Abstract
PURPOSE: To investigate the role of protein kinase G (PKG) in blocking post-shock mesenteric lymph (PSML) return ameliorating the calcium sensitivity in hemorrhagic shock rats. METHODS: Male Wistar rats were randomly divided into sham, shock, shock+ligation (shock plus mesenteric lymph duct ligation (MLDL)), shock+drainage (shock plus PSML drainage) groups. After shock (hypotension 40mmHg) for three hours or corresponding times, the superior mesenteric artery (SMA) was taken out for detecting the PKG and phospho PKG (p-PKG) contents, and the vascular rings of SMA were prepared for assaying the calcium sensitivity using an isolated organ perfusion system. RESULTS: The PKG and p-PKG contents of SMA in shock group were significantly increased than that of sham group, and MLDL or PSML drainage reducing the levels of PKG and p-PKG. Meanwhile, the vascular calcium sensitivity in shock group was significantly lower than that of sham group, MLDL or PSML drainage enhanced the calcium sensitivity. After incubating with PKG regulators in shock+ligation and shock+drainage groups, the PKG agonist 8Br-cGMP reduced the contractility of vascular rings to gradient calcium ions and Emax and the PKG inhibitor agonist KT5823 elevated the calcium sensitivity significantly. CONCLUSION: Protein kinase G plays an important role in post-shock mesenteric lymph blockage improving vascular calcium sensitivity.
Shock, Hemorrhagic; Protein Kinases; Calcium; Lymph, Rats
10 ORIGINAL ARTICLE
SHOCK
Role of protein kinase G on the post-shock mesenteric lymph blockage ameliorating vascular calcium sensitivity1 1 Research performed at Institute of Microcirculation, Hebei North University, Diamond South Road 11, Zhangjiakou Hebei, China.
Zi-gang ZhaoI; Yan-ling WeiII; Chun-yu NiuIII; Yu-ping ZhangIV; Li-min ZhangIV; Li-na JiangIV
IMaster, Full Professor, Institute of Microcirculation, Hebei North University, Zhangjiakou, China. Main author. Design of the study, manuscript writing, critical revision
IIMaster, Lecturer, Institute of Microcirculation, Hebei North University, Zhangjiakou, China. Acquisition of data
IIIPhD, Full Professor, Institute of Microcirculation, Hebei North University, Zhangjiakou, China. Main author. Design of the study, critical revision
IVMaster, Lecturer, Institute of Microcirculation, Hebei North University, Zhangjiakou, China. Acquisition of data
Correspondence Correspondence: Chun-yu Niu Institute of Microcirculation, Hebei North University Zhangjiakou Hebei, 075000, China Phone: (86)0313-4029168 ncylxf@126.com
ABSTRACT
PURPOSE: To investigate the role of protein kinase G (PKG) in blocking post-shock mesenteric lymph (PSML) return ameliorating the calcium sensitivity in hemorrhagic shock rats.
METHODS: Male Wistar rats were randomly divided into sham, shock, shock+ligation (shock plus mesenteric lymph duct ligation (MLDL)), shock+drainage (shock plus PSML drainage) groups. After shock (hypotension 40mmHg) for three hours or corresponding times, the superior mesenteric artery (SMA) was taken out for detecting the PKG and phospho PKG (p-PKG) contents, and the vascular rings of SMA were prepared for assaying the calcium sensitivity using an isolated organ perfusion system.
RESULTS: The PKG and p-PKG contents of SMA in shock group were significantly increased than that of sham group, and MLDL or PSML drainage reducing the levels of PKG and p-PKG. Meanwhile, the vascular calcium sensitivity in shock group was significantly lower than that of sham group, MLDL or PSML drainage enhanced the calcium sensitivity. After incubating with PKG regulators in shock+ligation and shock+drainage groups, the PKG agonist 8Br-cGMP reduced the contractility of vascular rings to gradient calcium ions and Emax and the PKG inhibitor agonist KT5823 elevated the calcium sensitivity significantly.
