Abstract
PURPOSE:
To evaluate the effect of ketamine S (+) 5% with no preservatives and administered as a subarachnoid single puncture on the spinal cord and meninges of rabbits.
METHODS:
Twenty young adult female rabbits, each weighing 3500-5000 g and having a spine length between 34 and 38 cm, were divided by lot into two groups (G): 0.9% saline in G1 and ketamine S (+) 5% in G2, by volume of 5 μg per cm column (0.18 mL). After intravenous anaesthesia with ketamine and xylazine, the subarachnoid space was punctured at S1-S2 under ultrasound guidance, and a random solution was injected. The animals remained in captivity for 21 days under medical observation and were sacrificed by decapitation. The lumbosacral spinal cord portion was removed for immunohistochemistry to assess the glial fibrillary acidic protein (GFAP), and histology was assessed using hematoxylin and eosin (HE) stain.
RESULTS:
No histological lesions were found in the nervous tissue (roots and cord) or meninges in either group.
CONCLUSION:
The ketamine S (+) 5% unpreserved triggered no neurological or histological lesions in the spinal cord or meninges of rabbits.
Anesthesia, Spinal; Ketamine; Injections, Spinal; Spinal Cord; Rabbits
Introduction
The initial sequence of events preceding any painful phenomenon is the transformation of aggressive stimuli into action potentials by nociceptors, which transfer the stimuli from the peripheral nerve fibres to the central nervous system11. Besson P, Perl ER. Responses of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969 Nov;32(6):1025-43. PMID: 5347705..
Specific pain receptors, known as nociceptors, are located in the nerve endings of the delta and C fibres, the latter of which are responsible for 70% to 80% of sensory afferent signal transduction. Nociceptors are found in superficial skin structures and the walls of the viscera and blood vessels in the musculoskeletal system; at different locations, they are capable of transmitting pain stimuli at different speeds. In addition, the nerve endings of nociceptive A delta and C fibres are able to respond to inflammatory mediators and to mechanical, thermal and chemical stimuli. Thus, such assaults on the transmission of translated electrical stimuli to the central nervous system are interpreted in the cerebral cortex as pain22. Piotrowski W, Foremam JC. Some effects of calcitonin gene-related peptide in human skin and on histamine release. Br J Dermatol. 1986 Jan;114(1):37-46. PMID: 2417614..
The sensitisation of nociceptors occurs by decreasing their excitability threshold33. O'Banion MK. Cyclooxygenase-2: molecular biology, pharmacology and neurobiology. Crit Rev Neurobiol. 1999;13(1):45-82. PMID: 10223523. due to the release of known antigenic chemicals that are present in the tissue environment44. Webster KE. Somaesthetic pathways. Br Med Bull. 1977 May;33(2):113-20. PMID: 324557., including bradykinin, acetylcholine, prostaglandins, histamine, serotonin, substance P, leukotrienes, platelet activating factor, acid radicals, potassium ions, thromboxanes, interleukins and tumour necrosis factor22. Piotrowski W, Foremam JC. Some effects of calcitonin gene-related peptide in human skin and on histamine release. Br J Dermatol. 1986 Jan;114(1):37-46. PMID: 2417614. among others. Nociceptors can also be sensitised by noradrenergic influences from the sympathetic efferent nerves and by the retrograde release of neurotransmitters from nerve fibres11. Besson P, Perl ER. Responses of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969 Nov;32(6):1025-43. PMID: 5347705..
The release of these neurotransmitters in the spinal cord generates excitatory postsynaptic potentials, which can be slow (conducted by C fibres ) or fast ( conducted by A delta fibres ), and activates specific receptors, including those involved in the mechanisms of action of such amino acids as N-methyl-D-aspartate (NMDA) receptors. These receptors are activated by glutamate and substance P, which in turn are modulated by promoting the increase in intracellular calcium and magnesium ion removal from inside the receptors. This action results in the amplification and prolongation of the response to painful stimuli55. Woolf CJ. Somatic pain - pathogenesis and prevention. Br J Anaesth. 1995 Aug;75(2):169-76. PMID: 7577250. , 66. Dickenson AH. Recent advances in the physiology and pharmacology of pain: plasticity and its implications for clinical analgesia. J Psychopharmacol. 1991 Jan;5(4):342-51.doi: 10.1177/026988119100500424.
https://doi.org/10.1177/0269881191005004...
