Abstract
PURPOSE: T
o investigate the possible protective effect of thymoquinone (TQ) in cisplatin (CP) induced myocardial injury.
METHODS:
A total of 28 adult male Wistar-Albino rats were randomly and equally divided into four groups as follows: Group 1 (control), Group 2 (CP at 15 mg/kg dose), Group 3 (TQ 40 mg/kg/day for two days prior to CP injection and on third day, CP at 15 mg/kg dose was intraperitoneally administered and TQ treatment continued until fifth day) and Group 4 (TQ at 40mg/kg/day dose for five days).
RESULTS:
There was a significant increment in CP group in terms of congestion, edema and pycnotic nuclei in myocardial fibers, comparing with other groups. TQ group exhibited significant increase in expression of antiapoptotic protein Bcl-2, comparing with CP group (p<0.05). In only CP administered group, expression of antiapoptotic protein Bcl-2 was lowest comparing with other groups.
CONCLUSION:
Established data indicate that cisplatin is cardiotoxic and thymoquinone may be useful in treating CP-induced cardiac injury.
Cisplatin; Cardiotoxicity; Genes, bcl-2; Rats
Introduction
Cisplatin is an effective chemotherapeutic drug that belonging to platinum relating agents used in oncological applications. CP-based treatment is very effective in different cancer types involving pulmonary, ovarian, cranial, cervical and gastric tissues cancers11. Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008 Mar;9(3):215-21. PMID: 18282805.,22. Tiseo M, Martelli O, Mancuso A, Sormani MP, Bruzzi P, Di Salvia R, De Marinis F, Ardizzoni A. Short hydration regimen and nephrotoxicity of intermediate to high-dose cisplatin-based chemotherapy for outpatient treatment in lung cancer and mesothelioma. Tumori. 2007 Mar-Apr;93(2):138-44. PMID: 17557559.. However, the optimal efficacy of CP use is generally restricted by toxic effects such as nephrotoxicity, ototoxicity and cardiovascular complications33. Santabarbara G, Maione P, Rossi AE, Gridelli C. Pharmacotherapeutic options for treating adverse effects of Cisplatin chemotherapy. Expert Opin Pharmacother. 2016 Mar;17(4):561-70. doi: 10.1517/14656566.2016.1122757.
https://doi.org/10.1517/14656566.2016.11...
,44. Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000 Apr;22(4):263-302. PMID: 10789823.. CP-induced cardiotoxicity which is characterized by various findings including electrocardiographic changes, arrhythmia, cardiomyopathy and congestive heart failure has been reported in numerous studies. Although huge efforts were made over many years to find out more powerful but less toxic agents, CP is still widely prescribed55. Yousef MI, Saad AA, El-Shennawy LK. Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem Toxicol. 2009 Jun;47(6):1176-83. PMID: 19425235.. Mechanisms underlying anti-tumoral activity of CP is well-known, however cellular and molecular factors which cause CP-induced cardiotoxicity are still unclear. On the other hand, several experimental and clinical studies support the idea that the enhancement of oxidative stress may involve in this insult66. Ma H, Jones KR, Guo R, Xu P, Shen Y, Ren J. Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: role of endoplasmic reticulum stress. Clin Exp Pharmacol Physiol. 2010 Apr;37(4):460-5. PMID: 19878217..
Thymoquinone is a bioactive compound mainly established from the Nigella sativa plant. TQ is known to have potent antioxidant and anti-inflammatory features, but it is also used in widely range of other fields such as cancer treatment77. Woo CC, Kumar AP, Sethi G, Tan KH. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol. 2012 Feb 15;83(4):443-51. PMID: 22005518.. In some previous studies, it has been reported that TQ treatment protected organs against oxidative damage induced by different free radical producing agents like carbon tetrachloride, cisplatin and doxorubicin88. Badary OA, Nagi MN, al-Shabanah OA, al-Sawaf HA, al-Sohaibani MO, al-Bekairi AM. Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can J Physiol Pharmacol. 1997 Dec;75(12):1356-61. PMID: 9534946.,99. Nagi MN, Mansour MA. Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol Res. 2000 Mar;41(3):283-9. PMID: 10675279.. Different agents with antioxidant, modulators of nitric oxide, diuretic, cytoprotective and antiapoptotic properties have been used to avoid CP nephrotoxicity1010. Ali BH, Al-Salam S, Al Husseini IS, Al-Lawati I, Waly M, Yasin J, Fahim M, Nemmar A. Abrogation of cisplatin-induced nephrotoxicity by emodin in rats. Fundam Clin Pharmacol. 2013 Apr;27(2):192-200. PMID: 22044459.
