Abstract
Purpose:
To investigate if the inorganic bovine bone matrix changes the bone formation in rats submitted to inhalation of cigarette smoke.
Methods:
Twenty Wistar rats were divided into two groups: Cigarette Clot Group (CCG), which in the inhalation chamber received the smoke of 10 cigarettes, 3 times a day, 10 minutes, for 30 days and had the surgical cavity filled by clot; Cigarette Biomaterial Group (CBG), submitted to the same inhalation technique but with the cavity filled by biomaterial.
Results:
In CCG there was a significant difference of new bone tissue in the analyzed periods (15 and 45 days), and in 15 days, there was 4.8 ± 0.42 of bone formed and 11.73 ± 0.59 (p <0.05) in 45 days. The CBG also showed a significant difference between the periods of 15 to 45 days, being respectively 6.16 ± 0.30 and 11.60 ± 0.61. However, when the groups were compared, within the same analyzed periods, a significant difference was observed only in the period of 15 days, with the new bone percentage being greater in the CBG.
Conclusion:
The bone matrix acted as an osteoinductive biomaterial, biocompatible and aided in the repair process, mainly in the initial period of recovery.
Key words:
Biocompatible Materials; Bone Regeneration; Bone Transplantation; Heterografts; Smoking; Rats.
Introduction
The various toxic effects of nicotine, due to smoking, constitute a serious public problem and an important risk factor for systemic health11 Lee PN, Fariss MW. A systematic review of possible serious adverse health`effects of nicotine replacement therapy. Arch Toxicol. 2017 Apr;91(4):1565-94. doi: 10.1007/s00204-016-1856-y.
https://doi.org/10.1007/s00204-016-1856-...
. Among the almost 5.300 toxic substances found in cigarettes, most are known for their toxic effects such as carbon monoxide, ammonia and nicotine, which have carcinogenic particles such as arsenic, polonium and dichlorodiphenyltrichloroethane (DDT)22 Almeida AÁ, Bandeira CM, Gonçalves AJ, Araújo AJ. Nicotine dependence and smoking habits in patients with head and neck cancer. J Bras Pneumol. 2014 May-Jun;40(3):286-93. PMID: 25029652..
Nicotine contributes to inflammatory periodontal disease, a change in gingival tissue, inhibiting bone formation and decreasing the synthesis of collagen and osteoblasts33 Torshabi M, Rezaei Esfahrood Z, Jamshidi M, Mansuri Torshizi A, Sotoudeh S. Efficacy of vitamins E and C for reversing the cytotoxic effects of nicotine and cotinine. Eur J Oral Sci. 2017 Dec;125(6):426-37. doi: 10.1111/eos.12375.
https://doi.org/10.1111/eos.12375...
. Smoking can interfere with vascular reactions because it causes vasoconstriction in the human gum, but when compared to other body regions it is less intense, for example vasoconstriction in the thumb skin44 Bassetti MA, Bassetti RG, Sculean A, Salvi GE, Bornstein MM, Ramseier CA. The impact of brief interventions for tobacco cessation on patients' awareness of cigarette smoking as a risk factor for chronic periodontitis. Oral Health Prev Dent. 2017;15(4):391-7. doi: 10.3290/j.ohpd.a38737.
https://doi.org/10.3290/j.ohpd.a38737...
.
Nicotine does not have a direct pharmacological effect on bone density but has an indirect effect on bone mineral density decrease and is an inducing factor in the occurrence of osteoporosis, aggravated if it is associated with chronic alcoholism55 Kallala R, Barrow J, Graham SM, Kanakaris N, Giannoudis PV. The in vitro and in vivo effects of nicotine on bone, bone cells and fracture repair. Expert Opin Drug Saf. 2013 Mar;12(2):209-33. doi: 10.1517/14740338.2013.770471.
https://doi.org/10.1517/14740338.2013.77...
6 Buchaim RL, Andreo JC, Rodrigues AC, Buchaim DV, Dias DV, Daré LR, Roque DD, Roque JS. The action of demineralized bovine bone matrix on bone neoformation in rats submitted to experimental alcoholism. Arq Bras Med Vet Zootec. 2013;65(3):715-721. doi: 10.1590/S0102-09352013000300016.
https://doi.org/10.1590/S0102-0935201300...
-77 Buchaim RL, Andreo JC, Rodrigues AC, Buchaim DV, Roque DD, Roque JS, Rosa Junior GM. Bovine bone matrix action associated with morphogenetic protein in bone defects in rats submitted to alcoholism. Int J Morphol. 2012 Mar;30(1):266-71. doi: 10.4067/S0717-95022012000100048.
https://doi.org/10.4067/S0717-9502201200...
.
These factors induce tooth loss more frequently, usually due to destructive periodontal disease, leading to greater loss of bone tissue and its appendixes88 Shintcovsk RL, Knop L, Tanaka OM, Maruo H. Nicotine effect on bone remodeling during orthodontic tooth movement: Histological study in rats. Dental Press J Orthod. 2014;19(2):96-107. doi: 10.1590/2176-9451.19.2.096-107.oar.
https://doi.org/10.1590/2176-9451.19.2.0...