.
The activation of NMDA receptors is the main mechanism of the sensitisation of the dorsal horn of the spinal cord, a process characterised by spontaneous activity. If the threshold is decreased or increased in its response to afferent impulses, prolonged discharges after repeated stimulation and expansions of receptive fields of spinal cord neurons can occur.
Ketamine, an anaesthetic agent that has been used for approximately forty years, is a derivative of phencyclidine and produces amnesia, dissociative anaesthesia and analgesia. It has been used in anaesthesia since 1970. Initially, it was synthesised as a racemic mixture. In the 1990s, ketamine S ( + ) was made to have four times the stereo selectivity of NMDA receptors; thus, it has greater analgesic and anaesthetic potency and fewer side effects, allowing its use as an adjuvant in anaesthesia77. Kohrs R, Durieux ME. Ketamine: teaching an old drug new trick. Anesth Analg. 1998 Nov;87(5):1186-93. PMID: 9806706. , 88. Dahl V, Raeder JC. Non-opioid postoperative analgesia. Acta Anaesthesiol Scand. 2000 Nov;44(10):1191-203. PMID: 11065198..
The mechanisms of action of ketamine include a competitive blockade of NMDA receptors, a non-competitive blockade of the glutamate NMDA receptors and interactions with opioid receptors, both spinal and supra segmental. Its methods of administration include intradermal, subcutaneous, intramuscular, intravenous, rectal, intranasal, oral, epidural and spinal77. Kohrs R, Durieux ME. Ketamine: teaching an old drug new trick. Anesth Analg. 1998 Nov;87(5):1186-93. PMID: 9806706. , 88. Dahl V, Raeder JC. Non-opioid postoperative analgesia. Acta Anaesthesiol Scand. 2000 Nov;44(10):1191-203. PMID: 11065198..
In the subarachnoid route, ketamine is used to treat cancer and neuropathic pain99. Walker SM, Goudas LC, Cousins MJ, Car DB. Combination spinal analgesic chemotherapy: a systemic review. Anesth Analg. 2002 Sep;95(3):674-715. PMID: 12198058.
10. Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003 Dec;97(6):1730-9. PMID: 14633551. - 1111. Benrath J, Scharbert G, Gustorff B, Adams HA, Kress HG. Long-term intrathecal S (+)-ketamine in a patient with cancer-related neuropathic pain. Br J Anaesth. 2005 Aug;95(2):247-9. PMID: 15951328.. Despite the present considerable clinical experience with ketamine, controversy lingers regarding the safety of ketamine administered in the subarachnoid route1212. Klimscha W, Horvath G, Szikszay M, Dobos I, Benedek G. Antinociceptive effect of the S (+) enantiomer of ketamine on carrageenan hyperalgesia after intrathecal administration in rats. Anesth Analg. 1998 Mar;86(3):561-5.doi: 10.1213/00000539-199803000-00023.
https://doi.org/10.1213/00000539-1998030...
. This is due to the potential toxicity of the agent's inclusion of preservatives (such as benzethonium chloride and chlorobutanol1313. Karpinski N, Dunn J, Hansen L, Masliah E. Subpial vacuolar myelopathy after intrathecal ketamine: report of a case. Pain. 1997 Oct;73(1):103-5. PMID: 9414063.
14. Brock-Utne JG, Mankowitz E, Lallichurum S. Effects of intrathecal saline and ketamine with and without preservative on the spinal nerve roots of monkeys. S Afr Med J. Mar 6;61(10):360-1. PMID: 6895951. - 1515. Malinovsky JM, Cozian A, Lepage JY, Mussini J, Pinaudt M, Souron R. Ketamine and midazolam neurotoxicity in the rabbit. Anesthesiology. 1991 Jul(1);75:91-7. PMID: 2064066.).
The aim of this study was to evaluate the effect of ketamine S (+) 5%, without preservatives and administered by an intrathecal route in a single puncture, on the spinal cord and meninges of rabbits.