CONCLUSION: Protein kinase G plays an important role in post-shock mesenteric lymph blockage improving vascular calcium sensitivity.
Key words: Shock, Hemorrhagic. Protein Kinases. Calcium. Lymph, Rats.
Introduction
One of the hallmarks of severe shock is refractory hypotension, during which the arteries lose responsiveness to vasoactive agents, such as norepinephrine (NE), epinephrine, and dopamine1. The vascular hyporeactivity is an important factor in irreversible shock2, meanwhile calcium desensitization is one of the mechanisms of vascular hyporeactivity3,4. Our previous study showed that the mesenteric lymph duct ligation (MLDL) and mesenteric lymph drainage (MLD), which blocking the post-shock mesenteric lymph (PSML) return, both improved the reactivity and calcium sensitivity of vascular rings isolated from severe hemorrhagic shock rats5. But the mechanism by which PSML blunts the vascular reactivity and calcium sensitivity remain unclear.
Protein kinase G (PKG) may phosphorylate Telokin (kinase-related protein of 17kDa), which has a similar amino acid sequence as myosin light chain kinase (MLCK) at COOH terminal with 156 amino acid, and promotes the dephosphorylation of myosin light chain 20 (MLC20), which is closed associated with the calcium sensitization6. The previous studies showed that PKG was involved in the calcium desensitization of vascular smooth muscle after hemorrhagic shock, which was possibly related to MLCK3,7. Meanwhile, an increase in PKG activity results in hypotension and vascular hyporeactivity in rats with endotoxemia8. Hence, PKG plays an important role on the regulation of calcium sensitivity. But whether PKG involved in PSML blockage improving calcium sensitivity is still unknown.
Therefore, to elucidate the role of PKG and gain more understanding about the mechanisms of PSML inducing vascular calcium desensitization, the superior mesenteric artery (SMA) obtained from hemorrhagic shock rats was used in the present study to investigate the role of PKG on PSML blockage increasing vascular calcium sensitivity in hemorrhagic shock.
Methods
One hundred and eight adult male Wistar rats weighing 260g to 280g were purchased from the Animal Breeding Center of the Chinese Academy of Medical Sciences (Beijing, China). These rats were fasted for 12h but allowed free access to water before the experiment. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee in Hebei North University according to the ethical code for animal experimentation (WHO Chronicle 1985;39(2):51-6). Meanwhile, the experimental protocol was conformed to the guidelines of ethical use of animals, and suffering was made to minimize.
Experimental protocol
The rats were randomly divided into: sham, shock, shock+ligation and shock+drainage groups. Among them, the hemorrhagic shock model was established in shock, shock+ligation and shock+drainage groups, and the MLDL or MLD were performed from the shock immediate to 3 h in shock+ligation group or shock+drainage group, respectively. Finally, the SMA were obtained from the sham-operated or shocked rats, the calcium sensitivity of SMA was determined in sham (n=6), shock (n=6), shock+ligation (n=18) and shock+drainage (n=18) groups; the rest of SMA tissue was used for the measurement of PKG and p-PKG levels (n=15/group).
Surgical procedures
After rats were anesthetized with pentobarbital sodium (1%, 50 mg/kg), the right femoral vein and artery were isolated, and heparin sodium (500 U/kg) was injected via the vein to prevent blood coagulation systematically, while a minimally heparinized polyethylene catheter were introduced into the artery to continuously monitor mean arterial pressure (MAP). The left femoral artery was also isolated and cannulated and attached in line to an NE-1000 automatic withdrawal-infusion machine (New Era Pump Systems Inc., Monrovia, California, USA) for the hemorrhage. A 3-cm midline laparotomy was performed to isolate the mesenteric lymph duct from the surrounding connective tissues by blunt dissection. After operation, all rats were allowed to stabilize for 30 min. Rats in the shock, shock+ligation and shock+drainage groups were hemorrhaged from the left femoral artery to produce a MAP of 40 mmHg within 10 min, slowly and uniformly. The MAP was maintained at 40±2 mmHg for 3h by withdrawing or returning shed blood as required for the preparation of hemorrhagic shock model3,5,7. When the MAP reached 40 mmHg, the mesenteric lymph duct was ligated in rats in the shock+ligation group; the mesenteric lymphatic duct was cannulated for lymph drainage using a homemade bending needle in rats in the shock+drainage group. Rats in the sham group were anesthetized and cannulated as described above, but no bleeding. After the in vivo experiments described above, the SMA was obtained from each group rats, and the adhering tissues were cleaned off carefully for the following experiments.