. The administration of cigarette smoke in rats within a short period of time suggests that nicotine has little significant effect on the bone regeneration process. In longer exposures, deleterious effects on the gene expression of angiogenic and osteogenic factors are observed99 Tamura K, Togo Y, Kaihara S, Hussain A, Takahashi K, Bessho K. The effect of nicotine on osteoinduction by recombinant human bone morphogenetic protein 2. Int J Oral Maxillofac Surg. 2014 Aug;43(8):1022-9. doi: 10.1016/j.ijom.2014.02.014.
https://doi.org/10.1016/j.ijom.2014.02.0...
.
Recent studies indicate that the harmful effects of nicotine are dose-dependent, that is, the higher the dose, the greater the negative effects1010 Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002 May;2(5):372-7. doi: 10.1038/nri803.
https://doi.org/10.1038/nri803...
. Despite this, even in low dosage, it can be considered a potential risk factor in the process of reconstitution of bone defects1111 Marinucci L, Balloni S, Fettucciari K, Bodo M, Talesa VN, Antognelli C. Nicotine induces apoptosis in human osteoblasts via a novel mechanism driven by H(2)O(2) and entailing Glyoxalase 1-dependent MG-H1 accumulation leading to TG2-mediated NF-kB desensitization: Implication for smokers-related osteoporosis. Free Radic Biol Med. 2018 Jan 20;117:6-17. [Epub ahead of print]. doi: 10.1016/j.freeradbiomed.2018.01.017.
https://doi.org/10.1016/j.freeradbiomed....
.
Currently there are alternatives that may help reduce the morbidities associated with loss of bone tissue. Among them, inorganic bovine bone matrices, which are potentially osteoinductive and show little immunological reaction1212 Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I, Grigolo B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater Sci Eng C Mater Biol Appl. 2017 Sep 1;78:1246-62. doi: 10.1016/j.msec.2017.05.017.
https://doi.org/10.1016/j.msec.2017.05.0...
. The inorganic bovine bone graft has shown promising results. It is based on the abundance of the matrix, low cost of bovine bone and in the appropriate mechanical and chemical preparation process1313 Curry AS, Pensa NW, Barlow AM, Bellis SL. Taking cues from the extracellular matrix to design bone-mimetic regenerative scaffolds. Matrix Biol. 2016 May-Jul;52-54:397-412. doi: 10.1016/j.matbio.2016.02.011.
https://doi.org/10.1016/j.matbio.2016.02...
.
The lyophilized inorganic (deproteinized) bovine bone follows the same process of preparation of the organic matrix, however, it does not undergo decalcification process, where all the mineral components of the bone are preserved and all organic parts (BMPs, collagen, proteins) are eliminated, meaning the inorganic part is preserved1414 Zizzari VL, Zara S, Tetè G, Vinci R, Gherlone E, Cataldi A. Biologic andm clinical aspects of integration of different bone substitutes in oral surgery: a literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016 Oct;122(4):392-402. doi: 10.1016/j.oooo.2016.04.010.
https://doi.org/10.1016/j.oooo.2016.04.0...
. It is an osteoconductive and biocompatible biomaterial, which serves as a scaffold for bone growth in contact with its surface1515 Gupta P, Adhikary M, M JC, Kumar M, Bhardwaj N, Mandal BB. Biomimetic, osteoconductive non-mulberry silk fiber reinforced tricomposite scaffolds for bone tissue engineering. ACS Appl Mater Interfaces. 2016 Nov 16;8(45):30797-810. PMID: 27783501..
Due to the lack of description in the world literature of similar study, we decided to carry out this study with the objective of observing whether the demineralized bovine bone matrix (GenOx, Baumer, Brazil) changes the bone neoformation in rats submitted to inhalation of cigarette smoke, using histomorphological and histomorphometric analysis.
Methods
Experimental design
Twenty male Wistar rats (Rattus norvegicus), with average weight of 271.5 grams, were obtained from the Central Animal House of the Universidade de Marília, after approval by the Committee of Ethics in Animal Use (protocol 10/2016).
The animals were kept in a 12 hour light / dark cycle environment, temperature of 22 ± 2°C and relative humidity of 60 ± 5% until the end of the experiment.
The animals were randomly divided into two experimental groups: Cigarette Clot Group (CCG) and Cigarette Biomaterial Group (CBG), which contained 10 animals in each group, as follows:
-
1) Cigarette Clot Group (CCG), which in the inhalation chamber received 10 cigarettes smoke (Mustang, Philip Morris, Brazil) 3 times a day for 10 minutes for 30 days and had the surgical cavity filled by clot.
-
2) Cigarette Biomaterial Group (CBG), which in the inhalation chamber received the smoke of 10 cigarettes (Mustang, Philip Morris, Brazil) 3 times a day for 10 minutes for 30 days and had the surgical cavity filled by inorganic bovine bone matrix (GenOx, Baumer, Mogi Mirim, SP, Brazil).