Methods
After approval from the Ethics Committee on Animal Experimentation of the Faculty of Medicine of Botucatu (Protocol 801), 20 young adult female rabbits from the breed Genetic Group Botucatu, each of which weighed between 3,500 grams and 5000 grams and had a spinal column length between 34 and 38 cm, were supplied by the animal colony of the Campus of Botucatu.
During the selection process, animals were excluded if they lacked a healthy appearance or required more than one spinal puncture. The animals were divided into two experimental groups, with 10 animals in each group. Each was subjected initially to intravenous anaesthesia with ketamine and xylazine and then to the spinal puncture. The groups differed by the type of solution administered, with group 1 (G1) receiving the volume control of saline, 0.9%, and group 2 (G2), the drug control of unpreserved ketamine S 5%.
In rabbits, the approach to the subarachnoid space between the vertebrae S1 and S2 is ample. To identify this space and to determine the site of the local spinal puncture, the iliac crests should first be palpated. Then, identifying the spinous process of the first sacral vertebra, a finger should be glided 1.5 cm to 2 cm in the caudal direction. The space of S1 - S2 is located 1 cm caudal to the spinous process of the first sacral vertebra.
The spinal block was guided by ultrasound imaging with a SonoSite brand apparatus (USA), model M -Turbo, which has the feature of wall tissue Doppler (TDI). A micro-linear transducer was used at a frequency of six to 13 MHz. Dural punctures were performed with a Quincke needle of 22G 11/2 using a medial approach and a tilt angle of approximately 45°. The needle was slowly introduced in the cephalic direction under ultrasound guidance even penetrating the subarachnoid space. After entering the subarachnoid space, the animals in G1 and G2 received their respective solutions. Any difficulties encountered in performing the punctures were recorded.
The animals were given 5 μl per centimetre of their spinal cord (0.2 ml), which was injected in 1 second using a 1 ml disposable syringe. The doses of 0.9% saline (Group 1) and 10 mg of S 5% ketamine without preservatives (G2) were synthesised by Cristália solutions.
After the injection of either solution into the spinal puncture site, the animals were removed from the operating table. When they had recovered from intravenous anaesthesia, they were assessed clinically as to their motor blockade and soreness. Their motor blocks were assessed by clinical observation, according to the criteria established by Drummond and Moore1616. Drummond JC, Moore SS. The influence of dextrose administration on neurological outcome after temporary spinal cord ischemia in the rabbit. Anesthesiology. 1989 Jan;70(1):64-70. PMID: 2912317. on the following scale: 0-free movement of the lower extremities; 1 - asymmetry and limitations sustaining the body, including potential changes in the lower extremities; 2 - inability to sustain the body in the lower extremities; and 3 - paralysis of the lower extremities. Next, the pain sensitivity was evaluated by gripping the lower and upper extremities, the ears and the skin in the regions of the sacral, lumbar, and thoracic dermatomes with the aid of rat tooth forceps.
The animals remained in captivity for 21 days under clinical observation. Motor changes were verified using the criteria of Drummond and Moore1616. Drummond JC, Moore SS. The influence of dextrose administration on neurological outcome after temporary spinal cord ischemia in the rabbit. Anesthesiology. 1989 Jan;70(1):64-70. PMID: 2912317.. Their soreness was assessed by observing the following signs, indicative of pain: paw retraction, changes in posture and grunting after the application of painful stimuli, as described above.
The animals were euthanised by decapitation after previous intravenous anaesthesia with sodium pentobarbital. The lumbar and sacral portions of the spinal cord, along with the roots and meninges, were removed in less than 3 minutes to minimise the risk of tissue damage triggered by ischemia and apoptosis. The medullary tissue, roots and meninges were fixed in 10% formalin solution for histological and immunohistochemical examination.
The specimens remained in the formalin solution for seven days. Transverse sections of the nervous tissue and meninges were started approximately ten inches above the point where the spinal puncture was performed and were continued at intervals of half a centimetre. The tissue slices were placed in paraffin blocks and stained with hematoxylin-eosin (HE) and glial fibrillary acidic protein (GFAP).
The GFAP-stained samples embedded in paraffin were sectioned at a thickness of 3 microns on a rotary microtome for immunohistochemistry and extended onto histological glass slides previously treated with organosilane. After 18 hours in an oven at 58°C, the slides were deparaffinised with xylene, three wells at a time, after spending 5 min in each tank. Thus, the slides were placed in four tanks with absolute ethanol for 5 minutes each before their hydration in running distilled water.
