinicopathological features of HC patients
To analyze the relationship between preoperative serum MMP-9 and clinicopathological features of HC patients, we classified the variables related to clinicopathological features according to their own characteristics. As shown in Table 1, preoperative serum MMP-9 levels were not associated with age, sex, tumor size, differentiation, nerve invasion, portal vein invasion, hepatic artery invasion, liver invasion, incised margin, total bilirubin, preoperative biliary drainage and postoperative radiotherapy, but were associated with Bismuth-Corlette classification (χ2=9.442, p=0.002) and lymph node metastasis (χ2=9.026, p=0.003).
Relationship between serum MMP-9 level and clinicopathological features of HC patients (n, %).
High MMP-9 levels are correlated with poor prognosis in HC patients
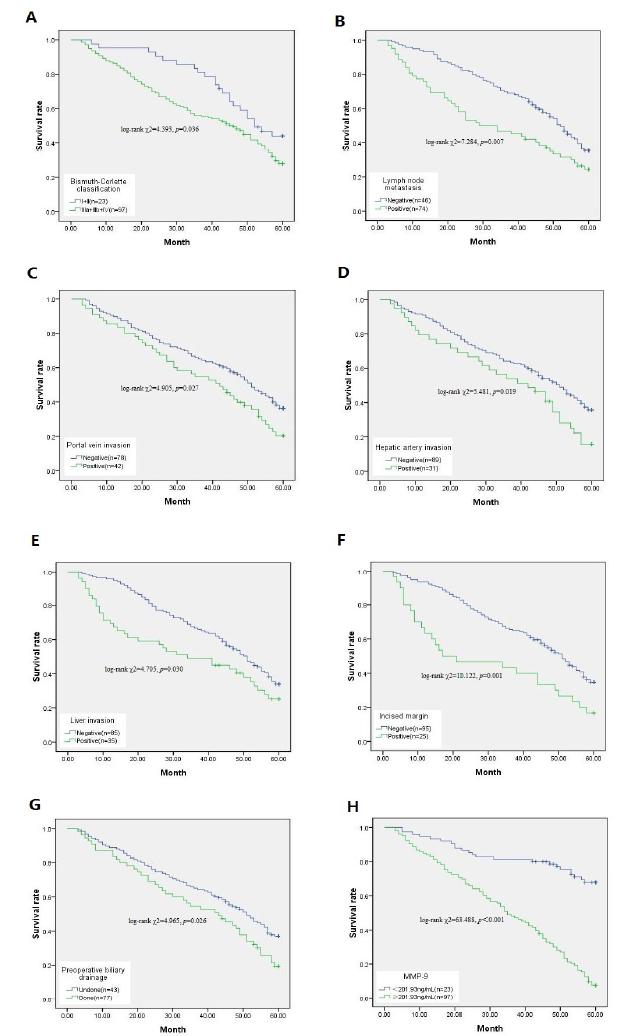
To explore whether clinical pathological parameters and preoperative serum MMP-9 levels were associated with OS, we utilized Kaplan Meier survival analysis and log-rank test (Table 2, Fig. 4). The median survival time was 34.5 months in patients with high MMP-9 (MMP-9≥201.93) and 50.9 months in patients with low MMP-9 (MMP-9<201.93 ng/mL). The Kaplan-Meier results showed (Table 2, Fig. 4H) that patients with high MMP-9 had a significantly lower median survival time than patients with low MMP-9 (log-rank χ2=63.488, p<0.001). Subsequently, Bismuth-Corlette classification (log-rank χ2=4.393, p=0.036), Lymph node metastasis (log-rank χ2=7.284, p=0.007), Portal vein invasion (log-rank χ2=4.905, p=0.027), Hepatic artery invasion (log-rank χ2=5.481, p=0.019), Liver invasion (log-rank χ2=4.705, p=0.030), Incised margin (log-rank χ2=10.122, p=0.001), and Preoperative biliary drainage (log-rank χ2=4.965, p=0.026) were also related to prognosis (Table 2, Fig. 4A-G,).
Relationship between clinical pathological parameters and preoperative serum MMP-9 and postoperative survival of HC patients. A: Bismuth-Corlette classification. B: Lymph node metastasis. C: Portal vein invasion. D: Hepatic artery invasion. E: Liver invasion. F: Incised margin. G: Preoperative biliary drainage. H: Preoperative serum MMP-9. “n” represents the number of deaths.
