Abstract
Purpose
To evaluate the neuroprotective effect of L-alanyl-glutamine in a gerbil model of brain ischemia-reperfusion injury based on immunohistochemical quantification of pro-inflammatory and cell activation biomarkers (TNF-α, NF-κB, IL-6 and HO-1).
Methods
Male gerbils weighing 100-180 g were pretreated with either 0.75 g/kg L-Ala-Gln (n=18) or 2.0 mL saline (n=18) administered i.v. 30 minutes before the bilateral ligation of the common carotid artery during 15 min and then the ligation was removed. Under anesthesia with urethane, brain tissue was harvested at 0 min (T0), 30 min (T30) and 60 min (T60) after reperfusion. The tissue was embedded in 10% formalin overnight and 4-μm sections were prepared for immunostaining with monoclonal antibodies. Immunostained cells were counted by optical microscopy. The statistical analysis used mean values based on 4 sections.
Results
The pretreatment with L-Ala-Gln animal group 1 demonstrated significantly lower levels of TNF-α, NF-κB and IL-6. On the other hand, the levels of HO-1 were significantly higher, suggesting a protective role in model of brain ischemia-reperfusion injury.
Conclusion
These findings suggest a protective effect of L-Ala-Gln by decreasing levels of TNF-alpha, IL-6 and NF-κB and Increasing levels of HO-1.
Anti-Inflammatory Agents; Glutamine; Immunohistochemistry; Dietary Supplements
Introduction
Cerebrovascular diseases are currently the third-most important cause of death in several developed and developing countries. The European incidence of cerebral infarction is 1.35-2.2 / 1,000 inhabitants, and 83% of cases are associated to ischemic etiology11. Pontes-Neto OM, Cougo P, Martins SC, Abud DG, Nogueira RG, Miranda M, Castro-Afonso, LH, Rebello LC, Caldas JGMP, Bazan R, Bezerra DC, Rezende MT, Freitas GR, Longo A, Magalhães P, Carvalho JJF, Montalverne FJ, Lima FO, Andrade GHV, Massaro AR, Oliveira-Filho J, Gagliardi R, Silva GS. Brazilian guidelines for endovascular treatment of patients with acute ischemic stroke. Arq Neuropsiquiatr. 2017;75(1):50-6. doi: 10.1590/0004-282X20160174.. According to the National Institute of Health, cerebrovascular diseases affect about 500,000 people every year in the U.S., with a mortality rate of 20-30% and a similar rate of severe disability22. Duarte SG, Campos AD, Colli BO. Functional evaluation of temporary focal cerebral ischemia: experimental model. Arq Neuropsiquiatr. 2003;61(3B):751-6. doi: 10.1590/s0004-282x2003000500009..
Cerebral ischemia is a reduction or total absence of oxygen and other metabolic substrates in the brain due to total or partial obstruction of the blood supply33. Moro MA, Almeida A, Bolanos JP, Lizasoain I. Mitochondrial respiratory chain and free radical generation in stroke. Free Radic Biol Med. 2005;39(10):1291-304. doi: 10.1016/j.freeradbiomed.2005.07.010.. Reperfusion after brain ischemia increases the levels of pro-oxidant reactive oxygen species (ROS) in the brain tissue and may lead to neuronal injury as ROS interact directly with macromolecules (including proteins, lipids and DNA) or indirectly affect cellular signaling pathways and the regulation of gene expression44. Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005;38(7):995-1014. doi: 10.1590/s0100-879x2005000700003..
The triggering and maintaining of the inflammatory state depend on several known mediators secreted by activated cells at the site of injury55. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428-35. doi: 10.1038/nature07201.,66. Valenca SS, Porto LC. Immunohistochemical study of lung remodeling in mice exposed to cigarette smoke*. J Bras Pneumol. 2008;34(10):787-95. doi: 10.1590/s1806-37132008001000006.
https://doi.org/10.1590/s1806-3713200800...
. IL-6 is a cytokine and its physiological role in injury-induced inflammation shows a potent pro-inflammatory role77. Xiao W. Advances in NF-kappaB signaling transduction and transcription. Cell Mol Immunol. 2004;1(6):425-35..
TNF-α acts on endothelial cells by promoting the migration of neutrophils and plays a key role stimulating endothelial cells to produce and release chemokines88. Roveran V, Silva MA, Yamano L, Rodrigues LP, Vasquez ML, Piato S. Local expression of tumor necrosis factor-alpha on premature rupture of membranes. Rev Bras Ginecol Obstet. 2009;31(5):249-53. doi: 10.1590/s0100-72032009000500008.
https://doi.org/10.1590/s0100-7203200900...
.
The nuclear factor kappa B (NF-κB) regulates the expression of genes essential for inflammation, cell survival, proliferation and apoptosis99. Yachie A, Toma T, Mizuno K, Okamoto H, Shimura S, Ohta K, Kasahara Y, Koizumi S. Heme oxygenase-1 production by peripheral blood monocytes during acute inflammatory illnesses of children. Exp Biol Med (Maywood). 2003;228(5):550-6. doi: 10.1177/15353702-0322805-26.. Microsomal heme oxygenase-1 (HO-1) is activated by oxidative stress or by the presence of proinflammatory cytokines, endotoxins, heme and nitric oxide1010. Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60(1):79-127. doi: 10.1124/pr.107.07104.,1111. Alves MA, Guimaraes SB, Dias DA, Vasconcelos PR, Coelho Vde P, Vasconcelos PR. Effects of L-alanyl-glutamine upon the blood and kidney biochemical parameters in the rat hind limb model of ischemia/reperfusion. Acta Cir Bras. 2005;20(6):445-9. doi: 10.1590/s0102-86502005000600009..