Animals from all groups received the same solid diet (Nuvilab CR 1 diet from Nuvital, Brazil) “ad libitum” throughout the experiment. After the inhalation period of 30 days all the animals were submitted to the experimental surgery. The animals of both groups continued to inhale the cigarette smoke after undergoing surgery until the period correspondent to euthanasia.
Experimental surgery
The animals were previously weighed and submitted to general anesthesia with injection of sodium pentobarbital intraperitoneally - IP (Nembutal, 30mg/kg). The tricotomy was performed in the ventral region of the posterior limb and the disinfection of the surgical area with 2% chlorhexidine (Riohex, Rioquímica, Brazil).
With a number 15 scalpel blade (Solidor, Brazil), a 20-mm linear incision was made in the craniocaudal direction in the left pelvic limb, cutting the skin and muscle fascia for exposure and divulsion of the muscular tissue involving the tibia.
The incision extended to the periosteum, allowing its displacement, moving it anterior-posteriorly, thus obtaining a large area of work on the tibia. With a number 6 spherical steel drill (Dentsply, Brazil) mounted on a low-rotation micro-engine (Dabi Atlante, Brazil), a cavity of approximately 3 mm in diameter was prepared and, in depth, reaching the bone marrow. This procedure was performed with abundant irrigation of 0.9% Sodium Chloride solution (Eurofarma, Brazil).
The cavity was made in the longitudinal axis of the bone, and the perforation was made in the proximal third of the tibial diaphysis, which was not filled by any material (clot only) in the Cigarette Clot Group (CCG) and filled with inorganic bovine bone matrix (GenOx, Baumer, Brazil) at the Cigarette Biomaterial Group (CBG).
The operatory acts were always performed by a single operator subjecting the animals to the same conditions. To complete the surgical procedures, the tissues were repositioned, and the sutures performed individually, first in the periosteum with resorbable suture (Shalon, Brazil) and then in the skin with uninterrupted stitches using 4-0 Silk suture (Shalon, Brazil).
Immediately after the surgical procedure, Tramadol (50mg/kg diluted in 500ml/water; EMS, Brazil) was administered for 3 days, followed by Paracetamol (200mg/kg diluted in 500ml/water), in drinking water, until the period of euthanasia. The animals were observed in the postoperative and postanesthetic period for 2 hours and, the next day, they returned to the inhalation routine of 10 cigarettes (Mustang, Philip Morris, Brazil), 3 times a day for 10 minutes, until the period of euthanasia.
Euthanasia of animals and processing of material
Fifteen and forty five days after the surgery, five animals from each group, per period, were euthanized. Barbiturate (Tiopental) was used in the dosage for rats (150 mg/kg) as follows: 2.5% intraperitoneal Sodium Thiopental applied in the lower left quadrant of the animal (associated with local anesthetic, lidocaine hydrochloride at the dosage of 10 mg/kg; EMS, Brazil).
The bone pieces removed from the tibia were fixed in 10% buffered formalin during 24 hours, washed in running water for 12 hours, and decalcified in sodium citrate solution and formic acid (Merck, Brazil) in equal portions for 45 days. The decalcifying solution, which contained the pieces, was changed every 3 days. Then, the pieces underwent the routine laboratory process for inclusion in paraffin.
After the blocks were obtained, longitudinal slices with a thickness of 6 micrometers were acquired on a rotating microtome (Leica RM 2245, Germany), resulting in semi-seriated cuts that were submitted to staining by hematoxylin and eosin for histomorphological and histomorphometric study under light microscopy and photographed in a photomicroscope (Olympus DP 71, Japan).
Stereological analysis was performed based on images obtained by 10x magnification according to the methodology described by Weibel et al.1616 Weibel ER, Kistler GS, Scherle WF. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23-38. PMID: 5338131. in order to quantify neoformed bone on lesion area.
Statistical analysis
Quantitative analysis was performed using the Image Pro-Plus 6.0 software (Media Cybernetics, USA). For the statistical analysis of the percentage of new bone tissue, in the different periods (15 and 45 days), the unpaired t-test was used in the same group. For the statistical analysis of the percentage of new bone tissue in the different groups (CCG and CBG) in the same period, ANOVA variance test was used along with Tukey post-test. The level of significance for the considerations was determined at p <0.05. The software used for statistical analysis was GraphPad Prism 5 (La Jolla, USA).
Results
Histomorphological analysis
Cigarette Clot Group (CCG)
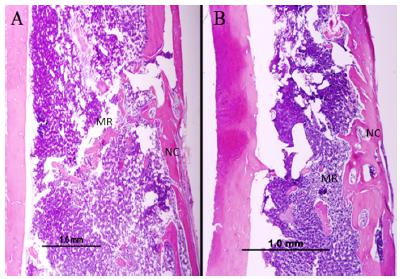
In the 15-day period, the ruptured cortical reconstitution was observed, but noticeably thinner and less organized than in the 45-day period. The site of surgical defect presented a reorganization of the medullar region in the two analyzed periods (Figure 1).