Results
The statistical analysis of the figures for the weight of the animals in group 1 was lower than in group 2 (p = 0.02). There was homogeneity between the groups with respect to the length of the spine (p = 1.0) and the volume of solution administered (p = 0.67) (Table 1).
Weight (kg), column length (cm) and administered volume (ml) in the animals belonging to the two groups. The results are expressed as the means and standard deviations.
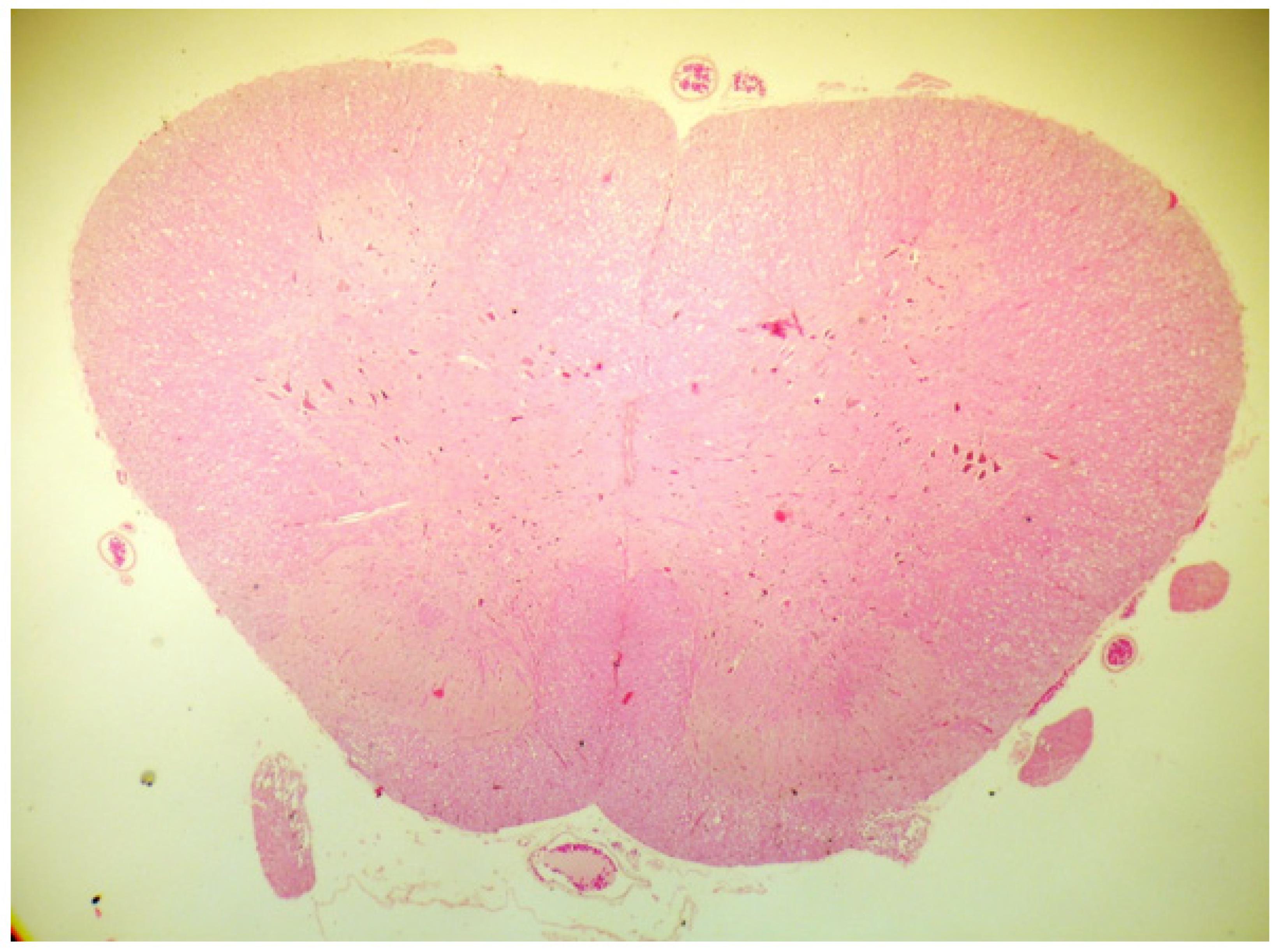
No animals were excluded from the experiment due to difficulties performing the puncture or death during the period of captivity. The recovery time from the intravenous anaesthesia was approximately 30 minutes, after which all of the animals demonstrated preserved motor function and pain sensitivity. The animals remained clinically normal during their captivity. No animals in either group showed histological changes in the nerve tissues within the meninges or vessels (Figures 1 to 5) based on optical microscopy.