Cox regression model
Next, we included the statistically significant differences in the results of the univariate analysis into the Cox regression model for multivariate analysis. Multivariate analysis confirmed that hepatic artery invasion (p=0.016), liver invasion (p=0.044), incised margin (p=0.012), and MMP-9 (p<0.001) have the potential to independently predicate prognosis in HC patients (Table 3).
Discussion
MMPs are one of the most important family of proteolytic enzymes that play an important role in embryonic development, differentiation, tumor angiogenesis, tumor invasion and metastasis99 Sun XF, Zhang H. Clinicopathological significance of stromal variables: angiogenesis, lymphangiogenesis, inflammatory infiltration, MMP and PINCH in colorectal carcinomas. Mol Cancer. 2006;5:43. doi: 10.1186/1476-4598-5-43.
https://doi.org/10.1186/1476-4598-5-43...
. It has been demonstrated that MMPs-mediated degradation of extracellular matrix can lead to invasion and metastasis of tumor cells1010 Fink K, Boratynski J. The role of metalloproteinases in modification of extracellular matrix in invasive tumor growth, metastasis and angiogenesis. Postepy Hig Med Dosw (Online). 2012;66:609-28. doi: 10.5604/17322693.1009705.
https://doi.org/10.5604/17322693.1009705...
,1111 Santibanez JF, Obradovic H, Kukolj T, Krstic J. Transforming growth factor-beta, matrix metalloproteinases, and urokinase-type plasminogen activator interaction in the cancer epithelial to mesenchymal transition. Dev Dyn. 2018;247(3):382-95. doi: 10.1002/dvdy.24554.
https://doi.org/10.1002/dvdy.24554...
, and abnormal expression of MMPs has prognostic significance in some human malignant tumors1212 Jensen AS, Vainer B, Bartels A, Brunner N, Sorensen JB. Expression of matrix metalloproteinase 9 (MMP-9) and tissue inhibitor of metalloproteinases 1 (TIMP-1) by colorectal cancer cells and adjacent stroma cells--associations with histopathology and patients outcome. Eur J Cancer. 2010;46(18):3233-42. doi: 10.1016/j.ejca.2010.07.046.
https://doi.org/10.1016/j.ejca.2010.07.0...
,1313 Molica S, Vitelli G, Levato D, Giannarelli D, Vacca A, Cuneo A, Cavazzini F, Squillace R, Mirabelli R, Digiesi, G. Increased serum levels of matrix metalloproteinase-9 predict clinical outcome of patients with early B-cell chronic lymphocytic leukaemia. Eur J Heamatol. 2003;70(6):373-8. doi: 10.1034/j.1600-0609.2003.00064.x.
https://doi.org/10.1034/j.1600-0609.2003...
. Deng et al.1414 Deng J, Chen W, Du Y, Wang W, Zhang G, Tang Y, Qian Z, Xu P, Cao Z, Zhou Y. Synergistic efficacy of Cullin1 and MMP-2 expressions in diagnosis and prognosis of colorectal cancer. Cancer Bio. 2017;19(1):57-64. doi: 10.3233/CBM-160341.
https://doi.org/10.3233/CBM-160341...
found that MMP-2 protein levels were significantly upregulated in colorectal cancer (CRC), and MMP-2 expression could be novel diagnostic and prognostic markers for CRC patients. Yao et al.1515 Yao Z, Yuan T, Wang H, Yao S, Zhao Y, Liu Y, Jin S, Chu J, Xu Y, Zhou W, Yang S, Liu Y. MMP-2 together with MMP-9 overexpression correlated with lymph node metastasis and poor prognosis in early gastric carcinoma. Tumour Biol. 2017;39(6):1010428317700411. doi: 10.1177/1010428317700411.
https://doi.org/10.1177/1010428317700411...
revealed that MMP-2 and MMP-9 expression were both independent factors of prognosis and lymphnode metastasis in patients with early gastric cancer. A meta-analysis also showed a significant poor prognostic effect of MMP-7 in gastric cancer survival1616 Soleyman-Jahi S, Nedjat S, Abdirad A, Hoorshad N, Heidari R, Zendehdel K. Prognostic significance of matrix metalloproteinase-7 in gastric cancer survival: a meta-analysis. PloS one. 2014;10(4):e0122316. doi: 10.1371/journal.pone.0122316.
https://doi.org/10.1371/journal.pone.012...