Several reports have been published investigating cerebral ischemia using animal models. Several species have been tested1111. Alves MA, Guimaraes SB, Dias DA, Vasconcelos PR, Coelho Vde P, Vasconcelos PR. Effects of L-alanyl-glutamine upon the blood and kidney biochemical parameters in the rat hind limb model of ischemia/reperfusion. Acta Cir Bras. 2005;20(6):445-9. doi: 10.1590/s0102-86502005000600009.; among these, the Mongolian gerbil (Meriones unguiculatus) is an adjusted methodological option due to its high susceptibility to experimental cerebral ischemia induced by ligation of the common carotid artery1212. Kanoria S, Jalan R, Seifalian AM, Williams R, Davidson BR. Protocols and mechanisms for remote ischemic preconditioning: a novel method for reducing ischemia reperfusion injury. Transplantation. 2007;84(4):445-58. doi: 10.1097/01.tp.0000228235.55419.e8..
Numerous studies have shown that L-alanyl-L-glutamine is an essential amino acid, which is actively transported and metabolized in all animal tissues1313. Muniz LRF, Faria MHG, Vasconcelos PRL. Metabolic evaluation of ischemic and reperfusion brain injury following bilateral occlusion of common carotid arteries: an experimental study in rats. Acta Cir Bras. 2004;19:529-34 doi: 10.1590/S0102-86502004000500012.
https://doi.org/10.1590/S0102-8650200400...
; the glutamate-glutamine cycle between glial cells and nerve endings is maintained through the uptake of gamma-amino butyric acid and glutamate by astrocytes. These amino acids are converted to glutamine1414. Pandya JD, Sullivan PG, Pettigrew LC. Focal cerebral ischemia and mitochondrial dysfunction in the TNFalpha-transgenic rat. Brain Res. 2011;1384:151-60. doi: 10.1016/j.brainres.2011.01.102.. Disturbances in synthesis may result in the accumulation of glutamate in glial cells leading to neurotoxicity.
Studies by Kanoria1212. Kanoria S, Jalan R, Seifalian AM, Williams R, Davidson BR. Protocols and mechanisms for remote ischemic preconditioning: a novel method for reducing ischemia reperfusion injury. Transplantation. 2007;84(4):445-58. doi: 10.1097/01.tp.0000228235.55419.e8. have shown that preconditioning with L-Ala-Gln protects against ischemia-reperfusion injury in several organs. Thus, the aim of this study was to evaluate the role of L-Ala-Gln to protect against brain ischemia-reperfusion injury in a gerbil model evaluated through immunohistochemistry (IHC) for the IL-6, TNF-α, NF-κB and HO-1.
Methods
The study was conducted in accordance with the International Guiding Principles for Biomedical Research Involving Animals (1990). The study protocol was approved in 2008 by the Research Ethics Committee (CEPA) of Universidade Federal do Ceará (UFC), #127/07. The gerbils were supplied by the experimental animal facility at Centro Universitário Christus and used at the laboratory of experimental surgery (UFC School of Medicine).
Experimental design
The sample consisted of 36 healthy, well-nourished male gerbils (Meriones ungiculatus) aged 8-16 months and weighing 100-180 g. The sample was divided into two groups: in Group 1 (n=18), the animals were pretreated with saline (control) and submitted to ischemia and reperfusion. In Group 2 (n=18), the animals were pretreated with L-Ala-Gln and submitted to ischemia and reperfusion. Each group was divided into three subgroups (n≥4) according to the time following reperfusion: 0 min (T0), 30 min (T30) and 60 min (T60)1515. Pires VL, Souza JR, Guimaraes SB, Silva Filho AR, Garcia JH, Vasconcelos PR. Preconditioning with L-alanyl-L-glutamine in a Mongolian gerbil model of acute cerebral ischemia/reperfusion injury. Acta Cir Bras. 2011;26 Suppl 1:14-20. doi: 10.1590/s0102-86502011000700004..
The animals were anesthetized with an intraperitoneal injection of urethane (1.5 g/kg) and submitted to osteotomy with a double-sided flexible grindstone attached to an electric motor, followed by the harvesting of brain tissue1515. Pires VL, Souza JR, Guimaraes SB, Silva Filho AR, Garcia JH, Vasconcelos PR. Preconditioning with L-alanyl-L-glutamine in a Mongolian gerbil model of acute cerebral ischemia/reperfusion injury. Acta Cir Bras. 2011;26 Suppl 1:14-20. doi: 10.1590/s0102-86502011000700004.,1616. Tripathy D, Yin X, Sanchez A, Luo J, Martinez J, Grammas P. Cerebrovascular expression of proteins related to inflammation, oxidative stress and neurotoxicity is altered with aging. J Neuroinflammation. 2010;7:63. doi: 10.1186/1742-2094-7-63.. Removal of internal pyramidal layer of the parietal region was performed using a method by Pires and cols. At the end of the procedure, the animals were euthanized by cervical dislocation.
Brain ischemia protocol
The surgical procedure started with trichotomy followed by an incision in the ventral region of the neck; the muscular and subcutaneous tissues were dissect with individualization and bilateral isolation of the common carotid arteries (CCAs) at 0.5 cm of bifurcation in the external carotid arteries and internal (occlusion point), followed by identification and clamping of the arteries, with bulldog type vascular clips, for a period of 15 minutes of ischemia followed by two reperfusion periods T30 and T60.
Chemicals
L-Ala-Gln was purchased from Fresenius Brazil. The 0.9% saline solution was purchased from Gaspar Viana and administered in a standardized volume of 2.0 mL.