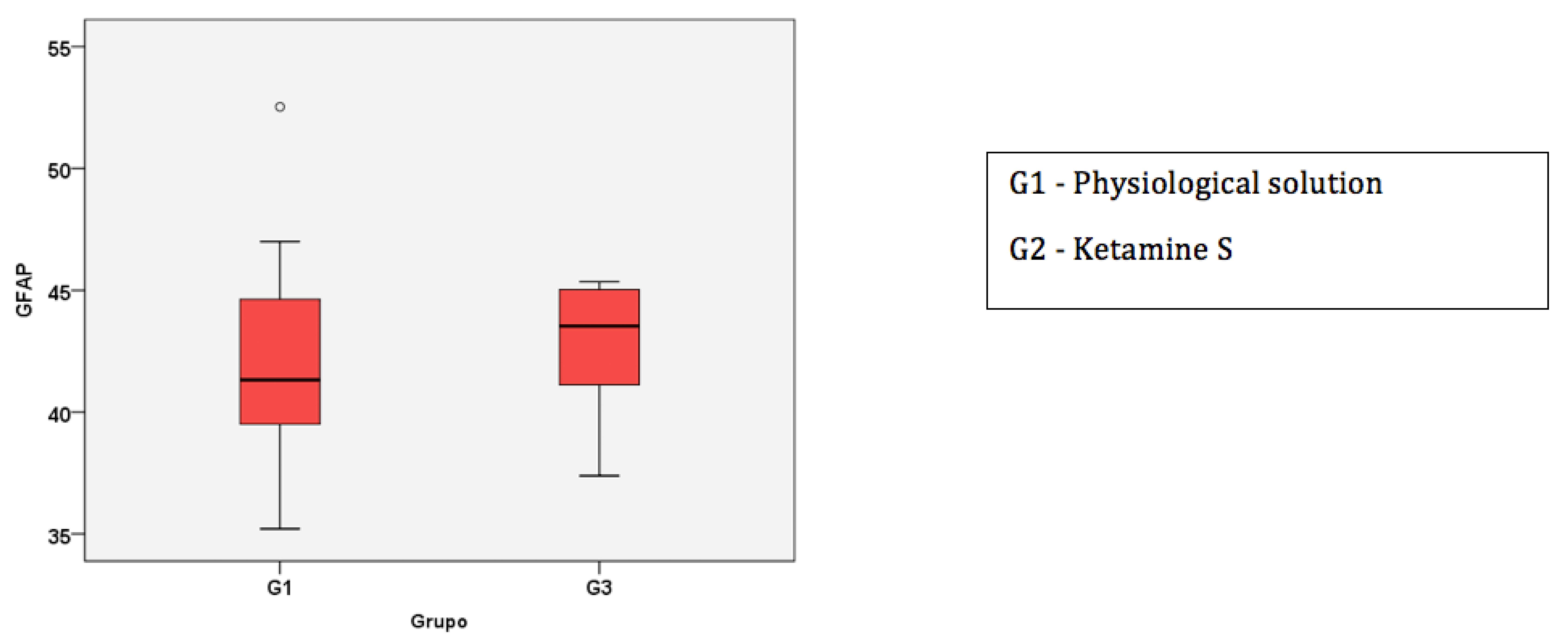
Box-Plot of the median and 1st and 3rd quartiles of the percentage of cells in the nervous tissue marked by GFAP.
The groups were similar regarding the percentage of labelled cells in the posterior horn marked by GFAP (p = 0.48). The median percentage of cells in the control group was 42%, with quartiles at 39% and 45%; in the ketamine group, the median was 43%, with quartiles at 41% and 45% (Figure 1).