11. Al-Kharusi N, Babiker HA, Al-Salam S, Waly MI, Nemmar A, Al-Lawati I, Yasin J, Beegam S, Ali BH. Ellagic acid protects against cisplatin-induced nephrotoxicity in rats: a dose-dependent study. Eur Rev Med Pharmacol Sci. 2013 Feb;17(3):299-310. PMID: 23426532.-1212. Ali BH, Al Za'abi M, Shalaby A, Manoj P, Waly MI, Yasin J, Fahim M, Nemmar A. The effect of thymoquinone treatment on the combined renal and pulmonary toxicity of cisplatin and diesel exhaust particles. Exp Biol Med (Maywood). 2015 Dec;240(12):1698-707. PMID: 25925792.. However, there are few studies focused on reducing the cardiotoxicity of CP in the literature.
In the current study, we aimed to investigate the protective and antiapoptotic effects of TQ on CP-induced cardiotoxicity by evaluating immunohistochemical and histopathological results. To best of our knowledge, this is the first study to evaluate the antiapoptotic and protective effects of TQ against myocardial damage induced by CP in rats.
Methods
This study was approved by the Ethical Committee on Animal Research of Afyon Kocatepe University. The experimental procedures were performed at Experimental Animals Studies Laboratory of Afyon Kocatepe University (Approval protocol number: AKÜHADYEK-328-14).
A total of 28 male Wistar-Albino rats weighing between 250 and 270 grams were included in this study. The animals were housed in separate plastic cages at a constant 24-26oC temperature and a humidity range of 40% to 60% with 12-hours of diurnal rhythm cycle, which comply with the protocols approved by the Animal Research Ethics Committee of our institution and the NIH Guide for the Care and Use of Laboratory Animals, NIH publication no. 85-23, 1985. Rats were orally fed by gavage and have access to free tap water from day 1 until 12 h before the sacrifice.
Experimental procedure
Animals were equally and randomly divided into four groups as follows:
Group I (Controls): Rats did not receive any treatment until the end of the study.
Group II (CP): Received intraperitoneal single injection of CP at 15 mg/kg1313. Sagit M, Korkmaz F, Akcadag A, Somdas MA. Protective effect of thymoquinone against cisplatin-induced ototoxicity. Eur Arch Otorhinolaryngol. 2013 Aug;270(8):2231-7. PMID: 23161274. dose on the third day (Cisplatin-Hospira 100mg/100 ml; UK).
Group III (CP+TQ): Thymoquinone (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) has been intraperitoneally administered at 40mg/kg/day1313. Sagit M, Korkmaz F, Akcadag A, Somdas MA. Protective effect of thymoquinone against cisplatin-induced ototoxicity. Eur Arch Otorhinolaryngol. 2013 Aug;270(8):2231-7. PMID: 23161274. dose for five days beginning two days prior to single CP injection. CP was administered at 15 mg/kg i.p. dose only on the third day of the experiment.
Group IV (TQ): This group received i.p. TQ injection at 40mg/kg/day dose for five days.
Termination of the experiment
At the end of the study, rats were anesthetized with 50 mg/kg of ketamine hydrochloride (Ketalar; Eczacibasi Ilac Sanayi ve Ticaret A.S., Luleburgaz, Turkey) and 10 mg/kg of xylazine (Alfazyne; Alfasan International B.V., Woerden, the Netherlands) and immediately sacrificed by exsanguination. Hearts were removed, cleaned and washed in ice-cold phosphate buffered saline (pH 7.4). One piece of each heart tissue was taken from left ventricule and this sample was fixed in 10% formalin solution for histopathological analysis.
Histopathological examination
Heart samples were fixed with 10% neutral formalin, have undergone routine tissue processing steps and embedded in paraffin. Sections at five micrometers thickness were cut from paraffin blocks and stained with hematoxylin-eosin (H&E) dye.
Immunohistochemical analysis
Tissue samples were deparaffinized and degraded to water. Citrate buffer (pH=6.0) was used for antigen retrieval for 20 min in a microwave. Endogenous peroxidase activity was blocked by %3 hydrogen peroxidase in methanol for 10 min. Then primary antibody (Bcl-2, Biogenex, 1:50 dilution) was dropped and incubated overnight at 40C. After incubation, HRP secondary antibody kit (Anti-polyvalent HRP, Labvision Corp, Fremont, CA, USA) was used as a secondary antibody and AEC kit (Labvision Corp, Fremont, CA, USA) for the chromogen. Stained slides were counterstained with Mayer's hematoxylin (Thermo Scientific Inc. Waltham, USA) and mounted with water based mounting medium.