IHC analysis
The brain tissue was submitted to immunohistochemical analysis with streptavidin-biotin to quantify TNF-α, NF-κB, IL-6 and HO-1. Initially, the tissue was fixed in 10% formalin for 24 hours, followed by embedding in paraffin. Sections measuring 4 μm were prepared with a microtome and placed on slides with poly-L-lysine, then dehydrated and hydrated with decreasing concentrations of alcohol and xylene. After heat-induced antigen retrieval (10min in citrate buffer pH=6.0), blocking with 3% hydrogen peroxide and rinsing with phosphate buffer solution (PBS), the samples were incubated overnight at 4ºC, according to manufacturer´s. Primary antibodies against IL-6 and TNF-α (goat polyclonal). NF-kB and HO-1 (mouse monoclonal) were used (1:200 diluted in 1 x PBS containing 5% BSA) (Santa Cruz, Texas, USA). The negative controls were treated with BSA but not with primary antibody.
After overnight incubation, the slides were washed in PBS and incubated with secondary mouse-anti-goat IgG-HRP for TNF-alpha and IL-6; and goat-anti-mouse for NF-kB and HO-1 both (1:100 diluted in 1 x PBS containing 5% BSA) (Santa Cruz, Texas, USA). Then, the slides were washed in PBS, and streptavidin- ABC complex conjugate (Santa Cruz) was added, followed by drying and the addition of 3,3-diaminobenzidine (Dako). Finally, the slides were mounted using Entellan (São Paulo, Brazil) and coverslips.
The evaluation of slides was performed by counting immunostained nerve cells, both neurons and neuroglia cells, observed at 400X magnification in an Olympus BX41 optical microscope. Ten fields of each slice were observed (x40, 10 oculars, 0.5024mm2 per field), with a total of 4 cuts per group, always trying to start with the internal pyramid layer. The analyses were performed by a pathologist. After the examiner’s analysis, the immunohistochemistry findings were sent for statistical tests of concordance.
Statistical analysis
The statistical analysis was performed with the software SPSS (v.17.0). Quantitative variables were initially analyzed with the Shapiro-Wilk test to verify the normality of the distribution. Having confirmed normality in all cases, mean values and standard deviations were calculated based on four sections. The groups and reperfusion times were compared with ANOVA and the post-hoc Tukey test. The level of statistical significance was set at 5% (p<0.05).
Results
Immunohistochemistry for TNF-α
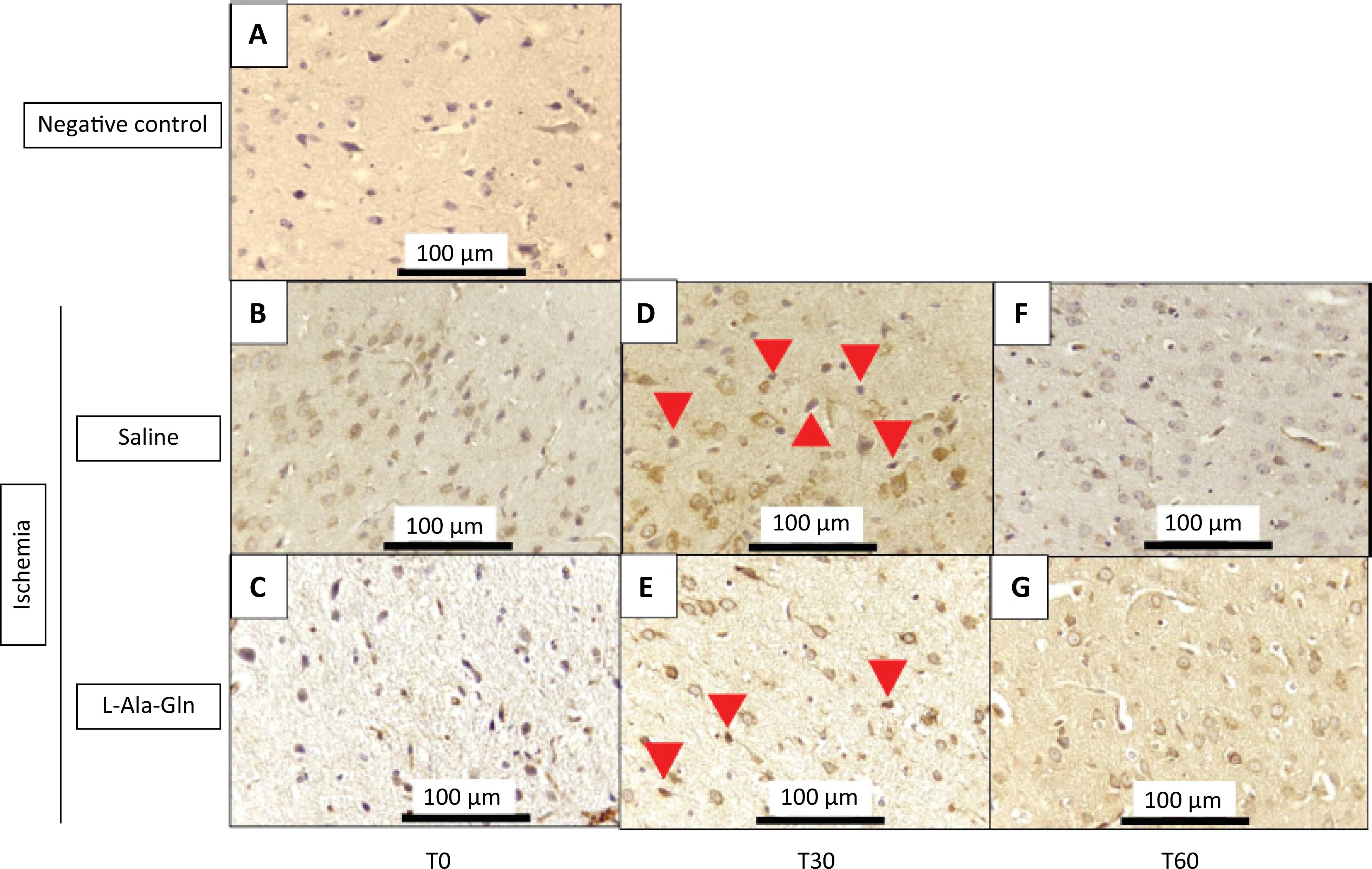
The IHC TNF-alpha immunostained cells in the internal pyramidal layer of parietal region of animals that received L-Ala-Gln were significantly reduced after 60 minutes when compared to control group (saline 0.9% NaCl) (Fig. 1). TNF-alpha levels were significantly decreased after 60 minutes of ischemia (p=0.001) (Table 1). The immunoreactivity after 30 minutes showed a discrete reduction (p>0.05) on immunostained cells in L-Ala-Gln group in comparison to control group.