Microscopic appearance of the surgical cavity of the Cigarette Clot Group (CCG), stained in HE, x4 objective. A) 15 days; B) 45 days. Medullar Region (MR), Neocortical (NC).
Cigarette Biomaterial Group (CBG)
In the 2 analyzed periods (15 and 45 days) it was observed that the cortical was reconstructed and embracing particles of the biomaterial (GenOx). The site of the defect showed evident presence of the grafted biomaterial and the visualization of the area undergoing tissue reorganization.
In the slides, the presence of new bone tissue around the biomaterial particles can be observed (Figure 2).
Microscopic appearance of the surgical cavity of the Cigarette Biomaterial Group (CBG) animals stained in HE. x4 objective. A) 15 days; B) 45 days. Medullar Region (MR), Neocortical (NC), Biomaterial (BM).
Histomorphometric Analysis
Cigarette Clot Group (CCG)
It was observed that, relatively to the percentage of bone tissue in formation, according to the analyzed periods, there was a significant difference between the periods (15 and 45 days) (Figure 3 and Table 1).
Graphical representation new bone tissue formation percentage, comparing the analyzed periods (15 and 45 days) in the CCG (clot). Different lowercase characters indicate a significant difference between the analyzed periods (p <0.05).
Cigarette Biomaterial Group (CBG)
It was observed that, relatively to the percentage of bone tissue in formation, according to the analyzed periods, a significant difference occurred between the periods (15 and 45 days) (Figure 4 and Table 2).
Graphical representation of new bone tissue formation percentage comparing the analyzed periods (15 and 45 days) in the CBG (Biomaterial). Different lowercase characters indicate a significant difference between the analyzed periods (p<0.05).
Cigarette Clot Group (CCG) x Cigarette Biomaterial Group (CBG)
When compared, data were obtained showing a higher percentage of new bone formation in the CBG group, with a significant difference in relation to CCG, in the period of 15 days. In the 45-day period, CCG and CBG did not present a statistically significant difference (Figure 5 and Table 3).
Graphical representation of new bone tissue formation percentage in the analyzed periods (15 and 45 days) comparing two experimental groups CCG (Clot) and CBG (Biomaterial). Different lowercase characters indicate significant difference between the analyzed groups (p<0.05).
Discussion
The present study aimed to analyze whether a demineralized bovine bone matrix changes the bone neoformation in rats submitted to inhalation of cigarette smoke (considering that these matrices are potentially osteoinductive), using histomorphological and histomorphometric analysis. It was observed that the grafted biomaterial assisted in the process of reconstitution of the injured bone in the initial period of recovery.
Several experiments already performed and published in the world scientific literature have already proven the deleterious effects of exposure to nicotine in several tissues, including bone tissue1717 Baljoon M, Natto S, Bergström J. Long-term effect of smoking on vertical periodontal bone loss. J Clin Periodontol. 2005 Jul;32(7):789-97. PMID: 15966888.
18 Pavone C, Nogueira AV, de Oliveira GJ, Scardueli CR, Cerri PS, Marcantonio E Jr, Marcantonio RA. Treatment of periodontal disease with an Er,Cr:YSGG laser in rats exposed to cigarette smoke inhalation. Lasers Med Sci. 2015 Nov;30(8):2095-103. doi: 10.1007/s10103-015-1731-8.
https://doi.org/10.1007/s10103-015-1731-...
-1919 Campos JM, Prati AJ, Cirano FR, Pimentel SP, Pastore GP, Pecorari VG, Ribeiro FV, Casati MZ, Casarin RC. Smoking modulates gene expression of type I collagen, bone sialoprotein, and osteocalcin in human alveolar bone. J Oral Maxillofac Surg. 2015 Nov;73(11):2123-31. doi: 10.1016/j.joms.2015.06.168.
https://doi.org/10.1016/j.joms.2015.06.1...
. However, little is known if the biomaterials grafted in bone defects of animals exposed to nicotine can help in the tissue repair mainly the bovine bone matrix (GenOx) used in the present research.
The use of the GenOx biomaterial led to the formation of tissue with characteristics similar to an osteoid matrix in the postoperative period of 6 months, according to da Silva et al.2020 da Silva RC, Crivellaro VR, Giovanini AF, Scariot R, Gonzaga CC, Zielak JC. Radiographic and histological evaluation of ectopic application of deproteinized bovine bone matrix. Ann Maxillofac Surg. 2016 Jan-Jun;6(1):9-14. doi: 10.4103/2231-0746.186150.
https://doi.org/10.4103/2231-0746.186150...