Image analysis
Sections were evaluated under light microscope (Eclipse E-600 Nikon, Japan) by using an image analysis software (NIS Elements, Nikon, Tokyo, Japan). Slides stained with hematoxyline-eosin were evaluated in terms of congestion, edema and pycnotic nuclei parameters in randomly chosen six different areas in each slide under x40 objective magnification. The slides were scored according to the method described by Demir et al.1414. Demir F, Güzel A, Kat C, Karadeniz C, Akdemir U, Okuyucu A, Gacar A, Özdemir S, Güvenç T. A combination of methylprednisolone and quercetin is effective for the treatment of cardiac contusion following blunt chest trauma in rats. Braz J Med Biol Res. 2014 Sep;47(9):766-72. PMID: 25098616. as follows: 0=no damage, 1=mild damage, 2=moderate damage and 3=severe damage. In immunohistochemical evaluation, Bcl-2 positivity was evaluated as per the amount and intensity of the cardiomyocyte staining in randomly chosen six different areas to under x200 objective magnification by two blinded histopathologists. Slides were semi-quantitatively scored according to the staining intensity of Bcl-2 as follows: 0=no staining, 1=mild staining, 2=moderate staining, 3=intense staining1515. Yang F, Wu WF, Yan YL, Pang Y, Kong Q, Huang YL. Expression of IL-23/Th17 pathway in a murine model of Coxsackie virus B3-induced viral myocarditis. Virol J. 2011 Jun 14;8:301. PMID: 21672246..
Statistical analysis
SOFA statistics for Windows v.1.4 (www.sofastatistics.com), a free and open source statistical analysis software was used for statistical analysis. Results of the descriptive statistics were expressed as mean ± standard deviation (SD) or median (range (minimum-maximum)) according to data's distribution type. Statistical comparisons of continuous variables among the groups were performed using Kruskal-Wallis tests based on their distribution type. Kruskal-Wallis test and post hoc analysis with Bonferroni-corrected Mann-Whitney U-test were used for analyzing difference between groups. P values less than 0.0125 were considered as statistically significant.
Results
The effects of thymoquinone on myocardial damage
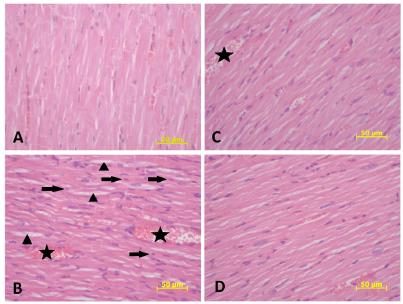
Routinely stained heart sections were given in Figure 1. In histopathological examination, myocardial fibers were observed normal morphology in control group (Figure 1A). There was an increase in congestion, edema, eosinophilic cytoplasm and pycnotic nuclei parameters in CP group than the controls (p=0.002, p=0.001, p=0.026 and p=0.001, respectively) (Figure 1B). There was a statistically significant difference between the CP and CP plus TQ groups with in these parameters except for eosinophilic cytoplasm (p=0.004, p=0.004, p=0.017 and p=0.001, respectively) and it was observed that additional TQ treatment led to an improvement in histopathological scores (Figure 1C). Only TQ given group exhibited less increment in mentioned parameters than the CP group (p=0.004, p=0.001, p=0.038 and p=0.001, respectively) (Figure 1D). There was no difference between the control and TQ groups here was no difference between the control and TQ groups here was no difference between the control and TQ groups. The related results were given in Table 1.
- A. Control group showing normal heart morphology. B. Cisplatin group (asterisks indicate congestion, arrows indicate pycnotic nuclei, arrowheads indicate edema). C. Cisplatin and Thymoquinon group showing lower congestion and pycnotic nuclei (asterisks indicate congestion). D. Thymoquinon group showing normal architecture. Hematoxylin-Eosin staining of the groups (x40, scale bar= 50 µm).
The effects of thymoquinone on expression of antiapoptotic protein Bcl-2
Sections stained with Bcl-2 primary antibody were represented in Figure 2. Diffuse and cytoplasmic stainings were assessed in myocardial tissue slides. In control group, some of the myocardial fibers have demonstrated moderate expressions of Bcl-2 (Figure 2A). In only CP administered group, expression of antiapoptotic protein Bcl-2 was lower than the controls (p=0.017). In CP and TQ given group, there was an increased expression of Bcl-2 in myocardial fibers than the CP group (p=0.026) (Figure 2C). Only TQ given group exhibited an increment in Bcl-2 expression (Figure 2D), when compared with the CP group (p=0.017). The related scores were given in Table 1.
- A. Control group showing moderate Bcl-2 expression. B. Cisplatin group showing mild Bcl-2 expression. C. Cisplatin and Thymoquinon group showing dense Bcl-2 expression. D. Thymoquinon group showing dense staining of Bcl-2 in myocardial fibers. Bcl-2 immunohistochemical staining of heart samples from the groups (x200, scale bar= 50 µm).
Discussion
Gradually increasing demand to develop new treatment modalities for cancer with minimal side effects has prompted an interest in exploiting the anticancer properties of dietary phytochemicals and other natural products. A perfect cardioprotective agent which is supposed to be used in combination with cisplatin chemotherapy should not compromise the antitumoral activity of chemotherapeutics. Similarly, a potential anticancer drug should also exhibit selective and specific cytotoxicity only on malignant cells.