Mean TNF-α levels (± SEM) in gerbil brain tissue according to time (T0, T30 and T60) and pretreatment with saline 0.9% vs. L-Ala-Gln (0.75g/kg). Representative photographs of, effect of pretreatment with L-Ala-Gln on internal pyramidal layer of parietal area, on immunoreactivity of TNF-alpha in the pyramidal layer in parietal cortex. (A) negative control (B, D and F) transient cerebral ischemia and saline 0,9% NaCl; C, E and G transient cerebral ischemia and L-Ala-Gln (0.75 g/kg): The figures B and C; D and E; and F and G are from T0, T30 and T60 minutes after the brain ischemia. Arrowheads show immunostained cells. Each group contained at least 4 animals. Bar=100 µm.
Immunoreactivity for TNF-a in brain tissue sections from internal pyramidal layer of the cerebral cortex positive cells for each field on 0, 30 and 60 minutes.
The number of TNF-alpha immunoreactive neurons in saline and L-Ala-Gln groups. The morphological analysis was performed evaluating the immunostained central nervous system nerve cells (neurons and neuroglia cells), observed at 400X magnification in an Olympus BX41 optical microscope. Ten fields of each cut were observed, with a total of 4 cuts per group, always trying to start with the internal pyramid layer (p<0.05).
Immunohistochemistry for NF-kB
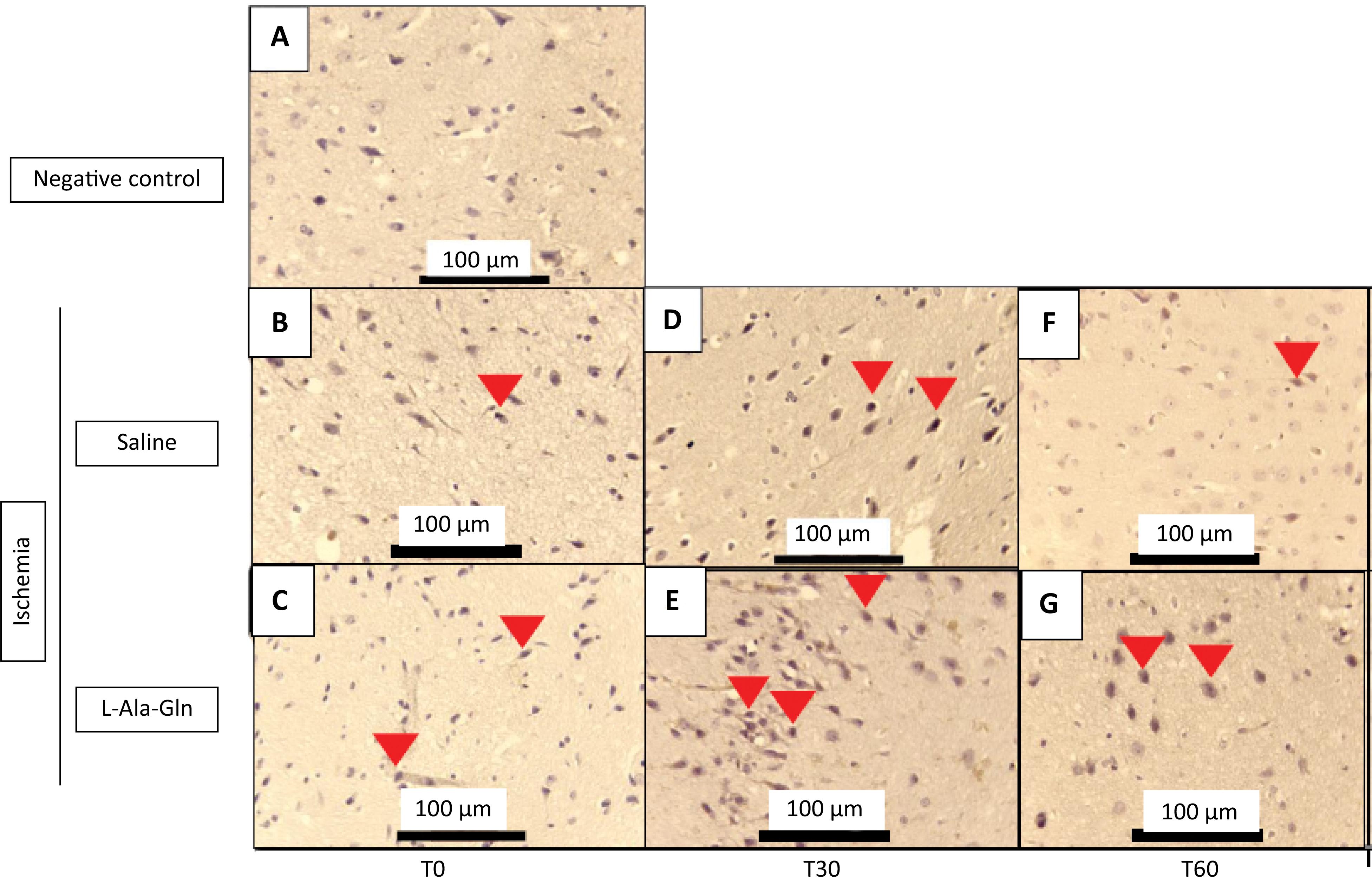
The animals that received L-Ala-Gln demonstrated significantly reduced positive-IHC neurons of NF-κB levels on the internal pyramidal layer of the parietal region, detected through immunohistochemistry in the cortex of parietal region, after 30 and 60 minutes, respectively when compared to saline (0.9% NaCl) group (p=0.001) (Table 2). In addition, the NF-kB immunoreactivity was weaker in L-Ala-Gln group compared to saline group levels (Fig. 2).
