Abstracts
PURPOSE: Compare fibrin glue (Tissucol®) and platelet-rich plasma in full-thickness mesh skin grafts in dogs. METHODS: Eighteen dogs were used, divided into two groups: fibrin glue (FG) and platelet-rich plasma (PRP). In all the animals, a full-thickness 3x3 cm mesh skin graft was implanted. In the left limb, the biomaterial was place between the graft and the receptor bed, according to the group, while the right limb served as the control group. All the animals were evaluated clinically every 48 hours until the 14th day, using the variables of exudation, coloration, edema and cosmetic appearance. Three animals were evaluated histologically, on the third, seventh and tenth postoperative days, using the variables of fibroblasts, collagen, granulation tissue, microscopic integration-adherence and acute inflammation. RESULTS: Clinical evaluations showed that the group CF showed better scores for all variables compared to PRP group. On the histological evaluations PRP group had a higher presence of fibroblasts in the seventh and fourteenth days. CONCLUSION: The fibrin glue group was clinically superior to the platelet-rich group when used on full-thickness skin grafts in dogs.
Surgical Flaps; Fibrin Tissue Adhesive; Platelet-Rich Plasma; Skin; Dogs
OBJETIVO: Comparar a cola de fibrina (Tissucol®) e o plasma rico em plaquetas em enxertos cutâneos de espessura completa em malha em cães. MÉTODOS: Foram utilizados 18 cães, distribuídos em dois grupos, cola de fibrina (CF) e plasma rico em plaquetas (PRP). Em todos os animais foi realizado um enxerto cutâneo de 3x3 cm, em malha de espessura completa. No membro esquerdo foi colocado o biomaterial entre o enxerto e o leito receptor, cada qual em seu grupo, o membro direito serviu como grupo controle. Todos os animais foram avaliados clinicamente a cada 48 horas até o décimo quarto dia, através das variáveis: exsudação, coloração, edema e aspecto cosmético; histologicamente em três animais, no terceiro, sétimo e décimo quarto dia de pós-operatório através das variáveis: fibroblastos, colágeno, tecido de granulação, integração-aderência microscópica e inflamação aguda. RESULTADOS: Avaliações clínicas demonstraram que o grupo CF apresentou melhor escores em todas variáveis quando comparado com o grupo PRP. Nas avaliações histológicas o grupo PRP apresentou maior presença de fibroblastos ao sétimo e décimo quarto dia. CONCLUSÃO: A cola de fibrina foi clinicamente superior ao grupo plasma rico em plaquetas quando usados em enxertos cutâneos de espessura completa em cães.
Retalhos Cirúrgicos; Adesivo Tecidual de Fibrina; Plasma Rico em Plaquetas; Pele; Cães
8 - ORIGINAL ARTICLE
PLASTIC SURGERY
Comparative study between fibrin glue and platelet rich plasma in dogs skin grafts1 1 Research performed at Division of Operative Technique and Experimental Surgery, Department of Surgery, Federal University of Mato Grosso do Sul (UFMS), Brazil. Part of Master Degree thesis, Postgraduate Program in Health and Development of the Center-West Region, UFMS, Mato Grosso do Sul-MS, Brazil Tutor: Rafael de Rossi.
Estudo comparativo entre a cola de fibrina e o plasma rico em plaquetas em enxertos cutâneos em cães
Larissa Correa HermetoI; Rafael de RossiII; Santiago Benites de PáduaIII; Elenir Rose Jardim PontesIV; Aureo Evangelista SantanaV
IFellow PhD degree, Postgraduate Program in Veterinary Surgery, School of Agrarian and Veterinary Sciences, UNESP, Jaboticabal-SP, Brazil. Main author. Acquisition of data, design of the study, surgical procedures, manuscript writing, critical revision
IIPhD, Associate Professor, Anesthesiology and Surgery Unit, Department of Veterinary Medicine, School of Veterinary Medicine and Animal Science, UFMS, Mato Grosso do Sul-MS, Brazil. Design of the study, surgical procedures, critical revision
IIIFellow Master degree, School of Agrarian and Veterinary Sciences, UNESP, Jaboticabal-SP, Brazil. Acquisition of data, surgical procedures
IVPhD, Associate Professor, Postgraduate Program in Health and Development of the Center-West Region, UFMS, Mato Grosso do Sul-MS, Brazil. Interpretation of data, design of the study, statistical analysis
VPhD, Chairman, Full Professor, Department of Veterinary Clinical Medicine and Surgery, School of Agrarian and Veterinary Sciences, UNESP, Jaboticabal-SP, Brazil. Conception and design of the study, analysis and interpretation of data, critical revision
Correspondence Correspondence: Larissa Correa Hermeto Rua Raposo Tavares, 508 79050-050 Campo Grande MS Brasil Tel.: (55 67)9258-9311 larissa_hermeto@yahoo.com.br
ABSTRACT
PURPOSE: Compare fibrin glue (Tissucol®) and platelet-rich plasma in full-thickness mesh skin grafts in dogs.