. In addition, when associated with animals submitted to chronic alcoholism, despite not accelerating the process of bone reconstitution, the demineralized bone matrix presented osteoconductive properties, with formation of new bone tissue aggregating the biomaterial particles66 Buchaim RL, Andreo JC, Rodrigues AC, Buchaim DV, Dias DV, Daré LR, Roque DD, Roque JS. The action of demineralized bovine bone matrix on bone neoformation in rats submitted to experimental alcoholism. Arq Bras Med Vet Zootec. 2013;65(3):715-721. doi: 10.1590/S0102-09352013000300016.
https://doi.org/10.1590/S0102-0935201300...
.
Exposure of the animal to nicotine can be accomplished in a number of ways, varying both in times of exposure as well as in the passive inhalation device2121 Brito MV, Yasojima EY, Silveira EL, Yamaki VN, Teixeira RK, Feijó DH, Gonçalves TB. New experimental model of exposure to environmental tobacco smoke. Acta Cir Bras. 2013 Dec;28(12):815-9. PMID: 24316853.. In this experiment, the option was made to use the method proposed by Cendon et al.2222 Cendon SP, Battlehner C, Lorenzi Filho G, Dohlnikoff M, Pereira PM, Conceição GM, Beppu OS, Saldiva PH. Pulmonary emphysema induced by passive smoking: an experimental study in rats. Braz J Med Biol Res. 1997 Oct;30(10):1241-7. PMID: 9496445. because, due to its simplicity, low cost and short duration, this technique proved to be a useful model for experiments in the area and animal model used.
In the histomorphological analysis, the formation of a new cortex and the reorganization of the medullar region were observed in the group in which the surgical cavity was filled only by clot (CCG). This finding is expected in the bone repair process in the model used, even with the presence of aggravating factors such as exposure to ethanol or nicotine66 Buchaim RL, Andreo JC, Rodrigues AC, Buchaim DV, Dias DV, Daré LR, Roque DD, Roque JS. The action of demineralized bovine bone matrix on bone neoformation in rats submitted to experimental alcoholism. Arq Bras Med Vet Zootec. 2013;65(3):715-721. doi: 10.1590/S0102-09352013000300016.
https://doi.org/10.1590/S0102-0935201300...
,77 Buchaim RL, Andreo JC, Rodrigues AC, Buchaim DV, Roque DD, Roque JS, Rosa Junior GM. Bovine bone matrix action associated with morphogenetic protein in bone defects in rats submitted to alcoholism. Int J Morphol. 2012 Mar;30(1):266-71. doi: 10.4067/S0717-95022012000100048.
https://doi.org/10.4067/S0717-9502201200...
,2323 Oballe HJ, Gaio EJ, Spuldaro T, Cavagni J, Gomez R, Rösing CK. Effects of alcohol and/or tobacco exposure on spontaneous alveolar bone loss in rat. Braz Dent J. 2014;25(3):197-202. PMID: 25252253.. Smoking leads to decreased bone mineral density, including the risk of osteoporosis in postmenopausal women2424 Lorentzon M, Mellstrom D, Haug E, Ohlsson C. Smoking is associated with lower bone mineral density and reduced cortical thickness in young man. J Clin Endocrinol Metab. 2007 Feb;92(2):497-503. PMID: 17077132.,2525 Giampietro PF, McCarty C, Mukesh B, McKiernan F, Wilson D, Shuldiner A, Liu J, LeVasseur J, Ivacic L, Kitchner T, Ghebranious N. The role of cigarette smoking and statins in the development of postmenopausal osteoporosis: a pilot study utilizing the Marshfield Clinic Personalized Medicine Cohort. Osteoporos Int. 2010 Mar;21(3):467-77. doi: 10.1007/s00198-009-0981-3.
https://doi.org/10.1007/s00198-009-0981-...
. This mechanism usually occurs due to osteocyte death, reduction of bone morphogenetic protein (BMP) and alkaline phosphatase concentration, consequently reducing bone mineral density2626 Soares EV, Favaro WJ, Cagnoh VH, Bertran CA, Camilli JA. Effects of alcohol and nicotine on the mechanical resistance of bone and bone neoformation around hydroxyapatite implants. J Bone Miner Metab. 2010;28(1):101-7. doi: 10.1007/s00774-009-0115-1.
https://doi.org/10.1007/s00774-009-0115-...
.
Placement of a graft material made from the inorganic bovine bone matrix provided the formation of new bone surrounding the particles, as well as the formation of a new cortical that was ruptured in the surgical act. The biomaterial used (GenOx) acts as an osteoconductive agent, being biocompatible, forming a scaffold for cell deposition and proliferation with osteoblastic activity2727 Marins LV, Cestari TM, Sottovia AD, Granjeiro JM, Taga R. Radiographic and histological study of perennial bone defect repair in rat calvaria after treatment with blocks of porous bovine organic graft material. J Appl Oral Sci. 2004 Mar;12(1):62-9. PMID: 21365154.,2828 Accorsi-Mendonça T, Conz MB, Barros TC, de Sena LA, Soares Gde A, Granjeiro JM. Physicochemical characterization of two deproteinized bovine xenografts. Braz Oral Res. 2008 Jan-Mar;22(1):5-10. PMID: 18425238..