Immunoreactivity for NF-kB in brain tissue sections from internal pyramidal layer of the cerebral cortex positive cells for each field on 0, 30 and 60 minutes.
Mean NF-κB levels (± SEM) in gerbil brain tissue according to time (T0, T30 and T60) and pretreatment with saline 0.9% vs. L-Ala-Gln (0.75g/kg). Representative photographs of, effect of pretreatment with L-Ala-Gln on internal pyramidal layer of parietal area, on immunoreactivity of NF-kB in the pyramidal layer in parietal cortex. (A) negative control (B, D and F) transient cerebral ischemia and saline 0,9% NaCl; C, E and G transient cerebral ischemia and L-Ala-Gln (0.75 g/kg): The figures B and C; D and E; and F and G are from T0, T30 and T60 minutes after the brain ischemia. Arrowheads show immunostained cells. Each group contained at least 4 animals. Bar=100 µm.
The number of NF-kB immunoreactive neurons in saline and L-Ala-Gln groups. The morphological analysis was performed evaluating the immunostained central nervous system nerve cells (neurons and neuroglia cells), observed at x400 magnification in an Olympus BX41 optical microscope. Ten fields of each cut were observed, with a total of 4 cuts per group, always trying to start with the internal pyramid layer. L-Ala-Gln vs. Saline group p<0.0001 at T30 and T60 after ischemia.
Immunohistochemistry for IL-6
The IL-6 immunostained positive cells had demonstrated a significant decrease after 30 minutes of ischemia injury on internal pyramidal layer of the parietal area from animals treated with L-Ala-Gln (Fig. 3). Although, after 60 minutes, the means of both groups are quite different (316.0 and 28.5); however, it cannot demonstrate the significance due to the standard error of mean (SEM) of the saline group (p=0.033) (Table 3).
Mean IL-6 levels (± SEM) in gerbil brain tissue according to time (T0, T30 and T60) and pretreatment with saline 0.9% vs. L-Ala-Gln (0.75g/kg). Representative photographs of, effect of pretreatment with L-Ala-Gln on internal pyramidal layer of parietal area, on immunoreactivity of IL-6 in the pyramidal layer in parietal cortex. (A) negative control (B, D and F) transient cerebral ischemia and saline 0,9% NaCl; C, E and G transient cerebral ischemia and L-Ala-Gln (0.75 g/kg): The figures B and C; D and E; and F and G are from T0, T30 and T60 minutes after the brain ischemia. Arrowheads show immunostained cells. Each group contained at least 4 animals. Bar=100 µm.
Immunoreactivity for IL-6 in brain tissue sections from internal pyramidal layer of the cerebral cortex positive cells for each field on 0, 30 and 60 minutes.
The number of IL-6 immunoreactive neurons in saline and L-Ala-Gln groups. The morphological analysis was performed evaluating the immunostained central nervous system nerve cells (neurons and neuroglia cells), observed at x400 magnification in an Olympus BX41 optical microscope. Ten fields of each cut were observed, with a total of 4 cuts per group, always trying to start with the internal pyramid layer. L-Ala-Gln vs. Saline group p<0.033 at T30 after ischemia.
Immunohistochemistry for HO-1
The HO-1 marker had demonstrated an significant increase on immunostained positive cells of internal pyramidal layer of parietal area from tissue brain of gerbils treated with L-Ala-Gln, after 0, 30 and 60 minutes of ischemia and reperfusion injuries in animals treated with L-Ala-Gln (p=0.015), (p=0.013) and (p=0.007) respectively, in comparison to gerbils that were administered saline 0.9% NaCl, observed in internal pyramidal layer of parietal region (Fig. 4, Table 4).
Mean HO-1 levels (± SEM) in gerbil brain tissue according to time (T0, T30 and T60) and pretreatment with saline 0.9% vs. L-Ala-Gln (0.75g/kg). Representative photographs of, effect of pretreatment with L-Ala-Gln on internal pyramidal layer of parietal area, on immunoreactivity of HO-1 in the pyramidal layer in parietal cortex. (A) negative control (B, D and F) transient cerebral ischemia and saline 0,9% NaCl; C, E and G transient cerebral ischemia and L-Ala-Gln (0.75 g/kg): The figures B and C; D and E; and F and G are from T0, T30 and T60 minutes after the brain ischemia. Arrowheads show immunostained cells. Each group contained at least 4 animals. Bar=100 µm.
Immunoreactivity for HO-1 in brain tissue sections from internal pyramidal layer of the cerebral cortex positive cells for each field on 0. 30 and 60 minutes.
The number of HO-1 immunoreactive cells in saline and L-Ala-Gln groups. The morphological analysis was performed evaluating the immunostained central nervous system cells (neurons and neuroglia cells), observed at 400X magnification in an Olympus BX41 optical microscope. Ten fields of each cut were observed. with a total of 4 cuts per group. always trying to start with the internal pyramid layer. The p=0.015, p=0.013, and p=0.007 vs 0.9% Na Cl control group after 0, 30 and 60 minutes of reperfusion respectively.