Established results so far indicate that TQ has these properties. TQ has attracted noteworthy scientific attention in last years for its high biological activity and low systemic toxicity that can make it a promising alternative to conventional therapeutic drugs. CP has well-known detrimental effects on cardiovascular system. In some previous clinical reports regarding the acute CP treatment, cardiotoxic aspects of CP chemotherapy have been reported as follows: Angina1616. Khan S, Chen CL, Brady MS, Parameswaran R, Moore R, Hassoun H, Carvajal RD. Unstable angina associated with cisplatin and carboplatin in a patient with advanced melanoma. J Clin Oncol. 2012 Jun 20;30(18):e163-4. PMID: 22585705., acute myocardial infarction1717. Ryberg M. Recent advances in cardiotoxicity of anticancer therapies. Am Soc Clin Oncol Educ Book. 2012:555-9. PMID: 24451795., thromboembolic events1818. Moore RA, Adel N, Riedel E, Bhutani M, Feldman DR, Tabbara NE, Soff G, Parameswaran R, Hassoun H. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011 Sep 1;29(25):3466-73. PMID: 21810688., hypertension1919. Amit L, Ben-Aharon I, Tichler T, Inbar E, Sulkes A, Stemmer S. Cisplatin-induced posterior reversible encephalopathy syndrome-brief report and review of the literature. J Behav Brain Sci. 2012;2(1):97-101. doi:10.4236/jbbs.2012.21011.
https://doi.org/10.4236/jbbs.2012.21011...
, hypotension2020. Dolci A, Dominici R, Cardinale D, Sandri MT, Panteghini M. Biochemical markers for prediction of chemotherapy-induced cardiotoxicity: systematic review of the literature and recommendations for use. Am J Clin Pathol. 2008 Nov;130(5):688-95. PMID: 18854260., myocarditis, pericarditis and severe congestive cardiomyopathy2121. Ozcan T, Cirit A, Kiykim A. Recurrent complete atrioventricular block during cisplatin infusion: a case report. J Clin Exp Cardiol. 2011;2:151. doi: 10.4172/2155-9880.1000151.
https://doi.org/10.4172/2155-9880.100015...
. Cardiotoxicity sustains a significant issue because of a high correlation between the stage of heart injury and the dosage of CP utilized2222. Low-Friedrich I, von Bredow F, SchoeppeW. In vitro studies on the cardiotoxicity of chemotherapeutics. Chemotherapy. 1990;36:416-21. PMID: 2292204.. CP is one of the most related chemotherapeutic agents with atrial fibrillation and it can improve the production of thromboxanes by platelets, activate platelet aggregation and induce thrombotic arachidonic acid pathway2323. Santini D, Tonini G, Abbate A, Di Cosimo S, Gravante G, Vincenzi B, Campisi C, Patti G, Di Sciascio G. Gemcitabine-induced atrial fibrillation: a hitherto unreported manifestation of drug toxicity. Ann Oncol. 2000 Apr;11(4):479-81. PMID: 10847470.,2424. Czaykowski PM, Moore MJ, Tannock IF. High risk of vascular events in patients with urothelial transitional cell carcinoma treated with cisplatin based chemotherapy. J Urol. 1998 Dec;160(6 Pt 1):2021-4. PMID: 9817314.. Dolci et al.2020. Dolci A, Dominici R, Cardinale D, Sandri MT, Panteghini M. Biochemical markers for prediction of chemotherapy-induced cardiotoxicity: systematic review of the literature and recommendations for use. Am J Clin Pathol. 2008 Nov;130(5):688-95. PMID: 18854260. have classified the chemotherapy induced cardiotoxicity in two patterns: (1) Acute or subacute cardiotoxicity and (2) chronic toxicity. The first pattern can be encountered at any time beginning from the initiation of the chemotherapy to two weeks after the termination of the treatment. The second form may occur in two years following the termination of the chemotherapy.