Discussion
In the study of neurotoxicity in living animals, the identification of the optimal positioning of the needle in the subarachnoid space is fundamental to the reliability of the results obtained. However, this fact excludes the possibility that trauma to the nervous tissue, blood vessel damage or intraneural injections of the solution may be responsible for assaulting the nervous tissue.
The use of ultrasound in regional anaesthesia has facilitated the implementation of anaesthetic techniques, which allow for better visibility of the structures to be addressed (nerves) and of the adjacent blood vessels, lungs and pleura. The adoption of this practice has supported proper needle positioning to avoid nerve injuries and intravascular injections of local anaesthetics due to the capacity to observe the dispersion area of the injected solution1717. Ting PH, Antonakakis JG. Evidence-based review of ultrasound imaging for regional anesthesia. Semin Anesth. 2007 Mar;26(3):218-28..
Thus, using ultrasound to guide the puncture sites in this study, we were able to visualise the subarachnoid space and dispersion of the solution administered, thereby confirming that the puncturing technique did not cause any possible changes in the tissues of the nerve cord and meninges.
Glial activation has been widely studied due to its role in the injury and repair of the central nervous system (CNS). It has long been recognised that microglial responses to most forms of CNS damage can be considered an early indicator of latent disease. Microglial activation involves a stereotypical pattern of cellular responses, including proliferation, recruitment at the site of injury and increased expression of immune molecules1818. Moore S, Thanos S. the concept of microglia in relation to central nervous system disease and regeneration. Prog Neurobiol. 1996 Mar-Apr;48(4-5):441-60. PMID: 8804116.. Astrocytes are among the first cells that respond to CNS injury. The hypertrophy and, to a lesser extent, proliferation of astrocytes are the first observed responses. Astrocytes increase in size because they contain large quantities of cytoplasmic organelles and, in particular, large amounts of GFAP1919. O'Callaghan JP, Miller D. Cerebellar hypoplasia in the Gunn rat is associated with quantitative changes in neurotypic and gliotypic proteins. J Pharmacol Exp Ther. 1985 Aug;234(2);522-33. PMID: 2410596.. There is evidence that microglia can directly contribute to or exacerbate neuronal degeneration2020. Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993 Jan;7(1):111-8. PMID: 8423058. , 2121. Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996 Aug;19(8):331-8. PMID: 8843602.. Therefore, the measurement of GFAP was used as an early marker of nerve injury2222. DeLeo JA, Colburn RW, Rickman AJ, Yeager MP. Intrathecal catheterization alone induces neuroimmune activation in the rat. Eur J Pain. 1997;1(2):115-22. PMID: 15102412..
It has been described that repeated doses of ketamine (S) can lead to excessive NMDA receptor antagonism. This antagonism can produce neurotoxicity due to the resultant inactivation of inhibitory mechanisms (involving blockade of the NMDA receptors in the interneuronal space, as mediated by y-amino butyric acid, which is in turn responsible for the inhibition of the tonic excitatory pathways) and the cellular necrosis and apoptosis that arises after such excitotoxic lesions2323. Farber NB, Kim SH, Dikranian K, Jiang XP, Heinkel C. Receptor mechanisms and circuitry underlying NMDA antagonist neurotoxicity. Mol Psychiatry. 2002;7(1):32-43. PMID: 11803444.. In addition, prolonged NMDA receptor antagonism prevents the activation of the endogenous mechanisms for neuronal survival and regeneration2424. Hoyte L, Barber PA, Buchan AM, Hill MD. The rise and fall of NMDA antagonists for ischemic stroke. Curr Mol Med. 2004 Mar;4(2):131-6. PMID: 15032709..
In dogs receiving a dose of 1 mg.kg-1 of 0.6 % to 1.5 % ketamine S ( + ) with no preservatives and through a subarachnoid route, no histological changes were found by optical microscopy in the spinal nerve tissue and meninges after 21 days2525. Rojas AC, Alves JG, Lima, RM, Marques MEA, Barros GAM, Fukushima FB, Ganem EM. The effects of subarachnoid administration of preservative-free S(+)-ketamine on spinal cord and meninges in dogs. Anesth Analg. 2012 Feb;114(2):450-5.doi: 10.1213/ANE.0b013e31823a5d1b.
https://doi.org/10.1213/ANE.0b013e31823a...