METHODS: Eighteen dogs were used, divided into two groups: fibrin glue (FG) and platelet-rich plasma (PRP). In all the animals, a full-thickness 3x3 cm mesh skin graft was implanted. In the left limb, the biomaterial was place between the graft and the receptor bed, according to the group, while the right limb served as the control group. All the animals were evaluated clinically every 48 hours until the 14th day, using the variables of exudation, coloration, edema and cosmetic appearance. Three animals were evaluated histologically, on the third, seventh and tenth postoperative days, using the variables of fibroblasts, collagen, granulation tissue, microscopic integration-adherence and acute inflammation.
RESULTS: Clinical evaluations showed that the group CF showed better scores for all variables compared to PRP group. On the histological evaluations PRP group had a higher presence of fibroblasts in the seventh and fourteenth days.
CONCLUSION: The fibrin glue group was clinically superior to the platelet-rich group when used on full-thickness skin grafts in dogs.
Key words: Surgical Flaps. Fibrin Tissue Adhesive. Platelet-Rich Plasma. Skin. Dogs.
RESUMO
OBJETIVO: Comparar a cola de fibrina (Tissucol®) e o plasma rico em plaquetas em enxertos cutâneos de espessura completa em malha em cães.
MÉTODOS: Foram utilizados 18 cães, distribuídos em dois grupos, cola de fibrina (CF) e plasma rico em plaquetas (PRP). Em todos os animais foi realizado um enxerto cutâneo de 3x3 cm, em malha de espessura completa. No membro esquerdo foi colocado o biomaterial entre o enxerto e o leito receptor, cada qual em seu grupo, o membro direito serviu como grupo controle. Todos os animais foram avaliados clinicamente a cada 48 horas até o décimo quarto dia, através das variáveis: exsudação, coloração, edema e aspecto cosmético; histologicamente em três animais, no terceiro, sétimo e décimo quarto dia de pós-operatório através das variáveis: fibroblastos, colágeno, tecido de granulação, integração-aderência microscópica e inflamação aguda.
RESULTADOS: Avaliações clínicas demonstraram que o grupo CF apresentou melhor escores em todas variáveis quando comparado com o grupo PRP. Nas avaliações histológicas o grupo PRP apresentou maior presença de fibroblastos ao sétimo e décimo quarto dia.
CONCLUSÃO: A cola de fibrina foi clinicamente superior ao grupo plasma rico em plaquetas quando usados em enxertos cutâneos de espessura completa em cães.
Descritores: Retalhos Cirúrgicos. Adesivo Tecidual de Fibrina. Plasma Rico em Plaquetas. Pele. Cães.
Introduction
Reconstructions on distal lesions are made more complicated because of the scarcity of local tissue and the frequent association between orthopedic injuries and skin loss. Second-intention healing or skin traction are used for wounds involving less than 30% of the limb circumference1.The method of bringing the wound edges together should only be used in situations in which no distorting or tensioning of the tissue occurs2.
Skin grafts are utilized in the treatment of acute ablative, traumatic and burn defects as well as with chronic venous leg ulcers and diabetic foot ulcers. Skin grafts assist wound healing by replacing dermal collagen and providing biological occlusion and protection of the wound3. Meshing a skin graft reduces the chance of haematoma collection and allows good draping within a concavity. These qualities ensure good contact with the graft bed and hence good graft take. It allows fluid and blood to pass easily through the graft via multiple fine perforations, enabling earlier intra-operative application to the bed without haematoma collection4.
The use of biological fibrin glue has been known since 1909, when Bergel documented the hemostatic effect of fibrin powder. The fibrin layer between the skin graft and its bed forms naturally during the healing process and is essential for graft survival. Fibrin plays a supporting role for fibroblasts to produce collagen; it increases phagocytosis by inhibiting the action of infectious agents; and it stabilizes the graft during revascularization5. The off-label sealing and adhesive applications are utilized by a wide variety of specialties and extend from fistula closure and seroma prevention to mesh fixation and stem cell implantation6. There is evidence that fibrin glue promotes healing independent of its adhesive properties, through the increased angiogenesis and growth of fibroblasts that are observed in the wound bed7, and an expanding number of research efforts are underway that are increasing the potential uses of this potent biologic material6.