In the histomorphometric analysis it was observed that, in the Cigarette Clot Group (CCG), there was a significant difference of new bone tissue formed in the analyzed periods (15 and 45 days), and in 15 days, there was 4.8 ± 0.42 of bone formation and 11.73 ± 0.59 (p <0.05) in 45 days. In the Cigarette Biomaterial Group (CBG) there were also significant differences between periods of 15 to 45 days, being respectively 6.16 ± 0.30 and 11.60 ± 0.61 (p <0.05). However, when the groups were compared, within the same periods analyzed, it was noted that bone neoformation was statistically significant only in the 15-day period, being higher in the CBG.
The graft with the biomaterial used in this study promoted osteoconduction which, in turn, refers to the ability of a surface to be favorable to adequate bone growth, usually through pores, channels or tubes. The biomaterial allows the recruitment and stimulation of pluripotent and undifferentiated cells into the form of bone-forming cells, such as pre-osteoblasts, osteoblasts, and ultimately osteocytes2929 Langhans MT, Yu S, Tuan RS. Stem cells in skeletal tissue engineering: technologies and models. Curr Stem Cell Res Ther. 2016;11(6):453-74. PMID: 26423296..
Future perspectives in this same line of research are evident in the range of biomaterials commercially produced worldwide that require detailed analysis in scientific research. In addition, the use of complementary therapies such as the low-level laser can help reduce physiological and functional recovery time by means of the photobiomodulatory effects that produce in the tissue in which it is applied3030 de Oliveira Gonçalves JB, Buchaim DV, de Souza Bueno CR, Pomini KT, Barraviera B, Júnior RSF, Andreo JC, de Castro Rodrigues A, Cestari TM, Buchaim RL. Effects of low-level laser therapy on autogenous bone graft stabilized with a new heterologous fibrin sealant. J Photochem Photobiol B. 2016 Sep;162:663-8. doi: 10.1016/j.jphotobiol.2016.07.023.
https://doi.org/10.1016/j.jphotobiol.201...
31 Buchaim RL, Andreo JC, Barraviera B, Ferreira Junior RS, Buchaim DV, Rosa Junior GM, de Oliveira AL, de Castro Rodrigues A. Effect of low-level laser therapy (LLLT) on peripheral nerve regeneration using fibrin glue derived from snake venom. Injury. 2015 Apr;46(4):655-60. doi: 10.1016/j.injury.2015.01.031.
https://doi.org/10.1016/j.injury.2015.01...
-3232 Franco GR, Laraia IO, Maciel AA, Miguel NM, Dos Santos GR, Fabrega-Carvalho CA, Pinto CA, Pettian MS, Cunha MR. Effects of chronic passive smoking on the regeneration of rat femoral defects filled with hydroxyapatite and stimulated by laser therapy. Injury. 2013 Jul;44(7):908-13. doi: 10.1016/j.injury.2012.12.022.
https://doi.org/10.1016/j.injury.2012.12...
.
Conclusion
The implanted bone matrix improved the process of reconstitution of the injured bone tissue in rats submitted to passive inhalation of cigarette smoke, mainly in the initial period of repair.
References
-
1Lee PN, Fariss MW. A systematic review of possible serious adverse health`effects of nicotine replacement therapy. Arch Toxicol. 2017 Apr;91(4):1565-94. doi: 10.1007/s00204-016-1856-y.
» https://doi.org/10.1007/s00204-016-1856-y -
2Almeida AÁ, Bandeira CM, Gonçalves AJ, Araújo AJ. Nicotine dependence and smoking habits in patients with head and neck cancer. J Bras Pneumol. 2014 May-Jun;40(3):286-93. PMID: 25029652.
-
3Torshabi M, Rezaei Esfahrood Z, Jamshidi M, Mansuri Torshizi A, Sotoudeh S. Efficacy of vitamins E and C for reversing the cytotoxic effects of nicotine and cotinine. Eur J Oral Sci. 2017 Dec;125(6):426-37. doi: 10.1111/eos.12375.
» https://doi.org/10.1111/eos.12375 -
4Bassetti MA, Bassetti RG, Sculean A, Salvi GE, Bornstein MM, Ramseier CA. The impact of brief interventions for tobacco cessation on patients' awareness of cigarette smoking as a risk factor for chronic periodontitis. Oral Health Prev Dent. 2017;15(4):391-7. doi: 10.3290/j.ohpd.a38737.
» https://doi.org/10.3290/j.ohpd.a38737 -
5Kallala R, Barrow J, Graham SM, Kanakaris N, Giannoudis PV. The in vitro and in vivo effects of nicotine on bone, bone cells and fracture repair. Expert Opin Drug Saf. 2013 Mar;12(2):209-33. doi: 10.1517/14740338.2013.770471.