Discussion
In this study, we found immunohistochemical evidence of the protective effect of L-Ala-Gln on the internal pyramidal layer of the parietal area of gerbil brain tissue exposed to ischemia and reperfusion injuries based on the quantification of inflammation and cell activation biomarkers (TNF-α, NF-κB, IL-6 and HO-1). In 2011, Pires and cols. using the same bilateral occlusion protocol of cerebral ischemia/reperfusion demonstrated that precondition with L-Ala-Gln reduced the oxidative stress in cerebral tissue1515. Pires VL, Souza JR, Guimaraes SB, Silva Filho AR, Garcia JH, Vasconcelos PR. Preconditioning with L-alanyl-L-glutamine in a Mongolian gerbil model of acute cerebral ischemia/reperfusion injury. Acta Cir Bras. 2011;26 Suppl 1:14-20. doi: 10.1590/s0102-86502011000700004.. Thus, this study evaluates the inflammatory aspect of cerebral ischemia/reperfusion.
The finding of significantly lower TNF-α levels in L-Ala-Gln group at T60 suggests L-Ala-Gln has a neuroprotective effect preventing cellular damage, on internal pyramidal layer of parietal area, induced by cytokines and proinflammatory mediators released in association with inflammatory microvascular injury and ROS-mediated cytotoxicity1717. Murphy CG, Chen G, Winter DC, Bouchier-Hayes DJ. Glutamine preconditioning protects against tourniquet-induced local and distant organ injury in a rodent ischemia-reperfusion model. Acta Orthop. 2007;78(4):559-66. doi: 10.1080/17453670710014220..
A redox-sensitive transcription factor, NF-κB activates the inflammatory transcription cascade, regulating an array of inflammatory genes in addition to certain mediators with anti-inflammatory action. NF-κB has therefore been proposed as a target for cell protection against oxidative stress, pro-inflammatory factors and sclerosis in several sites, including the myocardium and the brain1818. Moreira TJ, Cebere A, Cebers G, Ostenson CG, Efendic S, Liljequist S. Reduced HO-1 protein expression is associated with more severe neurodegeneration after transient ischemia induced by cortical compression in diabetic Goto-Kakizaki rats. J Cereb Blood Flow Metab. 2007;27(10):1710-23. doi: 10.1038/sj.jcbfm.9600479.. Our finding of significantly reduced levels of NF-κB in preconditioned animals with L-Ala-Gln at T60 is in concordance of other reports investigating the neuroprotective action of L-Ala-Gln and its interaction with glutaminergic NF-κB-dependent pathways1818. Moreira TJ, Cebere A, Cebers G, Ostenson CG, Efendic S, Liljequist S. Reduced HO-1 protein expression is associated with more severe neurodegeneration after transient ischemia induced by cortical compression in diabetic Goto-Kakizaki rats. J Cereb Blood Flow Metab. 2007;27(10):1710-23. doi: 10.1038/sj.jcbfm.9600479..
IL-6 is a multifunctional cytokine produced by several cell types, especially cells of the mononuclear phagocyte system. It plays an important role in lymphocyte (CD4+) differentiation, immunoglobulin secretion, formation of multipotent cell colonies in the bone marrow, and several proteins involved in the acute phase of systemic inflammation1919. Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28(2):289-309. doi: 10.1016/s0891-5849(99)00223-3..
The significantly lower IL-6 levels observed in L-Ala-Gln group at T30 suggests its protective against brain cell damage induced by cytokines and proinflammatory mediators released in association with microvascular injury. These findings agree with those of other studies employing cerebral ischemia/reperfusion models1919. Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28(2):289-309. doi: 10.1016/s0891-5849(99)00223-3.,2020. Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest. 2006;116(3):808-16. doi: 10.1172/JCI26857..
Some studies have shown that the induction of HO-1 promotes cellular protection against oxidative injury through different mechanisms, such as by controlling intracellular levels of free heme (an anti-oxidant), producing biliverdin (an anti-oxidant), improving perfusion of nutrients via the release of CO, and inducing ferritin synthesis through the release of free iron2121. Guan L, Zhang YL, Wen T, Wang XF, Zhu MX, Zhao JY. Dynamic changes of heme oxygenase-1 in the hippocampus of rats after acute carbon monoxide poisoning. Arch Environ Contam Toxicol. 2011;60(1):165-72. doi: 10.1007/s00244-010-9524-3.,2222. Morimoto K, Ohta K, Yachie A, Yang Y, Shimizu M, Goto C, Toma T, Kasahara Y, Yokohama H, Miyata T, Seki H, Koizumi S. Cytoprotective role of heme oxygenase (HO)-1 in human kidney with various renal diseases. Kidney Int. 2001;60(5):1858-66. doi: 10.1046/j.1523-1755.2001.01000.x..
Furthermore, low heme concentrations may have anti-inflammatory and cytoprotective effects by increasing the HO-1 expression and stimulating the formation of HO-1 and its products, such as CO and biliverdin. In the present study, HO-1 levels at T0, T30 and T60 were significantly higher in tissues from animals preconditioned with L-Ala-Gln, as shown elsewhere in the literature2121. Guan L, Zhang YL, Wen T, Wang XF, Zhu MX, Zhao JY. Dynamic changes of heme oxygenase-1 in the hippocampus of rats after acute carbon monoxide poisoning. Arch Environ Contam Toxicol. 2011;60(1):165-72. doi: 10.1007/s00244-010-9524-3.,2323. Alves WFG, SB Vasconcelos, PR Cavalcante, Vasconcelos PRL. Repercussões da L-alanil-glutamina sobre as concentrações de lactato e lactato desidrogenase (LDH) em pacientes com isquemia crítica dos membros inferiores submetidos a revascularização distal. Acta Cir Bras. 2003;18:209-15.
24. Barbosa RCC, Guimarães SB, Vasconcelos PRC, Chaves CR, Vasconcelos PRL. Metabolic effects of glutamine in rats subjected to scald burn. Acta Cir Bras. 2003;18(6):527-33. doi: 10.1016/j.burns.2005.12.014.
https://doi.org/10.1016/j.burns.2005.12....