Platelet-rich plasma (PRP) is defined as an autologous concentration of platelets in a small volume of plasma and has been used to accelerate the healing of both soft tissues and bones8, that promotes intensive stimulation of healing through releasing growth factors that are actively secreted by the platelets9. These growth factors include three isomers of platelet-derived growth factors (PDGF αα, PDGF ββ and PDGF αβ), two of the numerous types of transforming growth factor beta (TGF β1 and TGF β2), vascular endothelial growth factor (VEGF) and epithelial growth factor (EGF). PRP also has three blood proteins that are known to act as cell adhesion molecules for osteoinduction, bone matrix, connective tissue and epithelial migration. These cell adhesion molecules are fibrin itself, fibronectin and vitronectin8.
Recent trends in reconstructive surgery have demonstrated that use of PRP (generally prepared in gel form) has provided benefits in this field10, but there is some controversy in the literature in the relation to the benefits from this procedure. Although many authors have reported significant improvements in tissue healing and bone formation through using PRP, others have not observed this benefit8.Because of the growth factors present in PRP, which increase and stimulate deposition of extracellular matrix, it might be supposed that PRP would be more efficient than commercial fibrin glue, for increasing epidermal and dermal regeneration11. The PRP also induces little inflammatory reaction between the graft and the recipient area, resulting in favorable conditions for graft incorporation12.
The aim of this study was to compare PRP and fibrin glue in full-thickness mesh skin grafts in dogs.
Methods
The experimental protocol was approved by the Ethics Committee for Use of Animals (CEUA) of UFMS, under no. 198/2008, at a meeting held on November 24, 2008. The experiment was conducted in accordance with the ethical principles adopted by the Brazilian College of Animal Experimentation (COBEA). Measures to minimize the animals' distress were taken.
Eighteen animals of the canine species of no defined breed (NDB) were used, with a mean weight of 10kg. They were kept in individual kennels and were provided with water and feed ad libitum. The animals were randomly distributed into two groups of nine animals, thus: one group treated with Tissucol® fibrin glue (FG) and the other group treated with PRP.
After subjection to fasting for 12 hours, the dogs were tranquillized with acepromazine at a dose of 0.05mg/kg, in association with morphine, 0.5mg/kg, subcutaneously. Fifteen minutes later, anesthesia was induced using propofol, 5mg/kg. The animal was intubated and anesthesia was maintained using isoflurane.
The first procedure performed was to prepare the receptor area on the lateral face of the left forearm, using a scalpel, with the aid of a template made of brown packaging paper, measuring 3x3cm in area. A full-thickness skin graft was harvested from the left thoracic region, based on the same template. After harvesting, the subcutaneous tissue was removed with the aid of scissors, until the hair follicles could be seen. Using the scalpel, eight to ten sequential incisions of around 0.5cm were made in order to form the mesh. The donor site synthesis was done using a double-pedicle skin flap.
The animals were then repositioned in left lateral decubitus, and the right forearm was subjected to a procedure similar to what had been done to the left forearm. The difference was that eight drops of fibrin glue (Tissucol®) were applied to the left receptor bed in the FG group, followed by eight suturing stitches, while in the PRP group, platelet-rich plasma was applied to the left receptor bed, followed by eight suturing stitches. The right forelimb served as a control group, and the same technique was used, but without the biomaterial between the bed and the graft. The dogs received cephalexin at a dose of 30mg/kg every 12 hours for 10 days, and tramadol, 2mg/kg every eight hours, for four days.
The grafts were protected with a thin layer of neomycin and bacitracin ointment, overlain with rayon gauze and ordinary gauze, a layer of crepe bandage, a layer of orthopedic cotton wool, another layer of crepe bandage and surgical adhesive tape. The dressings were changed every 48 hours up to the seventh postoperative day and every 72 hours thereafter. To remove the dressings, the gauze was abundantly wetted with sterile physiological solution.