. MMP-9 is more well know in the MMPs family, which can degrade type IV collagen in ECM, allowing cancer cells to break through the basement membrane of the primary site1717 Zhang M, Zhu GY, Gao HY, Zhao SP, Xue Y. Expression of tissue levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in gastric adenocarcinoma. J Surg Oncol. 2011;103(3):243-7. doi: 10.1002/jso.21824.
https://doi.org/10.1002/jso.21824...
. MMP-9 can attenuate the basement membrane of blood vessels and lymphatic vessels, allowing cancer cells to infiltrate directly into the vasculature1818 Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27(31):5287-97. doi: 10.1200/jco.2009.23.5556.
https://doi.org/10.1200/jco.2009.23.5556...
to participate in tumor metastasis and invasion1919 Herszenyi L, Hritz I, Lakatos G, Varga MZ, Tulassay Z. The behavior of matrix metalloproteinases and their inhibitors in colorectal cancer. Int J Mol Sci. 2012;13(10):13240-63. doi: 10.3390/ijms131013240.
https://doi.org/10.3390/ijms131013240...
. Some scholars have conducted immunohistochemistry to confirm that MMP-9 has a high value in predicting the long-term prognosis of multiple tumors2020 Salem N, Kamal I, Al-Maghrabi J, Abuzenadah A, Peer-Zada AA, Qari Y, Al-Ahwal M, Al-Qahtani M, Buhmeida A. High expression of matrix metalloproteinases: MMP-2 and MMP-9 predicts poor survival outcome in colorectal carcinoma. Future Oncol (London, England). 2016;12(3):323-31. doi: 10.2217/fon.15.325.
https://doi.org/10.2217/fon.15.325...
,2121 Merdad A, Karim S, Schulten HJ, Dallol A, Buhmeida A, Al-Thubaity F, Gari MA, Chaudhary AG, Abuzenadah AM, Al-Qahtani MH. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer: MMP-9 as a potential biomarker for cancer invasion and metastasis. Anticancer Res. 2014;34(3):1355-66. doi: 10.1186/1471-2407-9-188.
https://doi.org/10.1186/1471-2407-9-188...
. Zhao et al.2222 Zhao S, Ma W, Zhang M, Tang D, Shi Q, Xu S, Zhang X, Liu Y, Song Y, Liu L, Zhang Q. High expression of CD147 and MMP-9 is correlated with poor prognosis of triple-negative breast cancer (TNBC) patients. Med Oncol. 2013;30(1):335. doi: 10.1007/s12032-012-0335-4.
https://doi.org/10.1007/s12032-012-0335-...
used immunohistochemistry to analyze the expression of MMP-9 in tumor tissues of 127 patients with tirple-negative breast cancer, and found that patients with high MMP-9 expression had poor prognosis. Interestingly, the results of some scholars have been relatively novel, which confirms that MMP-9 may be a protective factor in the carcinogenesis and metastasis of tumors. For example, MMP-9 expression is reduced in some head and neck malignancies with local metastasis2323 Stokes A, Joutsa J, Ala-Aho R, Pitchers M, Pennington CJ, Martin C, Premachandra DJ, Okada Y, Peltonen J, Grenman R, James HÁ, Edwards DR, Kahari VM. Expression profiles and clinical correlations of degradome components in the tumor microenvironment of head and neck squamous cell carcinoma. Clin Cancer Res. 2010;16(7):2022-35. doi: 10.1158/1078-0432.CCR-09-2525.
https://doi.org/10.1158/1078-0432.CCR-09...
and elevated MMP-9 expression may indicate better overall survival in salivary adenocarcinoma2424 Luukkaa H, Klemi P, Leivo I, Makitie AA, Irish J, Gilbert R, Perez-Ordonez B, Hirsimaki P, Vahlberg T, Kivisaari A, Kahari VM, Grenman R. Expression of matrix metalloproteinase-1, -7, -9, -13, Ki-67, and HER-2 in epithelial-myoepithelial salivary gland cancer. Head Neck. 2010;32(8):1019-27. doi: 10.1002/hed.21277.
https://doi.org/10.1002/hed.21277...