25. Campelo MW, Campelo AP, Lopes LG, Santos AA, Guimaraes SB, Vasconcelos PR. Effects of Rut-bpy (Cis-[Ru(bpy)2(SO3)(NO)]PF 6), a novel nitric oxide donor, in L-NAME-induced hypertension in rats. Acta Cir Bras. 2011;26 Suppl 1:57-9. doi: 10.1590/s0102-86502011000700012.
26. Yasuhara M. L-glutamine-induced heme oxygenase-1 protects small intestine from warm ischemia and reperfusion injury in the rat. Hokkaido J Med Sci. 2001;76:21-34.-2727. Wang HQ, Xu YX, Zhu CQ. Upregulation of heme oxygenase-1 by acteoside through ERK and PI3 K/Akt pathway confer neuroprotection against beta-amyloid-induced neurotoxicity. Neurotox Res. 2012;21(4):368-78. doi: 10.1007/s12640-011-9292-5..
L-Aln-Gln has been investigated extensively in order to evaluate tissue injury pathways and mechanisms in target organs in ischemia-reperfusion models1313. Muniz LRF, Faria MHG, Vasconcelos PRL. Metabolic evaluation of ischemic and reperfusion brain injury following bilateral occlusion of common carotid arteries: an experimental study in rats. Acta Cir Bras. 2004;19:529-34 doi: 10.1590/S0102-86502004000500012.
https://doi.org/10.1590/S0102-8650200400...
. The studies published so far provide strong evidence of a cytoprotective effect of L-Ala-Gln in various cell types and of the molecular and biochemical-signaling pathways implicated in antioxidant defense and the (probably sclerotic) anti-inflammatory action of L-Aln-Gln.
Preconditioning with L-Aln-Gln has shown to reduce the extension of myocardial cell damage associated with ischemia-reperfusion injury in experimental models. L-Aln-Gln is believed to inhibit the harmful effects of neuronal NO synthetase by nitrergic route and thereby inhibit glutamine synthetase. The blocking of NO synthesis may involve other enzymes such as glutamine synthase2828. Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579-88. doi: 10.1152/ajpheart.01064.2002.. It should be pointed out that the main metabolic changes are observed during the first minutes of reperfusion, indicating they represent a reaction to early reperfusion-induced oxidative stress1616. Tripathy D, Yin X, Sanchez A, Luo J, Martinez J, Grammas P. Cerebrovascular expression of proteins related to inflammation, oxidative stress and neurotoxicity is altered with aging. J Neuroinflammation. 2010;7:63. doi: 10.1186/1742-2094-7-63..
The epidemiological data of the non-transmissible diseases such as cerebrovascular ischemia increased in the last decade, requiring the development of a new tool to prevent or to reduce the potential negative effects of this kind of injury; thus, a translational approaching for this study could be done. Probably, the adoption of a diet rich in amino acids like Alanine and Glutamine could be a protective factor reducing the worst damages on neuronal tissue that led the patients to a several disabilities in many cases.
Further studies including a larger array of inflammatory and cell activation biomarkers are required to confirm the efficacy and safety of preconditioning with L-Ala-Gln against the deleterious effects of brain ischemia and reperfusion.
Conclusion
Preconditioning with L-Ala-Gln has a potentially protective role against inflammation induced by brain ischemia and reperfusion.
Acknowledgements
To Conceição da Silva Martins (laboratory technician) and Dra. Gerly Anne Castro Brito (coordinator) of the histology core facility at UFC; and to Dr. David Bollick from the University of Virginia for the English review.
References
-
1Pontes-Neto OM, Cougo P, Martins SC, Abud DG, Nogueira RG, Miranda M, Castro-Afonso, LH, Rebello LC, Caldas JGMP, Bazan R, Bezerra DC, Rezende MT, Freitas GR, Longo A, Magalhães P, Carvalho JJF, Montalverne FJ, Lima FO, Andrade GHV, Massaro AR, Oliveira-Filho J, Gagliardi R, Silva GS. Brazilian guidelines for endovascular treatment of patients with acute ischemic stroke. Arq Neuropsiquiatr. 2017;75(1):50-6. doi: 10.1590/0004-282X20160174.
-
2Duarte SG, Campos AD, Colli BO. Functional evaluation of temporary focal cerebral ischemia: experimental model. Arq Neuropsiquiatr. 2003;61(3B):751-6. doi: 10.1590/s0004-282x2003000500009.
-
3Moro MA, Almeida A, Bolanos JP, Lizasoain I. Mitochondrial respiratory chain and free radical generation in stroke. Free Radic Biol Med. 2005;39(10):1291-304. doi: 10.1016/j.freeradbiomed.2005.07.010.
-
4Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005;38(7):995-1014. doi: 10.1590/s0100-879x2005000700003.
-
5Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428-35. doi: 10.1038/nature07201.
-
6Valenca SS, Porto LC. Immunohistochemical study of lung remodeling in mice exposed to cigarette smoke*. J Bras Pneumol. 2008;34(10):787-95. doi: 10.1590/s1806-37132008001000006.
» https://doi.org/10.1590/s1806-37132008001000006 -
7Xiao W. Advances in NF-kappaB signaling transduction and transcription. Cell Mol Immunol. 2004;1(6):425-35.
-
8Roveran V, Silva MA, Yamano L, Rodrigues LP, Vasquez ML, Piato S. Local expression of tumor necrosis factor-alpha on premature rupture of membranes. Rev Bras Ginecol Obstet. 2009;31(5):249-53. doi: 10.1590/s0100-72032009000500008.
» https://doi.org/10.1590/s0100-72032009000500008 -
9Yachie A, Toma T, Mizuno K, Okamoto H, Shimura S, Ohta K, Kasahara Y, Koizumi S. Heme oxygenase-1 production by peripheral blood monocytes during acute inflammatory illnesses of children. Exp Biol Med (Maywood). 2003;228(5):550-6. doi: 10.1177/15353702-0322805-26.