Clinical evaluations were conducted on the grafts using an analysis matrix. The evaluations were performed on postoperative days 2, 4, 6, 8, 11 and 14. By means of the analysis matrix, notes were made about events that occurred, including: exudation (absence of exudate = 0; presence of exudate = 1; mild exudate = 2; large amount of exudate = 3), coloration (white = 0; pink = 1; purple = 2; black = 3), edema (no edema = 0; mild edema = 1; large amount of edema = 2); and cosmetic appearance (excellent = 1; good = 2; fair = 3; poor = 4). The evaluations were always made by the same observer, who did not know about the difference between the groups. For the microscopic evaluation, a fragment of the graft and receptor bed was taken from three animals on the third, seventh and fourteenth days after the operation. The slides made from this material were stained using the hematoxylin-eosin technique (HE). For this procedure, an anesthetic protocol similar to the one described above was used. After staining, the slides were evaluated by a single examiner who was unaware of the group to which each slide belonged. The histological analyses consisted of investigations on fibroblast proliferation, collagen, granulation tissue, acute inflammation and microscopic integration-adherence, and these were graded as absent (0), mild (1), moderate (2) or severe (3).
After the end of this study, all the animals were castrated and donated to people who were interested in having them.
The fibrin glue was prepared in accordance with the manufacturer's instructions, before the start of the surgical procedures, and the protocol for obtaining the PRP was as described below.
Preparation of platelet-rich plasma
Before the anesthetic medication was administered, 20ml of blood was collected from the jugular vein of each dog, in the form of two volumes of 10ml, each in a 15ml polypropylene Falcon tube, and eight drops of 10% sodium citrate was added to each tube. The tubes were centrifuged at 300g for ten minutes, at room temperature. Following this, 500μl of plasma was removed from the upper part of each tube and was transferred to another Falcon tube, which was marked as tube A. This was used to obtain autogenous thrombin. All the remaining plasma was then removed and transferred to another tube, marked as tube B, which was destined for production of PRP, and was left at room temperature.
To tube A, 300μl of 10% calcium gluconate were added. The mixture was shaken and incubated in a water bath at 37°C for 15 minutes. During this time, the remainder of the plasma and the fog zone were pipetted into another Falcon tube (Tube B). Tube A (after the water bath) and tube B were subjected to centrifugation again, which resulted in separation of a liquid rich in thrombin in tube A and in sedimentation of platelets (and a few red cells) at the bottom of tube B. The upper portion of the plasma in tube B was removed, such that the total volume of plasma in this tube was reduced by 50%. Tube B was then shaken to disperse the platelets in the remaining plasma, thereby obtaining the PRP. The thrombin obtained in tube A was added to tube B in the proportions 2:1 (2ml of PRP to 1ml of thrombin). After homogenization, the PRP was collected in a polypropylene receptacle and was left to rest at room temperature. After a few minutes, it became a gel.
For comparisons between the groups, the following tests were used: Mann-Whitney (independent samples), Wilcoxon (correlated samples) and Fisher (independent samples, classified into two categories). The Bio Estat software, version 5.0, was used and the significance level was taken to be 5%.
Results
Clinical analyses
Tables 1, 2 and 3 shows the results described below:
Regarding the exudation variable, it was seen that the FG group presented less exudation than the PRP group did. Regarding the coloration variable, we observed significant differences, thus demonstrating that the FG group was superior in the analyses, since this group followed the coloration pattern expected at different stages of graft revascularization, unlike the PRP group. Comparison between the FG group and its controls showed that the pink coloration was attained earlier in the treated limb (Figure 1), on the eighth day. In the PRP group, we observed that the coloration progressed to blackening, thus showing tissue necrosis.
The edema variable only presented a significant difference between the treated limbs and their controls on the second and fourth days, such that the treated limbs presented greater edema on these days.
Significant differences in the cosmetic appearance variable were observed, such that the FG group presented a better cosmetic appearance than the PRP group did (Figures 1).
Histological analyses
In the histological analyses, we observed that the group evaluated on the third day after grafting still did not present the signs that had been proposed for use in the evaluation. Only signs of ischemia were presented, which could not be used to analyze the variables laid out in the methodology.
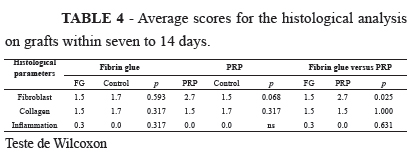
From Table 4, it can be seen that a significant difference was only noted in relation to the fibroblast variable, which was more abundant in the PRP group (Figure 2).