Western blotting analysis
Nine SMAs from each group were randomly divided into three groups (n=3). The proteins were extracted using a protein extraction kit and were quantified with the bicinchoninic acid (BCA) method. Total protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF blotting membranes. Following blocking with 5% skim milk for 1.5h, the membranes were incubated with 1,000-fold diluted anti-PKG which is a rabbit monoclonal antibody ( Enzo, USA) at room temperature for 30 min and then incubated overnight at 4°C. The blots were reprobed with β-actin (internal control) with a rabbit polyclonal antibody (1:10000 dilution, 30-min incubation). The membranes were washed five times with TBS-T, incubated with secondary goat anti-rabbit antibody at room temperature for 40 min, and washed five more times. The blots were developed using an ECL imaging system and grayscale images were captured and analyzed. The amount of protein was presented as the integrated optical density (IOD)/β-actin.
ELISA analysis
The SMA was triturated in liquid nitrogen and immigrated into EP tube with 0.2 ml lysis buffer (Tritox-100 100 μl, PMSF 100 μl, AProtein 10 μl, Leupeptin 10.1 μl, Pepstatin 0.707 μl, and adding PBS buffer solution to 10 ml), and was homogenized using an SM-6500 ultrasonic cell disruptor (Shunma Instrument Equipment Inc., Nanjing, China) for 15 min. Then, the homogenate was centrifuged at 14.000 g for 5 min at 4°C using Labofuge 400R supercentrifuge (Thermo Fisher Scientific, San Jose, California, USA), and the supernatant was collected. The phospho-PKG (p-PKG) levels in SMA tissues (n=6 per group) were determined using a rat p-PKG ELISA kit (R&D Systems, USA) after a standard curve had been plotted (y = 0.036x + 0.000000589x3, r2 = 0.998). Protein quantification was performed using the Coomassie brilliant blue colorimetric method for each sample.
Measurement of calcium sensitivity
SMA ring was identified and transfered into the chamber of a wire myograph system and two stainless-steel wire hooks were cannulated through the lumen of SMA ring. One connected with a micrometer and the other was linked to a force transducer, then the SMA ring was immersed into Krebs-Hensley (K-H) solution (in mmol/L): 118 NaCl, 4.7 KCl, 1.2 MgSO4, 25 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 11 Glucose at pH 7.3-7.4, which was continuously bubbled with 95% O2/5% CO2 and its temperature was maintained at 37°C. A 0.5-g preload was exerted and the K-H solution was replaced every 20 min. The tension of the SMA ring was determined using a Power Lab System.
After 1.5h equilibration, the solution was replaced with depolarizing solution containing (in mmol/L): 2.7 NaCl, 120 KCl, 1.2 MgSO4, 25 NaHCO3, 1.2 KH2PO4, 11 Glucose; pH 7.3-7.4. After 15-min equilibration, the contractile responses of SMA rings to Ca2+ (3×10-5, 1×10-4, 3×10-4, 1×10-3, 3×10-3, 1×10-2 and 3×10-2 mol/L) in each group (n=6) were determined using a concentration accumulation method as described previously3,7. Tension/vascular ring wet weight (g/mg) was calculated and cumulative concentration-response curves for the responses of artery rings to Ca2+ were generated. The values of maximal contraction (Emax) and pD2 (-log 50% effective concentration) were obtained from the concentration-response curves and were used to compare calcium sensitivity9,10.