. Even more surprising is that in breast cancer2525 Bendrik C, Robertson J, Gauldie J, Dabrosin C. Gene transfer of matrix metalloproteinase-9 induces tumor regression of breast cancer in vivo. Cancer Res. 2008;68(9):3405-12. doi: 10.1158/0008-5472.CAN-08-0295.
https://doi.org/10.1158/0008-5472.CAN-08...
and enteritis-related cancers2626 Garg P, Sarma D, Jeppsson S, Patel NR, Gewirtz AT, Merlin D, Sitaraman SV. Matrix metalloproteinase-9 functions as a tumor suppressor in colitis-associated cancer. Cancer Res. 2010;70(2):792-801. doi: 10.1158/0008-5472.CAN-09-3166.
https://doi.org/10.1158/0008-5472.CAN-09...
, MMP-9 expression also indicates a relatively good prognosis. These results suggest that MMP-9 can act as both a tumor-promoting factor and a protective factor, and its relationship with disease prognosis may depend on the specific environment of action.
Sun et al.2727 Sun Q, Zhao C, Xia L, He Z, Lu Z, Liu C, Jia M, Wang J, Niu J. High expression of matrix metalloproteinase-9 indicates poor prognosis in human hilar cholangiocarcinoma. Int J Clin Exp Pathol. 2014;7(9):6157-64. PMID: 25337264. found that MMP-9 overexpression was observed in tumor tissues of 46.5% of patients with cholangiocarcinoma of the liver. Their study also found that although MMP-9 overexpression was not associated with patient clinicopathological parameters, overall survival was significantly lower in patients with high MMP-9 expression than in patients with negative or low MMP-9 expression, suggesting that the expression of MMP-9 in tissues is of great significance in the evaluation of postoperative prognosis of intrahepatic cholangiocarcinoma. Recent studies have shown that real-time monitoring of non-invasive serum biomarkers targeting prognosis has greater safety and efficacy2828 Kurosaki A, Hasegawa K, Kato T, Abe K, Hanaoka T, Miyara A, O'Shannessy DJ, Somers EB, Yasuda M, Sekino T, Fujiwara K. Serum folate receptor alpha as a biomarker for ovarian cancer: Implications for diagnosis, prognosis and predicting its local tumor expression. Int J Cancer. 2016;138(8):1994-2002. doi: 10.1002/ijc.29937.
https://doi.org/10.1002/ijc.29937...
. MMP-9 levels in the peripheral circulation have also been shown to be associated with metastasis and long-term prognosis in a variety of cancers. Sung et al.2929 Sung H, Choi JY, Lee AS, Lee KM, Han S, Jeon S, Song M, Lee Y, Park SK, Yoo KY, Noh DY, Ahn SH, Kang D. The association between the preoperative serum levels of lipocalin-2 and matrix metalloproteinase-9 (MMP-9) and prognosis of breast cancer. BMC Cancer. 2012;12:193. doi: 10.1186/1471-2407-12-193.
https://doi.org/10.1186/1471-2407-12-193...
tested the preoperative serum MMP-9 levels in breast cancer patients and found that elevated MMP-9 levels were associated with decreased breast cancer survival rate. Lin et al.3030 Lin SY, Wang YY, Sheu WH. Preoperative plasma concentrations of vascular endothelial growth factor and matrix metalloproteinase 9 are associated with stage progression in papillary thyroid cancer. Clin Endocrino. 2003;58(4):513-8. doi: 10.1046/j.1365-2265.2003.01749.x.
https://doi.org/10.1046/j.1365-2265.2003...
also reported that plasma MMP-9 was significantly elevated in thyroid cancer patients with lymph node invasion and distant metastasis, and suggested that regular detection of plasma MMP-9 may help to determine the distant metastasis tendency. However, the relationship between preoperative serum MMP-9 levels and postoperative prognosis in HC patients has not been reported. Therefore, we focused on serum MMP-9 levels and studied the relationship between preoperative serum MMP-9 levels and postoperative prognosis in HC patients.
We used the ROC curve method to analyze the MMP-9 cut-off point, which has the most prognostic value after HC radical surgery. The results showed that the area under the ROC curve was the largest (AUC=0.896) when the cut-off point was 201.93 ng/mL, which was the most valuable value. Kaplan-Meier analysis confirmed that the median survival time of HC patients with low serum MMP-9 levels was significantly higher than that of patients with high serum MMP-9 levels. These results confirm that preoperative serum MMP-9 can be used as a useful marker to predict postoperative prognosis in HC patients. Furthermore, we found that preoperative serum MMP-9 levels were associated with Bismuth-Corlette classification and Lymph node metastasis, which may be related to the lower sensitivity of MMP-9 in predicting the prognosis of HC patients. Our results suggested that MMP-9 may be involved in the progression and metastasis of HC disease, which may be related to the potential carcinogenesis of MMP-9, but the related mechanism remains to be further explored. We further included statistically significant clinical pathological factors and preoperative serum MMP-9 levels in the multivariate survival analysis model. It was found that high serum MMP-9 levels is an independent risk factor for predicting the prognosis of HC patients. Additionally, Cox analysis showed that risk factors associated with prognosis of patients with HC include portal vein invasion, hepatic artery invasion, liver invasion, preoperative biliary drainage, etc., which may also affect the sensitivity of MMP-9 by affecting the immune response state of the body.