Cardiotoxic effects can be placed in a wide spectrum from simple ECG changes to death2020. Dolci A, Dominici R, Cardinale D, Sandri MT, Panteghini M. Biochemical markers for prediction of chemotherapy-induced cardiotoxicity: systematic review of the literature and recommendations for use. Am J Clin Pathol. 2008 Nov;130(5):688-95. PMID: 18854260.. Increased amount of oxidative stress and apoptosis related to cardiotoxicity have led to a restricted clinical usage of CP as an anti-tumoral agent. CP-induced cardiotoxicity is related to excessive oxidative stress and apoptosis2525. El-Awady el-SE, Moustafa YM, Abo-Elmatty DM, Radwan A. Cisplatin-induced cardiotoxicity: Mechanisms and cardioprotective strategies. Eur J Pharmacol. 2011 Jan 10;650(1):335-41. PMID: 21034734.. Ma et al.66. Ma H, Jones KR, Guo R, Xu P, Shen Y, Ren J. Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: role of endoplasmic reticulum stress. Clin Exp Pharmacol Physiol. 2010 Apr;37(4):460-5. PMID: 19878217. have reported that depressed cardiomyocyte contraction and mitochondrial abnormalities, increased endoplasmic reticulum stress and associated apoptosis caused cardiac damage following CP treatment. Cardiomyocytes containing numerous mitochondria constitute an important site for CP accumulation and this condition eventually can lead to mitochondrial DNA damage2626. Qian W, Nishikawa M, Haque AM, Hirose M, Mashimo M, Sato E, Inoue M. Mitochondrial density determines the cellular sensitivity to cisplatin-induced cell death. Am J Physiol Cell Physiol. 2005 Dec;289(6):C1466-75. PMID: 16107504.. In some previous studies, it has been reported that a considerable incrementing caspase-3 activity, marked nuclear DNA fragmentation and reduced cardiomyocyte numbers were observed in the cross-sectional evaluation of the heart tissues following CP administration, which in turn may suggest apoptotic cell death2727. El-Sawalhi MM, Ahmed LA. Exploring the protective role of apocynin, a specific NADPH oxidase inhibitor, in cisplatin-induced cardiotoxicity in rats. Chem Biol Interact. 2014 Jan 25;207:58-66. doi: 10.1016/j.cbi.2013.11.008.
https://doi.org/10.1016/j.cbi.2013.11.00...
28. Connell BJ, Saleh MC, Khan BV, Saleh TM. Apocynin may limit total cell death following cerebral ischemia and reperfusion by enhancing apoptosis. Food Chem Toxicol. 2011 Dec;49(12):3063-9. PMID: 21946070.-2929. Sishi BJ, Loos B, van Rooyen J, Engelbrecht AM. Autophagy upregulation promotes survival and attenuates doxorubicin-induced cardiotoxicity. Biochem Pharmacol. 2013 Jan 1;85(1):124-34. PMID: 23107818.. Also, degradation of the mitochondrial transmembrane potential by cisplatin may give rise to a disruption in mitochondrial functionality and thereby trigger apoptosis.
TQ can potentiate the effect of conventional chemotherapeutic drugs. Its utilization in combined therapies may be at lower dosage, reduce the dosage of the concomitant drug optimizing efficacy and toxicity and it might also overcome the drug resistance problem3030. Jafri SH, Glass J, Shi R, Zhang S, Prince M, Kleiner-Hancock H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J Exp Clin Cancer Res. 2010 Jul 1;29:87. PMID: 20594324.,3131. Banerjee S1, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S, Sarkar FH, Mohammad RM. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009 Jul 1;69(13):5575-83. doi: 10.1158/0008-5472.
https://doi.org/10.1158/0008-5472...
. In previous studies, it has been reported that TQ led to a decrease in mean arterial blood pressure and heart rate in hypertensive rats in a dose dependent manner3131. Banerjee S1, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S, Sarkar FH, Mohammad RM. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009 Jul 1;69(13):5575-83. doi: 10.1158/0008-5472.
https://doi.org/10.1158/0008-5472...
. The robust free radical scavenger feature and antioxidant capacity of TQ may affect the cardiac status and may explain its potency as a cardioprotective agent. Myocardial protective effects of TQ have also been demonstrated in cardiac damage induced by isoproterenol, cyclophosphamide and doxorubicin3333. Randhawa MA, Alghamdi MS, Maulik SK. The effect of thymoquinone, an active component of Nigella sativa, on isoproterenol induced myocardial injury. Pak J Pharm Sci. 2013 Nov;26(6):1215-9. PMID: 24191329.
34. Nagi MN, Al-Shabanah OA, Hafez MM, Sayed-Ahmed MM. Thymoquinone supplementation attenuates cyclophosphamide-induced cardiotoxicity in rats. J Biochem Mol Toxicol. 2011 May-Jun;25(3):135-42. PMID: 20957680.-3535. Effenberger-Neidnicht K, Schobert R. Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin. Cancer Chemother Pharmacol.. 2011 Apr;67(4):867-74. PMID: 20582416..
In the current study, protective effect of TQ in myocardial tissue was showed by light microscopic examination. In CP administered group, there were prominent histopathological changes including congestion, edema, pycnotic nuclei and eosinophylic appearance in myocardial tissues. However, it has been shown that TQ exhibited counteraction against the mentioned changes associated with CP administration. In our study, normal myocardial morphology structure was observed in rats treated with TQ.