Others SMA rings obtained form rats in shock+ligation and shock+drainage groups were incubated with 8-Bromo-cGMP (8Br-cGMP, 100 μmol/L) (Alexis Inc, Lausen, Switzerland) and KT5823 (1 μmol/L, Alexis Inc), n=6, respectively, for 10 min, then the calcium sensitivity of SMA were observed as described above. The dosages of 8Br-cGMP and KT5823 used in this study were according to the previous reports3.
Statistical analysis
All data were shown as the mean ± standard deviation (SD) and were analyzed using SPSS version 16.0 software (SPSS Inc, Chicago, USA). The difference between experimental groups was analyzed by one-way analysis of variance, followed by Kruskal-Wallis test. P<0.05 was considered significant.
Results
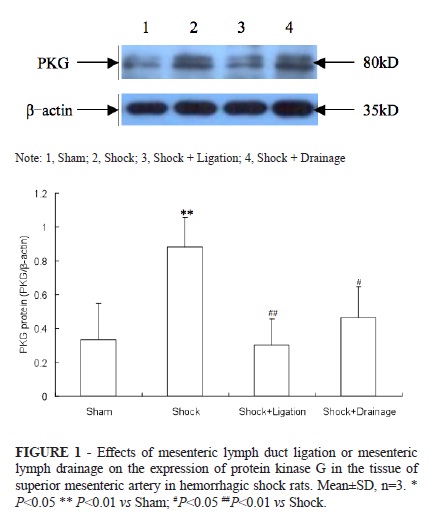
Effect of MLDL or MLD on PKG and p-PKG levels of SMA tissue in hemorrhagic shock rats
The levels of PKG and p-PKG in SMA tissues of shock group were significantly increased compared with that of the sham group (Figures 1 and 2); the levels of PKG and p-PKG in shock+ligation and shock+drainage groups were observably decreased than that of shock group.
Effects of PKG on MLDL enhancing vascular calcium sensitivity in hemorrhagic shock rats
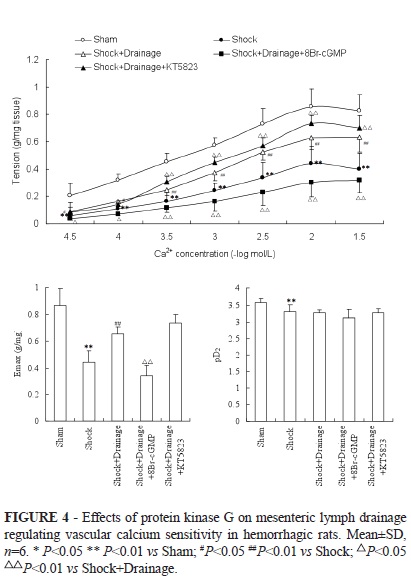
The contractile tension of SMA to gradient Ca2+ and the values of Emax, pD2 in shock group were remarkably decreased compared with the sham group (Figure 3); and the tension and Emax of SMA in shock+ligation shock were observably increased than that of the shock group. After vascular rings obtained from shock+ligation groups incubated with tool agents, KT5823 significantly enhanced the contractile response to Ca2+ from the concentration of 3×10-4 mol/L and the Emax; meanwhile, 8Br-cGMP significantly declined the vascular tension to Ca2+ at any concentrations and the values of Emax and pD2.
Effects of PKG on MLD enhancing vascular calcium sensitivity in hemorrhagic shock rats
The contractile response to gradient Ca2+ of SMA obtained from rats in shock+drainage group was significantly increased compared with the shock group, moreover, so do the Emax (Figure 4). After SMA rings in shock+ligation groups incubated with 8Br-cGMP, the reactivity to gradient Ca2+ and the Emax, pD2 were remarkably decreased. Meanwhile, after SMA rings in shock+ligation groups incubated with KT5823, the response to gradient Ca2+ was remarkably increased.