-
10Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60(1):79-127. doi: 10.1124/pr.107.07104.
-
11Alves MA, Guimaraes SB, Dias DA, Vasconcelos PR, Coelho Vde P, Vasconcelos PR. Effects of L-alanyl-glutamine upon the blood and kidney biochemical parameters in the rat hind limb model of ischemia/reperfusion. Acta Cir Bras. 2005;20(6):445-9. doi: 10.1590/s0102-86502005000600009.
-
12Kanoria S, Jalan R, Seifalian AM, Williams R, Davidson BR. Protocols and mechanisms for remote ischemic preconditioning: a novel method for reducing ischemia reperfusion injury. Transplantation. 2007;84(4):445-58. doi: 10.1097/01.tp.0000228235.55419.e8.
-
13Muniz LRF, Faria MHG, Vasconcelos PRL. Metabolic evaluation of ischemic and reperfusion brain injury following bilateral occlusion of common carotid arteries: an experimental study in rats. Acta Cir Bras. 2004;19:529-34 doi: 10.1590/S0102-86502004000500012.
» https://doi.org/10.1590/S0102-86502004000500012 -
14Pandya JD, Sullivan PG, Pettigrew LC. Focal cerebral ischemia and mitochondrial dysfunction in the TNFalpha-transgenic rat. Brain Res. 2011;1384:151-60. doi: 10.1016/j.brainres.2011.01.102.
-
15Pires VL, Souza JR, Guimaraes SB, Silva Filho AR, Garcia JH, Vasconcelos PR. Preconditioning with L-alanyl-L-glutamine in a Mongolian gerbil model of acute cerebral ischemia/reperfusion injury. Acta Cir Bras. 2011;26 Suppl 1:14-20. doi: 10.1590/s0102-86502011000700004.
-
16Tripathy D, Yin X, Sanchez A, Luo J, Martinez J, Grammas P. Cerebrovascular expression of proteins related to inflammation, oxidative stress and neurotoxicity is altered with aging. J Neuroinflammation. 2010;7:63. doi: 10.1186/1742-2094-7-63.
-
17Murphy CG, Chen G, Winter DC, Bouchier-Hayes DJ. Glutamine preconditioning protects against tourniquet-induced local and distant organ injury in a rodent ischemia-reperfusion model. Acta Orthop. 2007;78(4):559-66. doi: 10.1080/17453670710014220.
-
18Moreira TJ, Cebere A, Cebers G, Ostenson CG, Efendic S, Liljequist S. Reduced HO-1 protein expression is associated with more severe neurodegeneration after transient ischemia induced by cortical compression in diabetic Goto-Kakizaki rats. J Cereb Blood Flow Metab. 2007;27(10):1710-23. doi: 10.1038/sj.jcbfm.9600479.
-
19Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28(2):289-309. doi: 10.1016/s0891-5849(99)00223-3.
-
20Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest. 2006;116(3):808-16. doi: 10.1172/JCI26857.
-
21Guan L, Zhang YL, Wen T, Wang XF, Zhu MX, Zhao JY. Dynamic changes of heme oxygenase-1 in the hippocampus of rats after acute carbon monoxide poisoning. Arch Environ Contam Toxicol. 2011;60(1):165-72. doi: 10.1007/s00244-010-9524-3.
-
22Morimoto K, Ohta K, Yachie A, Yang Y, Shimizu M, Goto C, Toma T, Kasahara Y, Yokohama H, Miyata T, Seki H, Koizumi S. Cytoprotective role of heme oxygenase (HO)-1 in human kidney with various renal diseases. Kidney Int. 2001;60(5):1858-66. doi: 10.1046/j.1523-1755.2001.01000.x.
-
23Alves WFG, SB Vasconcelos, PR Cavalcante, Vasconcelos PRL. Repercussões da L-alanil-glutamina sobre as concentrações de lactato e lactato desidrogenase (LDH) em pacientes com isquemia crítica dos membros inferiores submetidos a revascularização distal. Acta Cir Bras. 2003;18:209-15.
-
24Barbosa RCC, Guimarães SB, Vasconcelos PRC, Chaves CR, Vasconcelos PRL. Metabolic effects of glutamine in rats subjected to scald burn. Acta Cir Bras. 2003;18(6):527-33. doi: 10.1016/j.burns.2005.12.014.
» https://doi.org/10.1016/j.burns.2005.12.014 -
25Campelo MW, Campelo AP, Lopes LG, Santos AA, Guimaraes SB, Vasconcelos PR. Effects of Rut-bpy (Cis-[Ru(bpy)2(SO3)(NO)]PF 6), a novel nitric oxide donor, in L-NAME-induced hypertension in rats. Acta Cir Bras. 2011;26 Suppl 1:57-9. doi: 10.1590/s0102-86502011000700012.
-
26Yasuhara M. L-glutamine-induced heme oxygenase-1 protects small intestine from warm ischemia and reperfusion injury in the rat. Hokkaido J Med Sci. 2001;76:21-34.
-
27Wang HQ, Xu YX, Zhu CQ. Upregulation of heme oxygenase-1 by acteoside through ERK and PI3 K/Akt pathway confer neuroprotection against beta-amyloid-induced neurotoxicity. Neurotox Res. 2012;21(4):368-78. doi: 10.1007/s12640-011-9292-5.
-
28Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579-88. doi: 10.1152/ajpheart.01064.2002.
-
1
Research performed at Laboratório de Cirurgia Experimental (LABCEX), Universidade Federal do Ceará (UFC), Fortaleza-CE, Brazil.
-
Financial source: CNPq
Publication Dates
-
Publication in this collection
20 July 2020 -
Date of issue
2020
History
-
Received
25 Feb 2020 -
Reviewed
22 Apr 2020 -
Accepted
23 May 2020