Bcl-2 is considered to be an important cellular component that not only guards against apoptotic cell death, but also impinges on multiple cellular events. It was reported that the prevention of apoptosis is associated with increased abundance of Bcl-2 and decreased levels of Bax3636. Shihab FS, Andoh TF, Tanner AM, Yi H, Bennett WM. Expression of apoptosis regulatory genes in chronic cyclosporine nephrotoxicity favors apoptosis. Kidney Int. 1999 Dec;56(6):2147-59. PMID: 10594790.. Various substrates like bax, bak and caspase trigger the apoptotic process; however bcl-2, bcl-xL or mcl-1 family proteins generally act as antiapoptotic mediators, which may cause delay or inhibition in apoptotic enzyme release. Moreover, overexpression of antiapoptotic proteins provide a survival benefit in cells treated with chemotherapeutic agents3737. Yildirim S, Kisa F, Karadeniz A, Yildirim A, Karakoc A, Can I, Kara A, Simsek N. Effects of pomegranate seed extract on liver paraoxonase and bcl-xL activities in rats treated with cisplatin. J Med Plants Res. 2012;6(12):2317-23. doi: 10.5897/JMPR011.788.
https://doi.org/10.5897/JMPR011.788...
. Wetzel and Berberich3737. Yildirim S, Kisa F, Karadeniz A, Yildirim A, Karakoc A, Can I, Kara A, Simsek N. Effects of pomegranate seed extract on liver paraoxonase and bcl-xL activities in rats treated with cisplatin. J Med Plants Res. 2012;6(12):2317-23. doi: 10.5897/JMPR011.788.
https://doi.org/10.5897/JMPR011.788...
have reported that cisplatin-induced apoptosis was believed to be the result of DNA damage in remaining survivor cells. CP binds to the nuclear and mitochondrial DNA of the neurons, which may trigger the activation of RhoA GTPase enzyme and induction of the inflammatory pathways3838. Wetzel CC, Berberich SJ. p53 binds to cisplatin-damaged DNA. Biochim Biophys Acta. 2001 Feb 16;1517(3):392-7. PMID: 11342217.,3939. Karavelioglu E, Boyaci MG, Simsek N, Sonmez MA, Koc R, Karademir M, Guven M, Eser O. Selenium protects cerebral cells by cisplatin induced neurotoxicity. Acta Cir Bras. 2015 Jun;30(6):394-400. PMID: 26108027.. Nuclear DNA damage leads to p53 activation and triggers apoptosis. Taken together, these results suggest that increase in bax activity may be correlated with CP-induced apoptosis.
In this study, we have observed that TQ treatment significantly increased the antiapoptotic Bcl-2 expression, which was mainly reduced by CP administration alone and we have also found that the amount of apoptotic cardiomyocytes were remarkably higher in the CP group. TQ treatment reduced the reactivity and the number of apoptotic cardiomyocytes. Given these results, it can be suggested that TQ has antiapoptotic and protective properties. To best of our knowledge, this would be the first study to evaluate the antiapoptotic and protective effects of TQ against myocardial damage induced by CP in rats. We have observed that single dose of CP (15 mg/kg) led to severe heart injury in rats and that TQ established a prominent protective effect against this insult. CP-based treatment is very effective in different cancer types involving pulmonary, ovarian, cranial, cervical and gastric tissues cancers and its clinical use is gradually increasing. In the current study, we observed CP can be cardiotoxic and it could also trigger apoptosis in myocytes especially at higher doses. Cardiotoxic aspect of CP should be kept in mind when CP treatment is planned for patients with oncological conditions. More comprehensive studies are required in which different and multiple doses of CP and TQ would be administered in various time lapses in order to get more insight into the underlying mechanisms by which TQ exerts its protective effect against CP-induced myocardial damage.
Conclusion
Thymoquinone attenuated cardiomyocyte necrosis and apoptosis induced by cisplatin treatment.
References
-
1Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008 Mar;9(3):215-21. PMID: 18282805.
-
2Tiseo M, Martelli O, Mancuso A, Sormani MP, Bruzzi P, Di Salvia R, De Marinis F, Ardizzoni A. Short hydration regimen and nephrotoxicity of intermediate to high-dose cisplatin-based chemotherapy for outpatient treatment in lung cancer and mesothelioma. Tumori. 2007 Mar-Apr;93(2):138-44. PMID: 17557559.
-
3Santabarbara G, Maione P, Rossi AE, Gridelli C. Pharmacotherapeutic options for treating adverse effects of Cisplatin chemotherapy. Expert Opin Pharmacother. 2016 Mar;17(4):561-70. doi: 10.1517/14656566.2016.1122757.
» https://doi.org/10.1517/14656566.2016.1122757 -
4Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000 Apr;22(4):263-302. PMID: 10789823.
-
5Yousef MI, Saad AA, El-Shennawy LK. Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem Toxicol. 2009 Jun;47(6):1176-83. PMID: 19425235.