Conclusions
As far as we know, this is the only study to evaluate the relationship between preoperative serum MMP-9 levels and postoperative prognosis in HC patients. In this study, we showed that high MMP-9 levels have a high predictive value for postoperative prognosis in patients with HC undergoing radical resection, and are one of the independent factors influencing postoperative prognosis. Therefore, evaluation of serum MMP-9 levels may be helpful in guiding the prognosis of HC patients and subsequent treatment options. However, more studies are necessary to confirm our data.
References
-
1Soares KC, Kamel I, Cosgrove DP, Herman JM, Pawlik T M. Hilar cholangiocarcinoma: diagnosis, treatment options, and management. Hepatobiliary Surg Nutr. 2014;3(1):18-34. doi: 10.3978/j.issn.2304-3881.2014.02.05.
» https://doi.org/10.3978/j.issn.2304-3881.2014.02.05 -
2Sudan D, DeRoover A, Chinnakotla S, Fox I, Shaw Jr B, McCashland T, Sorrell M, Tempero M, Langnas A. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2(8):774-9. PMID: 12243499.
-
3Matsuyama R, Mori R, Ota Y, Homma Y, Kumamoto T, Takeda K, Morioka D, Maegawa J, Endo I. Significance of vascular resection and reconstruction in surgery for hilar cholangiocarcinoma: with special reference to hepatic arterial resection and reconstruction. Ann Surg Oncol. 2016;23(Suppl 4):475-84. doi: 10.1245/s10434-016-5381-2.
» https://doi.org/10.1245/s10434-016-5381-2 -
4Lee SG, Song GW, Hwang S, Ha TY, Moon DB, Jung DH, Kim KH, Ahn CS, Kim MH, Lee SK, Sung KB, Ko GY, Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci. 2010;17(4):476-89. doi: 10.1007/s00534-009-0204-5.
» https://doi.org/10.1007/s00534-009-0204-5 -
5Vafadari B, Salamian A, Kaczmarek L. MMP-9 in translation: from molecule to brain physiology, pathology, and therapy. J Neurochem. 2016;139 Suppl 2:91-114. doi: 10.1111/jnc.13415.
» https://doi.org/10.1111/jnc.13415 -
6Dragutinovic VV, Radonjic NV, Petronijevic ND, Tatic SB, Dimitrijevic IB, Radovanovic NS, Krivokapic ZV. Matrix metalloproteinase-2 (MMP-2) and -9 (MMP-9) in preoperative serum as independent prognostic markers in patients with colorectal cancer. Mol Cell Biochem. 2011;355(1-2):173-8. doi: 10.1007/s11010-011-0851-0.
» https://doi.org/10.1007/s11010-011-0851-0 -
7Burlaka AP, Ganusevich II, Gafurov MR, Lukin SM, Sidorik EP. Stomach cancer: interconnection between the Redox state, activity of MMP-2, MMP-9 and stage of tumor growth. Cancer Microenviron. 2016;9(1):27-32. doi: 10.1007/s12307-016-0182-5.
» https://doi.org/10.1007/s12307-016-0182-5 -
8Schveigert D, Cicenas S, Bruzas S, Samalavicius NE, Gudleviciene Z, Didziapetriene J. The value of MMP-9 for breast and non-small cell lung cancer patients' survival. Adv Med Sci. 2013;58(1):73-82. doi: 10.2478/v10039-012-0066-y.
» https://doi.org/10.2478/v10039-012-0066-y -
9Sun XF, Zhang H. Clinicopathological significance of stromal variables: angiogenesis, lymphangiogenesis, inflammatory infiltration, MMP and PINCH in colorectal carcinomas. Mol Cancer. 2006;5:43. doi: 10.1186/1476-4598-5-43.