» https://doi.org/10.1517/14740338.2013.770471 -
6Buchaim RL, Andreo JC, Rodrigues AC, Buchaim DV, Dias DV, Daré LR, Roque DD, Roque JS. The action of demineralized bovine bone matrix on bone neoformation in rats submitted to experimental alcoholism. Arq Bras Med Vet Zootec. 2013;65(3):715-721. doi: 10.1590/S0102-09352013000300016.
» https://doi.org/10.1590/S0102-09352013000300016 -
7Buchaim RL, Andreo JC, Rodrigues AC, Buchaim DV, Roque DD, Roque JS, Rosa Junior GM. Bovine bone matrix action associated with morphogenetic protein in bone defects in rats submitted to alcoholism. Int J Morphol. 2012 Mar;30(1):266-71. doi: 10.4067/S0717-95022012000100048.
» https://doi.org/10.4067/S0717-95022012000100048 -
8Shintcovsk RL, Knop L, Tanaka OM, Maruo H. Nicotine effect on bone remodeling during orthodontic tooth movement: Histological study in rats. Dental Press J Orthod. 2014;19(2):96-107. doi: 10.1590/2176-9451.19.2.096-107.oar.
» https://doi.org/10.1590/2176-9451.19.2.096-107.oar -
9Tamura K, Togo Y, Kaihara S, Hussain A, Takahashi K, Bessho K. The effect of nicotine on osteoinduction by recombinant human bone morphogenetic protein 2. Int J Oral Maxillofac Surg. 2014 Aug;43(8):1022-9. doi: 10.1016/j.ijom.2014.02.014.
» https://doi.org/10.1016/j.ijom.2014.02.014 -
10Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002 May;2(5):372-7. doi: 10.1038/nri803.
» https://doi.org/10.1038/nri803 -
11Marinucci L, Balloni S, Fettucciari K, Bodo M, Talesa VN, Antognelli C. Nicotine induces apoptosis in human osteoblasts via a novel mechanism driven by H(2)O(2) and entailing Glyoxalase 1-dependent MG-H1 accumulation leading to TG2-mediated NF-kB desensitization: Implication for smokers-related osteoporosis. Free Radic Biol Med. 2018 Jan 20;117:6-17. [Epub ahead of print]. doi: 10.1016/j.freeradbiomed.2018.01.017.
» https://doi.org/10.1016/j.freeradbiomed.2018.01.017 -
12Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I, Grigolo B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater Sci Eng C Mater Biol Appl. 2017 Sep 1;78:1246-62. doi: 10.1016/j.msec.2017.05.017.
» https://doi.org/10.1016/j.msec.2017.05.017 -
13Curry AS, Pensa NW, Barlow AM, Bellis SL. Taking cues from the extracellular matrix to design bone-mimetic regenerative scaffolds. Matrix Biol. 2016 May-Jul;52-54:397-412. doi: 10.1016/j.matbio.2016.02.011.
» https://doi.org/10.1016/j.matbio.2016.02.011 -
14Zizzari VL, Zara S, Tetè G, Vinci R, Gherlone E, Cataldi A. Biologic andm clinical aspects of integration of different bone substitutes in oral surgery: a literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016 Oct;122(4):392-402. doi: 10.1016/j.oooo.2016.04.010.
» https://doi.org/10.1016/j.oooo.2016.04.010 -
15Gupta P, Adhikary M, M JC, Kumar M, Bhardwaj N, Mandal BB. Biomimetic, osteoconductive non-mulberry silk fiber reinforced tricomposite scaffolds for bone tissue engineering. ACS Appl Mater Interfaces. 2016 Nov 16;8(45):30797-810. PMID: 27783501.
-
16Weibel ER, Kistler GS, Scherle WF. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23-38. PMID: 5338131.
-
17Baljoon M, Natto S, Bergström J. Long-term effect of smoking on vertical periodontal bone loss. J Clin Periodontol. 2005 Jul;32(7):789-97. PMID: 15966888.
-
18Pavone C, Nogueira AV, de Oliveira GJ, Scardueli CR, Cerri PS, Marcantonio E Jr, Marcantonio RA. Treatment of periodontal disease with an Er,Cr:YSGG laser in rats exposed to cigarette smoke inhalation. Lasers Med Sci. 2015 Nov;30(8):2095-103. doi: 10.1007/s10103-015-1731-8.
» https://doi.org/10.1007/s10103-015-1731-8 -
19Campos JM, Prati AJ, Cirano FR, Pimentel SP, Pastore GP, Pecorari VG, Ribeiro FV, Casati MZ, Casarin RC. Smoking modulates gene expression of type I collagen, bone sialoprotein, and osteocalcin in human alveolar bone. J Oral Maxillofac Surg. 2015 Nov;73(11):2123-31. doi: 10.1016/j.joms.2015.06.168.
» https://doi.org/10.1016/j.joms.2015.06.168 -
20da Silva RC, Crivellaro VR, Giovanini AF, Scariot R, Gonzaga CC, Zielak JC. Radiographic and histological evaluation of ectopic application of deproteinized bovine bone matrix. Ann Maxillofac Surg. 2016 Jan-Jun;6(1):9-14. doi: 10.4103/2231-0746.186150.