-
6Ma H, Jones KR, Guo R, Xu P, Shen Y, Ren J. Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: role of endoplasmic reticulum stress. Clin Exp Pharmacol Physiol. 2010 Apr;37(4):460-5. PMID: 19878217.
-
7Woo CC, Kumar AP, Sethi G, Tan KH. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol. 2012 Feb 15;83(4):443-51. PMID: 22005518.
-
8Badary OA, Nagi MN, al-Shabanah OA, al-Sawaf HA, al-Sohaibani MO, al-Bekairi AM. Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can J Physiol Pharmacol. 1997 Dec;75(12):1356-61. PMID: 9534946.
-
9Nagi MN, Mansour MA. Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol Res. 2000 Mar;41(3):283-9. PMID: 10675279.
-
10Ali BH, Al-Salam S, Al Husseini IS, Al-Lawati I, Waly M, Yasin J, Fahim M, Nemmar A. Abrogation of cisplatin-induced nephrotoxicity by emodin in rats. Fundam Clin Pharmacol. 2013 Apr;27(2):192-200. PMID: 22044459.
-
11Al-Kharusi N, Babiker HA, Al-Salam S, Waly MI, Nemmar A, Al-Lawati I, Yasin J, Beegam S, Ali BH. Ellagic acid protects against cisplatin-induced nephrotoxicity in rats: a dose-dependent study. Eur Rev Med Pharmacol Sci. 2013 Feb;17(3):299-310. PMID: 23426532.
-
12Ali BH, Al Za'abi M, Shalaby A, Manoj P, Waly MI, Yasin J, Fahim M, Nemmar A. The effect of thymoquinone treatment on the combined renal and pulmonary toxicity of cisplatin and diesel exhaust particles. Exp Biol Med (Maywood). 2015 Dec;240(12):1698-707. PMID: 25925792.
-
13Sagit M, Korkmaz F, Akcadag A, Somdas MA. Protective effect of thymoquinone against cisplatin-induced ototoxicity. Eur Arch Otorhinolaryngol. 2013 Aug;270(8):2231-7. PMID: 23161274.
-
14Demir F, Güzel A, Kat C, Karadeniz C, Akdemir U, Okuyucu A, Gacar A, Özdemir S, Güvenç T. A combination of methylprednisolone and quercetin is effective for the treatment of cardiac contusion following blunt chest trauma in rats. Braz J Med Biol Res. 2014 Sep;47(9):766-72. PMID: 25098616.
-
15Yang F, Wu WF, Yan YL, Pang Y, Kong Q, Huang YL. Expression of IL-23/Th17 pathway in a murine model of Coxsackie virus B3-induced viral myocarditis. Virol J. 2011 Jun 14;8:301. PMID: 21672246.
-
16Khan S, Chen CL, Brady MS, Parameswaran R, Moore R, Hassoun H, Carvajal RD. Unstable angina associated with cisplatin and carboplatin in a patient with advanced melanoma. J Clin Oncol. 2012 Jun 20;30(18):e163-4. PMID: 22585705.
-
17Ryberg M. Recent advances in cardiotoxicity of anticancer therapies. Am Soc Clin Oncol Educ Book. 2012:555-9. PMID: 24451795.
-
18Moore RA, Adel N, Riedel E, Bhutani M, Feldman DR, Tabbara NE, Soff G, Parameswaran R, Hassoun H. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011 Sep 1;29(25):3466-73. PMID: 21810688.
-
19Amit L, Ben-Aharon I, Tichler T, Inbar E, Sulkes A, Stemmer S. Cisplatin-induced posterior reversible encephalopathy syndrome-brief report and review of the literature. J Behav Brain Sci. 2012;2(1):97-101. doi:10.4236/jbbs.2012.21011.
» https://doi.org/10.4236/jbbs.2012.21011 -
20Dolci A, Dominici R, Cardinale D, Sandri MT, Panteghini M. Biochemical markers for prediction of chemotherapy-induced cardiotoxicity: systematic review of the literature and recommendations for use. Am J Clin Pathol. 2008 Nov;130(5):688-95. PMID: 18854260.
-
21Ozcan T, Cirit A, Kiykim A. Recurrent complete atrioventricular block during cisplatin infusion: a case report. J Clin Exp Cardiol. 2011;2:151. doi: 10.4172/2155-9880.1000151.
» https://doi.org/10.4172/2155-9880.1000151 -
22Low-Friedrich I, von Bredow F, SchoeppeW. In vitro studies on the cardiotoxicity of chemotherapeutics. Chemotherapy. 1990;36:416-21. PMID: 2292204.
-
23Santini D, Tonini G, Abbate A, Di Cosimo S, Gravante G, Vincenzi B, Campisi C, Patti G, Di Sciascio G. Gemcitabine-induced atrial fibrillation: a hitherto unreported manifestation of drug toxicity. Ann Oncol. 2000 Apr;11(4):479-81. PMID: 10847470.