. However, other authors with similar methodologies and model animals have obtained different results2626. Gomes LMRS, Garcia JBSG, Ribamar JS, Nascimento AGP. Neurotoxicity of subaracnoid preservative-free S (+) Ketamine in dogs. Pain Physician. 2011 Jan-Fev;14(1):83-90. PMID: 21267045.. After the subarachnoid injection of lower doses of preservative-free ketamine S ( + ) ( 0.5 mg.kg-1 and 0.7 mg.kg-1), another study reported gliosis, axonal edema, central chromatolysis, lymphocytic infiltration, axonal degeneration, vasculitis, and thickening of the dura mater. Traumatic punctures were also observed in five dogs, although the authors did not specify to which group the animals belonged or what percentage of the histological field was compromised by damage to the nerve tissue and meninges.
In recent years, related experimental research has been published on rat models. These studies found ischemia of the spinal cord after the subarachnoid injection of ketamine S ( + ); this result suggests neuroprotective benefits for the treatment. It is possible that these effects could be further strengthened by combining these injections with corticosteroids in similar situations to those described above2727. Kose EA, Bakar B, Ayva SK, Kilinc K, Apan A. , Neuroprotective effects of racemic ketamine and (S)-ketamine on spinal cord injury in rat. Injury. 2012 Jul;43(7):1124-30.doi: 10.1016/j.injury.2012.02.022.
https://doi.org/10.1016/j.injury.2012.02...
.
Although the results of this study suggest that administration of a single dose of ketamine S (+) without preservatives in the subarachnoid space of rabbits does not trigger lesions in the spinal nerve tissue and meninges, there is no consensus on this topic. Therefore, further studies are needed to elucidate this issue.
Conclusion
This rabbit experimental model of ketamine S (+) 5%, with no preservatives and administered in the subarachnoid space in a single dose, did not induce histological changes within the nervous tissue or the meninges.
References
-
1Besson P, Perl ER. Responses of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969 Nov;32(6):1025-43. PMID: 5347705.
-
2Piotrowski W, Foremam JC. Some effects of calcitonin gene-related peptide in human skin and on histamine release. Br J Dermatol. 1986 Jan;114(1):37-46. PMID: 2417614.
-
3O'Banion MK. Cyclooxygenase-2: molecular biology, pharmacology and neurobiology. Crit Rev Neurobiol. 1999;13(1):45-82. PMID: 10223523.
-
4Webster KE. Somaesthetic pathways. Br Med Bull. 1977 May;33(2):113-20. PMID: 324557.
-
5Woolf CJ. Somatic pain - pathogenesis and prevention. Br J Anaesth. 1995 Aug;75(2):169-76. PMID: 7577250.
-
6Dickenson AH. Recent advances in the physiology and pharmacology of pain: plasticity and its implications for clinical analgesia. J Psychopharmacol. 1991 Jan;5(4):342-51.doi: 10.1177/026988119100500424.
» https://doi.org/10.1177/026988119100500424 -
7Kohrs R, Durieux ME. Ketamine: teaching an old drug new trick. Anesth Analg. 1998 Nov;87(5):1186-93. PMID: 9806706.
-
8Dahl V, Raeder JC. Non-opioid postoperative analgesia. Acta Anaesthesiol Scand. 2000 Nov;44(10):1191-203. PMID: 11065198.
-
9Walker SM, Goudas LC, Cousins MJ, Car DB. Combination spinal analgesic chemotherapy: a systemic review. Anesth Analg. 2002 Sep;95(3):674-715. PMID: 12198058.
-
10Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003 Dec;97(6):1730-9. PMID: 14633551.
-
11Benrath J, Scharbert G, Gustorff B, Adams HA, Kress HG. Long-term intrathecal S (+)-ketamine in a patient with cancer-related neuropathic pain. Br J Anaesth. 2005 Aug;95(2):247-9. PMID: 15951328.
-
12Klimscha W, Horvath G, Szikszay M, Dobos I, Benedek G. Antinociceptive effect of the S (+) enantiomer of ketamine on carrageenan hyperalgesia after intrathecal administration in rats. Anesth Analg. 1998 Mar;86(3):561-5.doi: 10.1213/00000539-199803000-00023.