Discussion
The studies aimed to pathogenesis of shock vascular hypo-reactivity found that vascular contractile activity not only depends on the concentration of Ca2+ in the VSMC, but also has a close relationship with the efficiency of Ca2+ utilization. The concentration of Ca2+ in the VSMC is not lowered but overloaded in the late stage of hemorrhagic shock, which suggests that the reduced calcium sensitivity involves in the pathogenesis of shock induced vascular hypo-reactivity3,4. On the basis of an animal experiment that the PSML return involved in the vascular hypo-reactivity and calcium desensitization induced by hemorrhagic shock5, the present paper documented that the mesenteric lymph duct ligation or mesenteric lymph drainage decreased the elevated expression of PKG protein and activity of p-PKG induced by hemorrhagic shock in the tissue of SMA. The present experiment indicates that the mechanism by which prevention of PSML return ameliorates vascular calcium sensitivity may be associated with PKG.
Using the technique of vascular ring strain measurement in vitro, we found that the calcium sensitivity of SMA in shock group was significantly reduced compared with sham group, the calcium sensitivity of SMA in shock+ligation and shock+drainage groups were significantly increased than that of shock group at several concentration points, these results are consistent with our previous study5. The SMA rings obtained from rats subjected to mesenteric lymphatic duct ligation or mesenteric lymph drainage were incubated with tool agents of PKG, the results showed that 8Br-cGMP, an agonist of PKG, decreased the contractility of SMA to calcium gradient concentration as well as the Emax values in varying degrees in both groups; KT5823, a selective inhibitor of PKG, elevated the contractility reactivity of SMA rings to gradient calcium and the Emax values in shock+ligation and shock+drainage groups. These results suggested that PKG play a certain role in the increasing of vascular calcium sensitivity by blockading the PSML return.
Our results also found that KT5823 had no obvious effect in pD2 on SMA obtained from shock+ligation and shock+drainage groups; it indicated that there were the other factors excepted to PKG. Previous studies showed that there were many factors related to the calcium desensitization, such as Rho-kinase, protein kinase C (PKC), PKG, etc3,9,10. Meanwhile, there was interaction effect of Rho-kinase and PKC on regulating vascular contraction11. Our previous experiments found that there were effects of Rho-kinase, PKC on blocking the return of PSML improving calcium sensitivity, respectively12,13. But, whether there are interaction effects among these signal molecules during the PSML decreasing the calcium sensitivity following hemorrhagic shock, it still not clears. At the same time, whether there are effects of these drugs on animal, it is unknown. Therefore, more studies need to confirm because this work was only a preliminary study.
It should be pointed out that the hemorrhagic shock model employed in this study was controlled without fluid resuscitation, which may have less direct clinical significance for patients with hemorrhagic shock. However, it should also be noted that a number of shock patients do not receive timely fluid resuscitation14-16 and thus we felt that it was essential to determine the alterations during shock. Nonetheless, further studies are needed to investigate the mechanism of blocking PSML return improving the calcium sensitivity in hemorrhagic shock model with fluid resuscitation.
Conclusion
Protein kinase G plays an important role in post-shock mesenteric lymph blockage improving vascular calcium sensitivity.
Received: March 25, 2013
Review: May 27, 2013
Accepted: June 24, 2013
Conflict of interest: none
Financial source: National Natural Science Foundation of China (30971203)
- 1. Zhao KS, Huang X, Liu J, Huang Q, Jin C, Jiang Y, Jin J, Zhao G. New approach to treatment of shock-restitution of hyporeactivity. Shock. 2002;18(2):189-92.
- 2. Zhao Q, Zhao KS. Inhibition of L-type calcium channels in arteriolar smooth muscle cells is involved in the pathogenesis of vascular hyporeactivity in severe shock. Shock. 2007;28(6):717-21.