» https://doi.org/10.4103/2231-0746.186150 -
21Brito MV, Yasojima EY, Silveira EL, Yamaki VN, Teixeira RK, Feijó DH, Gonçalves TB. New experimental model of exposure to environmental tobacco smoke. Acta Cir Bras. 2013 Dec;28(12):815-9. PMID: 24316853.
-
22Cendon SP, Battlehner C, Lorenzi Filho G, Dohlnikoff M, Pereira PM, Conceição GM, Beppu OS, Saldiva PH. Pulmonary emphysema induced by passive smoking: an experimental study in rats. Braz J Med Biol Res. 1997 Oct;30(10):1241-7. PMID: 9496445.
-
23Oballe HJ, Gaio EJ, Spuldaro T, Cavagni J, Gomez R, Rösing CK. Effects of alcohol and/or tobacco exposure on spontaneous alveolar bone loss in rat. Braz Dent J. 2014;25(3):197-202. PMID: 25252253.
-
24Lorentzon M, Mellstrom D, Haug E, Ohlsson C. Smoking is associated with lower bone mineral density and reduced cortical thickness in young man. J Clin Endocrinol Metab. 2007 Feb;92(2):497-503. PMID: 17077132.
-
25Giampietro PF, McCarty C, Mukesh B, McKiernan F, Wilson D, Shuldiner A, Liu J, LeVasseur J, Ivacic L, Kitchner T, Ghebranious N. The role of cigarette smoking and statins in the development of postmenopausal osteoporosis: a pilot study utilizing the Marshfield Clinic Personalized Medicine Cohort. Osteoporos Int. 2010 Mar;21(3):467-77. doi: 10.1007/s00198-009-0981-3.
» https://doi.org/10.1007/s00198-009-0981-3 -
26Soares EV, Favaro WJ, Cagnoh VH, Bertran CA, Camilli JA. Effects of alcohol and nicotine on the mechanical resistance of bone and bone neoformation around hydroxyapatite implants. J Bone Miner Metab. 2010;28(1):101-7. doi: 10.1007/s00774-009-0115-1.
» https://doi.org/10.1007/s00774-009-0115-1 -
27Marins LV, Cestari TM, Sottovia AD, Granjeiro JM, Taga R. Radiographic and histological study of perennial bone defect repair in rat calvaria after treatment with blocks of porous bovine organic graft material. J Appl Oral Sci. 2004 Mar;12(1):62-9. PMID: 21365154.
-
28Accorsi-Mendonça T, Conz MB, Barros TC, de Sena LA, Soares Gde A, Granjeiro JM. Physicochemical characterization of two deproteinized bovine xenografts. Braz Oral Res. 2008 Jan-Mar;22(1):5-10. PMID: 18425238.
-
29Langhans MT, Yu S, Tuan RS. Stem cells in skeletal tissue engineering: technologies and models. Curr Stem Cell Res Ther. 2016;11(6):453-74. PMID: 26423296.
-
30de Oliveira Gonçalves JB, Buchaim DV, de Souza Bueno CR, Pomini KT, Barraviera B, Júnior RSF, Andreo JC, de Castro Rodrigues A, Cestari TM, Buchaim RL. Effects of low-level laser therapy on autogenous bone graft stabilized with a new heterologous fibrin sealant. J Photochem Photobiol B. 2016 Sep;162:663-8. doi: 10.1016/j.jphotobiol.2016.07.023.
» https://doi.org/10.1016/j.jphotobiol.2016.07.023 -
31Buchaim RL, Andreo JC, Barraviera B, Ferreira Junior RS, Buchaim DV, Rosa Junior GM, de Oliveira AL, de Castro Rodrigues A. Effect of low-level laser therapy (LLLT) on peripheral nerve regeneration using fibrin glue derived from snake venom. Injury. 2015 Apr;46(4):655-60. doi: 10.1016/j.injury.2015.01.031.
» https://doi.org/10.1016/j.injury.2015.01.031 -
32Franco GR, Laraia IO, Maciel AA, Miguel NM, Dos Santos GR, Fabrega-Carvalho CA, Pinto CA, Pettian MS, Cunha MR. Effects of chronic passive smoking on the regeneration of rat femoral defects filled with hydroxyapatite and stimulated by laser therapy. Injury. 2013 Jul;44(7):908-13. doi: 10.1016/j.injury.2012.12.022.
» https://doi.org/10.1016/j.injury.2012.12.022
-
Financial source:
none
-
1
Research performed at Laboratory of Human Morphology, Division of Anatomy, Faculty of Medicine, Universidade de Marília (UNIMAR), Brazil.
Publication Dates
-
Publication in this collection
Apr 2018
History
-
Received
08 Dec 2017 -
Reviewed
07 Feb 2018 -
Accepted
09 Mar 2018