Discussion
From the analysis on the results, the FG treated group presented a better cosmetic appearance than shown by the controls, and this was also reported by Branski et al.13. This was similar to the findings of Mittermayer et al.14 that compared the slow-clotting version of fibrin sealant to sutured grafts in a porcine wound healing model, and found decreased seroma and hematoma formation, less graft dislocation and necrosis, and an improved graft take. Erba et al.15 obtained good results and conclued that the spray application technique provides an effective method of application of fibrin glue in extended fasciocutaneous flap surgery and results in decreased drainage output and time. For the authors, the application of fibrin glue seems to favor flap adhesion in deep pressure sores, where tight contact of the flap with the wound bed is essential to prevent the complications associated with dead space formation. Simillar to Foster et al.16, a number of efficacy endpoints were analyzed and conclued better results in treated sites than for control sites: hematoma/seroma were significantly lower, engraftment and wound closure were better too. Likewise, the adhesive properties of fibrin glue may reduce seroma formation via continuous full surface adhesion of the graft to the wound bed, closing the space that exists when grafts are attached using point fixation techniques such as staples. The authors described that reduction in hematoma and seroma is clinically significant because of the additional procedures that are required to deal with these complications in certain cases.
In the PRP group, we observed that the control limbs presented a better cosmetic appearance, less exudation and better coloring that shown by the treated limbs in this group. Moreover, the FG group continued to present the best assessment. These results can be compared with those of O'Grady et al.17, who concluded that using thick layers of fibrin glue between the receptor bed and the graft could result in graft adhesion failure. They reported that the group with thin layers in the graft and the control group presented a better final cosmetic appearance and a better percentage of graft survival. These results are similar to those of the present study, in comparison with the PRP group. According to this author, the thick layer of biomaterial blocked the movement of cell components and vascular elements needed for achieving graft survival, which suggests that the thickness of PRP used was the main cause of failure of graft survival in this group. Vendramin et al.18 also questioned whether the PRP could disrupt the phenomena of inosculation and revasculariozation, and stated that the higher the density of the fibrin mesh, the more likely it adversely affect wound healing, preventing the arrival of platelets and their growth factors, so, the excess fibrin is harmful. Further studies are needed in order to investigate whether different thicknesses of PRP might influence a better response from the skin graft, in the light of their properties and possible benefits.
In the same line of reasoning, Altmeppen et al.11 conducted a study in which they evaluated the characteristics and composition of PRP, transformed the PRP into glue and compared this with commercially available fibrin glues. They also assessed the adhesion strength of this preparation. However, PRP does not function as a glue but, rather, as a factor for improving the healing process. These authors demonstrated that the adhesion strength decreased as the platelet concentration increased, which may explain how high platelet concentrations can block formation of a fibrin network. On the other hand, high platelet concentrations might improve healing because growth factors are released. This was observed in the present study, in which intense growth of fibroblasts was observed, but revascularization of the graft did not occur in the PRP group, the same conclusion in the study conducted by Vendramin et al.18.
Differing from what was found in the present study, Bhanot and Alex19 stated that the platelet gel used in plastic surgery presented advantages that included faster re-epithelialization and reduction of the postoperative edema, as well as Chen et al.12. that sprayed skin ulcers with PRP, and put a thin split-thickness skin graft with multiple slits on the wound bed, and conclude that most skin grafts took well. Li et al.20 assessed whether the application of a platelet-rich plasma gel would increase skin flap survival in an experimental rat model. And unlike our results, for the authors, platelet-rich plasma administration could be an important technique for starting angiogenesis by recruiting the endothelial cells that line the blood vessels. They conclude that a subcutaneous platelet rich plasma injection significantly improves the survival of cutaneous flaps and is strongly correlated with angiogenesis. It is important to highlight that Li et al.20 had used skin flaps and not skin grafts, and Chen et al.12 sprayed fibrin glue on the skin graft, and these differences in methodology may have resulted in different conclusions.
Conclusions
The fibrin glue group presented a result that was clinically superior to the platelet-rich group, when these agents were used on full-thickness skin grafts in dogs. However, it has to be emphasized that the histological analysis demonstrated greater presence of fibroblasts in the group treated with platelet-rich plasma.
Acknowledgements
The authors wish to express their immense gratitude to the company Baxter Healthcare Brazil for donating samples of Tissucol®, to Prof. Dr. Alexandre Nakao Odashiro for carrying out the histological examinations and the Research Internationalization Program of UNESP.