- 3. Xu J, Liu L. The role of calcium desensitization in vascular hyporeactivity and its regulation after hemorrhagic shock in the rat. Shock. 2005;23(6):576-81.
- 4. Sandoval RJ, Injeti ER, Gerthoffer WT, Pearce WJ. Postnatal maturation modulates relationships among cytosolic Ca2+, myosin light chain phosphorylation, and contractile tone in ovine cerebral arteries. Am J Physiol Heart Circ Physiol. 2007;293(4):H2183-92.
- 5. Zhao ZG, Niu CY, Wei YL, Zhang YP, Si YH, Zhang J. Mesenteric lymph return is an important contributor to vascular hyporeactivity and calcium desensitization after hemorrhagic shock. Shock. 2012;38(2):186-95.
- 6. Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275(28):21722-9.
- 7. Yang G, Liu L, Xu J, Li T. Effects of MCI-154 on vascular reactivity and its mechanisms after hemorrhagic shock in rats. J Cardiovasc Pharmacol. 2006;47(6):751-7.
- 8. Korkmaz B, Buharalioglu K, Sahan-Firat S, Cuez T, Tuncay Demiryurek A, Tunctan B. Activation of MEK1/ERK1/2/iNOS/sGC/PKG pathway associated with peroxynitrite formation contributes to hypotension and vascular hyporeactivity in endotoxemic rats. Nitric Oxide. 2011;24(3):160-72.
- 9. Hu Y, Li T, Tang XF, Chen K, Liu L. Effects of ischemic preconditioning on vascular reactivity and calcium sensitivity after hemorrhagic shock and their relationship to the Rho A-Rho-kinase pathway in rats. J Cardiovasc Pharmacol. 2011;57(2):231-9.
- 10. Xu J, Li T, Yang GM, Liu LM. Protein kinase C isoforms responsible for the regulation of vascular calcium sensitivity and their relationship to integrin-linked kinase pathway after hemorrhagic shock. J Trauma. 2010;69(5):1274-81.
- 11. Kandabashi T, Shimokawa H, Miyata K, Kunihiro I, Eto Y, Morishige K, Matsumoto Y, Obara K, Nakayama K, Takahashi S, Takeshita A. Evidence for protein kinase C-mediated activation of Rho-kinase in a porcine model of coronary artery spasm. Arterioscler Thromb Vasc Biol. 2003;23(12):2209-14.
- 12. Zhao ZG, Niu CY, Wei YL, Zhang YP, Si YH, Zhang J. Role of Rho kinase in blocking the return of mesenteric lymph to improve vascular calcium sensitivity in hemorrhagic shock rats. Zhongguo Bing Li Sheng Li Zazhi. 2012;28(1):11-5.
- 13. Niu CY, Zhao ZG, Wei YL, Zhang YP, Zhang J. Involvement of protein kinase C in enhancement of vascular calcium sensitivity by blocking mesenteric lymph return in hemorrhagic shock rats. Sheng Li Xue Bao. 2012;64(2):213-9.
- 14. Xiao N, Wang XC, Diao YF, Liu R, Tian KL. Effect of initial fluid resuscitation on subsequent treatment in uncontrolled hemorrhagic shock in rats. Shock. 2004;21(2):276-80.
- 15. Radhakrishnan RS, Xue H, Weisbrodt N, Moore FA, Allen SJ, Laine GA, Cox CS Jr. Resuscitation-induced intestinal edema decreases the stiffness and residual stress of the intestine. Shock. 2005;24(2):165-70.
- 16. Santry HP, Alam HB. Fluid resuscitation: past, present, and future. Shock. 2010;33(2):229-41
Correspondence:
Publication Dates
-
Publication in this collection
01 July 2013 -
Date of issue
July 2013
History
-
Received
25 Mar 2013 -
Accepted
24 June 2013 -
Reviewed
27 May 2013