» https://doi.org/10.1213/00000539-199803000-00023 -
13Karpinski N, Dunn J, Hansen L, Masliah E. Subpial vacuolar myelopathy after intrathecal ketamine: report of a case. Pain. 1997 Oct;73(1):103-5. PMID: 9414063.
-
14Brock-Utne JG, Mankowitz E, Lallichurum S. Effects of intrathecal saline and ketamine with and without preservative on the spinal nerve roots of monkeys. S Afr Med J. Mar 6;61(10):360-1. PMID: 6895951.
-
15Malinovsky JM, Cozian A, Lepage JY, Mussini J, Pinaudt M, Souron R. Ketamine and midazolam neurotoxicity in the rabbit. Anesthesiology. 1991 Jul(1);75:91-7. PMID: 2064066.
-
16Drummond JC, Moore SS. The influence of dextrose administration on neurological outcome after temporary spinal cord ischemia in the rabbit. Anesthesiology. 1989 Jan;70(1):64-70. PMID: 2912317.
-
17Ting PH, Antonakakis JG. Evidence-based review of ultrasound imaging for regional anesthesia. Semin Anesth. 2007 Mar;26(3):218-28.
-
18Moore S, Thanos S. the concept of microglia in relation to central nervous system disease and regeneration. Prog Neurobiol. 1996 Mar-Apr;48(4-5):441-60. PMID: 8804116.
-
19O'Callaghan JP, Miller D. Cerebellar hypoplasia in the Gunn rat is associated with quantitative changes in neurotypic and gliotypic proteins. J Pharmacol Exp Ther. 1985 Aug;234(2);522-33. PMID: 2410596.
-
20Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993 Jan;7(1):111-8. PMID: 8423058.
-
21Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996 Aug;19(8):331-8. PMID: 8843602.
-
22DeLeo JA, Colburn RW, Rickman AJ, Yeager MP. Intrathecal catheterization alone induces neuroimmune activation in the rat. Eur J Pain. 1997;1(2):115-22. PMID: 15102412.
-
23Farber NB, Kim SH, Dikranian K, Jiang XP, Heinkel C. Receptor mechanisms and circuitry underlying NMDA antagonist neurotoxicity. Mol Psychiatry. 2002;7(1):32-43. PMID: 11803444.
-
24Hoyte L, Barber PA, Buchan AM, Hill MD. The rise and fall of NMDA antagonists for ischemic stroke. Curr Mol Med. 2004 Mar;4(2):131-6. PMID: 15032709.
-
25Rojas AC, Alves JG, Lima, RM, Marques MEA, Barros GAM, Fukushima FB, Ganem EM. The effects of subarachnoid administration of preservative-free S(+)-ketamine on spinal cord and meninges in dogs. Anesth Analg. 2012 Feb;114(2):450-5.doi: 10.1213/ANE.0b013e31823a5d1b.
» https://doi.org/10.1213/ANE.0b013e31823a5d1b -
26Gomes LMRS, Garcia JBSG, Ribamar JS, Nascimento AGP. Neurotoxicity of subaracnoid preservative-free S (+) Ketamine in dogs. Pain Physician. 2011 Jan-Fev;14(1):83-90. PMID: 21267045.
-
27Kose EA, Bakar B, Ayva SK, Kilinc K, Apan A. , Neuroprotective effects of racemic ketamine and (S)-ketamine on spinal cord injury in rat. Injury. 2012 Jul;43(7):1124-30.doi: 10.1016/j.injury.2012.02.022.
» https://doi.org/10.1016/j.injury.2012.02.022
-
Financial source: National Council for Scientific and Technological Development (CNPq)
-
1
Research performed at Department of Anesthesiology, Botucatu Medical School, Paulista State University (UNESP), Botucatu-SP, Brazil. Part of PhD degree thesis, Postgraduate Program in Anesthesiology, UNESP. Tutor: Eliana Marisa Ganem.
Publication Dates
-
Publication in this collection
July 2014
History
-
Received
12 Feb 2014 -
Reviewed
14 Apr 2014 -
Accepted
19 May 2014