» https://doi.org/10.1186/1476-4598-5-43 -
10Fink K, Boratynski J. The role of metalloproteinases in modification of extracellular matrix in invasive tumor growth, metastasis and angiogenesis. Postepy Hig Med Dosw (Online). 2012;66:609-28. doi: 10.5604/17322693.1009705.
» https://doi.org/10.5604/17322693.1009705 -
11Santibanez JF, Obradovic H, Kukolj T, Krstic J. Transforming growth factor-beta, matrix metalloproteinases, and urokinase-type plasminogen activator interaction in the cancer epithelial to mesenchymal transition. Dev Dyn. 2018;247(3):382-95. doi: 10.1002/dvdy.24554.
» https://doi.org/10.1002/dvdy.24554 -
12Jensen AS, Vainer B, Bartels A, Brunner N, Sorensen JB. Expression of matrix metalloproteinase 9 (MMP-9) and tissue inhibitor of metalloproteinases 1 (TIMP-1) by colorectal cancer cells and adjacent stroma cells--associations with histopathology and patients outcome. Eur J Cancer. 2010;46(18):3233-42. doi: 10.1016/j.ejca.2010.07.046.
» https://doi.org/10.1016/j.ejca.2010.07.046 -
13Molica S, Vitelli G, Levato D, Giannarelli D, Vacca A, Cuneo A, Cavazzini F, Squillace R, Mirabelli R, Digiesi, G. Increased serum levels of matrix metalloproteinase-9 predict clinical outcome of patients with early B-cell chronic lymphocytic leukaemia. Eur J Heamatol. 2003;70(6):373-8. doi: 10.1034/j.1600-0609.2003.00064.x.
» https://doi.org/10.1034/j.1600-0609.2003.00064.x -
14Deng J, Chen W, Du Y, Wang W, Zhang G, Tang Y, Qian Z, Xu P, Cao Z, Zhou Y. Synergistic efficacy of Cullin1 and MMP-2 expressions in diagnosis and prognosis of colorectal cancer. Cancer Bio. 2017;19(1):57-64. doi: 10.3233/CBM-160341.
» https://doi.org/10.3233/CBM-160341 -
15Yao Z, Yuan T, Wang H, Yao S, Zhao Y, Liu Y, Jin S, Chu J, Xu Y, Zhou W, Yang S, Liu Y. MMP-2 together with MMP-9 overexpression correlated with lymph node metastasis and poor prognosis in early gastric carcinoma. Tumour Biol. 2017;39(6):1010428317700411. doi: 10.1177/1010428317700411.
» https://doi.org/10.1177/1010428317700411 -
16Soleyman-Jahi S, Nedjat S, Abdirad A, Hoorshad N, Heidari R, Zendehdel K. Prognostic significance of matrix metalloproteinase-7 in gastric cancer survival: a meta-analysis. PloS one. 2014;10(4):e0122316. doi: 10.1371/journal.pone.0122316.
» https://doi.org/10.1371/journal.pone.0122316 -
17Zhang M, Zhu GY, Gao HY, Zhao SP, Xue Y. Expression of tissue levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in gastric adenocarcinoma. J Surg Oncol. 2011;103(3):243-7. doi: 10.1002/jso.21824.
» https://doi.org/10.1002/jso.21824 -
18Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27(31):5287-97. doi: 10.1200/jco.2009.23.5556.
» https://doi.org/10.1200/jco.2009.23.5556 -
19Herszenyi L, Hritz I, Lakatos G, Varga MZ, Tulassay Z. The behavior of matrix metalloproteinases and their inhibitors in colorectal cancer. Int J Mol Sci. 2012;13(10):13240-63. doi: 10.3390/ijms131013240.
» https://doi.org/10.3390/ijms131013240 -
20Salem N, Kamal I, Al-Maghrabi J, Abuzenadah A, Peer-Zada AA, Qari Y, Al-Ahwal M, Al-Qahtani M, Buhmeida A. High expression of matrix metalloproteinases: MMP-2 and MMP-9 predicts poor survival outcome in colorectal carcinoma. Future Oncol (London, England). 2016;12(3):323-31. doi: 10.2217/fon.15.325.
» https://doi.org/10.2217/fon.15.325 -
21Merdad A, Karim S, Schulten HJ, Dallol A, Buhmeida A, Al-Thubaity F, Gari MA, Chaudhary AG, Abuzenadah AM, Al-Qahtani MH. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer: MMP-9 as a potential biomarker for cancer invasion and metastasis. Anticancer Res. 2014;34(3):1355-66. doi: 10.1186/1471-2407-9-188.