Received: June 21, 2012
Review: August 23, 2012
Accepted: September 28, 2012
Conflict of interest: none
Financial source: none
- 1. Fowler D. Distal limb and paw injuries. Vet Clin Small Anim. 2006;36:819-45.
- 2. Jensen AR, Klein MB, Ver Halen JP, Wright AS, Horvath KD. Skin flaps and grafts: a primer for the national technical skills curriculum advanced tissue-handling module. J Surg Educ. 2008;65 (3):191-9.
- 3. Molnar JA, DeFranzo AJ, Hadaegh A, Morykwas MJ, Shen P, Argenta LC. Acceleration of Integra incorporation in complex tissue defects with subatmospheric pressure. Plast Reconstr Surg. 2004;113:133946.
- 4. McCulley SJ. Sideways meshing of split-thickness skin grafts - a useful technique. Burns. 1999;25:453-4.
- 5. Saltz R, Sierra D, Feldman D, Saltz MB, Dimick AD, Vasconez LO. Experimental and clinical applications of fibrin glue. Plast Reconstr Surg. 1991;88(6):1005-15.
- 6. Spotnitz WD. Fibrin sealant: past, present, and future: a brief review. World J Surg. 2010;34:6324.
- 7. Gosain AK, Lyon VB. The current status of tissue glues: Part II. For adhesion of soft tissues. Plast Reconstr Surg. 2002;110(6):1581-4.
- 8. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-96.
- 9. Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, Moroni M, Carabelli A. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci. 2004;30(2):145-51.
- 10. Henkel G. Platelet gel in facial surgery: is the excitement warranted? ENToday. 2006;1(23). http://www.enttoday.org/details/article/531875/Platelet_Gel_in_Facial_Surgery_Is_The_Excitement_Warranted.html
- 11. Altmeppen J, Hansen E, Bonnlander GL, Horch RE, Jeschke MG. Composition and characteristics of an autologous thrombocyte gel. J Surg Res. 2004;117:202-7.
- 12. Chen TM, Tsai JS, Burnouf T. A novel technique combining platelet gel, skin graft, and fibrin glue for healing recalcitrant lower extremity ulcers. Dermatol Surg. 2010;36:453-60.
- 13. Branski LK, Mittermayr R, Herndon DN, Jeschke MG, Hofmann M, Masters OE, Norbury WB, Traber DL, Tangl S, Redl H. Fibrin sealant improves graft adherence in a porcine full-thickness burn wound model. Burns. 2011;37:1360-6.
- 14. Mittermayr R, Wassermann E, Thurnher M, Simunek M, Redl H. Skin graft fixation by slow clotting fibrin sealantapplied as a thin layer. Burns. 2006;32:30511.
- 15. Erba P, di Summa PG, Wettstein R, Raffoul W, Kalbermatten D.F. Fibrin sealant for fasciocutaneous flaps. J Reconstr Microsurg. 2010;26:213-7.
- 16. Foster K, Greenhalgh D, Gamelli RL, Mozingo D, Gibran N, Neumeister M, Abrams SZ, Hantak E, Grubbs L, Ploder B, Schofield N, Riina LH, FS 4IU VH S/D Clinical Study Group. Efficacy and safety of a fibrin sealant for adherence of autologous skin grafts to burn wounds: results of a phase 3 clinical study. J Burn Care Res. 2008;29:293303.
- 17. O'Grady KM, Agrawal A, Bhattacharyya TK, Shah A, Toriumi DM. An Evaluation of fibrin tissue adhesive concentration and application thickness on skin graft survival. Laryngoscope. 2000;110:1931-5.
- 18. Vendramin FS, Franco D, Schamall RF, Franco TR. Utilização do plasma rico em plaquetas (PRP) autólogo em enxertos cutâneos em coelhos. Rev Bras Cir Plast. 2010;25(1):4-10.
- 19. Bhanot S, Alex JC. Current applications of platelet gels in facial plastic surgery. Facial Plast Surg. 2002;18(1):27-33.
- 20. Li W, Enomoto M, Ukegawa M, Hirai T, Sotome S, Wakabayashi Y, Shinomiya K, Okawa KS. Subcutaneous injections of platelet-rich plasma into skin flaps modulate proangiogenic gene expression and improve survival rates. Plast Reconstr Surg. 2012;129:858-66.
Publication Dates
-
Publication in this collection
29 Oct 2012 -
Date of issue
Nov 2012
History
-
Received
21 June 2012 -
Accepted
28 Aug 2012 -
Reviewed
23 Aug 2012