-
24Czaykowski PM, Moore MJ, Tannock IF. High risk of vascular events in patients with urothelial transitional cell carcinoma treated with cisplatin based chemotherapy. J Urol. 1998 Dec;160(6 Pt 1):2021-4. PMID: 9817314.
-
25El-Awady el-SE, Moustafa YM, Abo-Elmatty DM, Radwan A. Cisplatin-induced cardiotoxicity: Mechanisms and cardioprotective strategies. Eur J Pharmacol. 2011 Jan 10;650(1):335-41. PMID: 21034734.
-
26Qian W, Nishikawa M, Haque AM, Hirose M, Mashimo M, Sato E, Inoue M. Mitochondrial density determines the cellular sensitivity to cisplatin-induced cell death. Am J Physiol Cell Physiol. 2005 Dec;289(6):C1466-75. PMID: 16107504.
-
27El-Sawalhi MM, Ahmed LA. Exploring the protective role of apocynin, a specific NADPH oxidase inhibitor, in cisplatin-induced cardiotoxicity in rats. Chem Biol Interact. 2014 Jan 25;207:58-66. doi: 10.1016/j.cbi.2013.11.008.
» https://doi.org/10.1016/j.cbi.2013.11.008 -
28Connell BJ, Saleh MC, Khan BV, Saleh TM. Apocynin may limit total cell death following cerebral ischemia and reperfusion by enhancing apoptosis. Food Chem Toxicol. 2011 Dec;49(12):3063-9. PMID: 21946070.
-
29Sishi BJ, Loos B, van Rooyen J, Engelbrecht AM. Autophagy upregulation promotes survival and attenuates doxorubicin-induced cardiotoxicity. Biochem Pharmacol. 2013 Jan 1;85(1):124-34. PMID: 23107818.
-
30Jafri SH, Glass J, Shi R, Zhang S, Prince M, Kleiner-Hancock H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J Exp Clin Cancer Res. 2010 Jul 1;29:87. PMID: 20594324.
-
31Banerjee S1, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S, Sarkar FH, Mohammad RM. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009 Jul 1;69(13):5575-83. doi: 10.1158/0008-5472.
» https://doi.org/10.1158/0008-5472 -
32el Tahir KE, Ashour MM, al-Harbi MM. The cardiovascular actions of the volatile oil of the black seed (Nigella sativa) in rats: elucidation of the mechanism of action. Gen Pharmacol. 1993 Sep;24(5):1123-31. PMID: 8270171.
-
33Randhawa MA, Alghamdi MS, Maulik SK. The effect of thymoquinone, an active component of Nigella sativa, on isoproterenol induced myocardial injury. Pak J Pharm Sci. 2013 Nov;26(6):1215-9. PMID: 24191329.
-
34Nagi MN, Al-Shabanah OA, Hafez MM, Sayed-Ahmed MM. Thymoquinone supplementation attenuates cyclophosphamide-induced cardiotoxicity in rats. J Biochem Mol Toxicol. 2011 May-Jun;25(3):135-42. PMID: 20957680.
-
35Effenberger-Neidnicht K, Schobert R. Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin. Cancer Chemother Pharmacol.. 2011 Apr;67(4):867-74. PMID: 20582416.
-
36Shihab FS, Andoh TF, Tanner AM, Yi H, Bennett WM. Expression of apoptosis regulatory genes in chronic cyclosporine nephrotoxicity favors apoptosis. Kidney Int. 1999 Dec;56(6):2147-59. PMID: 10594790.
-
37Yildirim S, Kisa F, Karadeniz A, Yildirim A, Karakoc A, Can I, Kara A, Simsek N. Effects of pomegranate seed extract on liver paraoxonase and bcl-xL activities in rats treated with cisplatin. J Med Plants Res. 2012;6(12):2317-23. doi: 10.5897/JMPR011.788.
» https://doi.org/10.5897/JMPR011.788 -
38Wetzel CC, Berberich SJ. p53 binds to cisplatin-damaged DNA. Biochim Biophys Acta. 2001 Feb 16;1517(3):392-7. PMID: 11342217.
-
39Karavelioglu E, Boyaci MG, Simsek N, Sonmez MA, Koc R, Karademir M, Guven M, Eser O. Selenium protects cerebral cells by cisplatin induced neurotoxicity. Acta Cir Bras. 2015 Jun;30(6):394-400. PMID: 26108027.
-
Financial source: none
-
1Research performed at Department of Cardiovascular Surgery, Faculty of Medicine, Afyon Kocatepe University, Afyon, Turkey.
Publication Dates
-
Publication in this collection
Apr 2016
History
-
Received
18 Dec 2015 -
Reviewed
20 Feb 2016 -
Accepted
22 Mar 2016