» https://doi.org/10.1186/1471-2407-9-188 -
22Zhao S, Ma W, Zhang M, Tang D, Shi Q, Xu S, Zhang X, Liu Y, Song Y, Liu L, Zhang Q. High expression of CD147 and MMP-9 is correlated with poor prognosis of triple-negative breast cancer (TNBC) patients. Med Oncol. 2013;30(1):335. doi: 10.1007/s12032-012-0335-4.
» https://doi.org/10.1007/s12032-012-0335-4 -
23Stokes A, Joutsa J, Ala-Aho R, Pitchers M, Pennington CJ, Martin C, Premachandra DJ, Okada Y, Peltonen J, Grenman R, James HÁ, Edwards DR, Kahari VM. Expression profiles and clinical correlations of degradome components in the tumor microenvironment of head and neck squamous cell carcinoma. Clin Cancer Res. 2010;16(7):2022-35. doi: 10.1158/1078-0432.CCR-09-2525.
» https://doi.org/10.1158/1078-0432.CCR-09-2525 -
24Luukkaa H, Klemi P, Leivo I, Makitie AA, Irish J, Gilbert R, Perez-Ordonez B, Hirsimaki P, Vahlberg T, Kivisaari A, Kahari VM, Grenman R. Expression of matrix metalloproteinase-1, -7, -9, -13, Ki-67, and HER-2 in epithelial-myoepithelial salivary gland cancer. Head Neck. 2010;32(8):1019-27. doi: 10.1002/hed.21277.
» https://doi.org/10.1002/hed.21277 -
25Bendrik C, Robertson J, Gauldie J, Dabrosin C. Gene transfer of matrix metalloproteinase-9 induces tumor regression of breast cancer in vivo. Cancer Res. 2008;68(9):3405-12. doi: 10.1158/0008-5472.CAN-08-0295.
» https://doi.org/10.1158/0008-5472.CAN-08-0295 -
26Garg P, Sarma D, Jeppsson S, Patel NR, Gewirtz AT, Merlin D, Sitaraman SV. Matrix metalloproteinase-9 functions as a tumor suppressor in colitis-associated cancer. Cancer Res. 2010;70(2):792-801. doi: 10.1158/0008-5472.CAN-09-3166.
» https://doi.org/10.1158/0008-5472.CAN-09-3166 -
27Sun Q, Zhao C, Xia L, He Z, Lu Z, Liu C, Jia M, Wang J, Niu J. High expression of matrix metalloproteinase-9 indicates poor prognosis in human hilar cholangiocarcinoma. Int J Clin Exp Pathol. 2014;7(9):6157-64. PMID: 25337264.
-
28Kurosaki A, Hasegawa K, Kato T, Abe K, Hanaoka T, Miyara A, O'Shannessy DJ, Somers EB, Yasuda M, Sekino T, Fujiwara K. Serum folate receptor alpha as a biomarker for ovarian cancer: Implications for diagnosis, prognosis and predicting its local tumor expression. Int J Cancer. 2016;138(8):1994-2002. doi: 10.1002/ijc.29937.
» https://doi.org/10.1002/ijc.29937 -
29Sung H, Choi JY, Lee AS, Lee KM, Han S, Jeon S, Song M, Lee Y, Park SK, Yoo KY, Noh DY, Ahn SH, Kang D. The association between the preoperative serum levels of lipocalin-2 and matrix metalloproteinase-9 (MMP-9) and prognosis of breast cancer. BMC Cancer. 2012;12:193. doi: 10.1186/1471-2407-12-193.
» https://doi.org/10.1186/1471-2407-12-193 -
30Lin SY, Wang YY, Sheu WH. Preoperative plasma concentrations of vascular endothelial growth factor and matrix metalloproteinase 9 are associated with stage progression in papillary thyroid cancer. Clin Endocrino. 2003;58(4):513-8. doi: 10.1046/j.1365-2265.2003.01749.x.
» https://doi.org/10.1046/j.1365-2265.2003.01749.x
-
Financial source:
none -
1
Research performed at Department of Hepatobiliary Surgery, Hunan Provincial People’s Hospital, People’s Republic of China.
Publication Dates
-
Publication in this collection
29 Apr 2019 -
Date of issue
2019
History
-
Received
19 Dec 2018 -
Reviewed
16 Feb 2019 -
Accepted
14 Mar 2019