Abstracts
A small circuit system of anesthesia was developed by Fonseca and Goldenberg in 1993. The authors used in this study New Zealand White (NZW) rabbits under closed system anesthetic regiment by insoflurane. Twenty male adult New Zealand rabbits were distributed in two groups of ten animals. No premedicant drugs were given. Endotraqueal intubation was made after intravenous administration of propofol (10mg/kg). Insoflurane was used to anesthesia management, administred by lowflow closed system technique with cooper kettle vaporizer, fixed by pre-calculated vaporizing flow in double times intervals. The group II underwent surgical periostal scratching in the medial tibial surface at the proximal shaft. Rabbits breathed spontaneously. Hypotensio, hypercapnia and respiratory acidosis were characteristic of the cardiopulmonary effects of the anesthesia. The corneal reflex and pinch reflex was useful as reliable indicators of anesthesic depth. Manual or mechanical ventilation should be considered as a way of improving alveolar ventilation and normalize blood-gas values. The system developed by Fonseca and Goldenberg was considered suitable for anesthesic management in rabbits.
Anesthesia; Anesthesia; Rabbits
Um sistema circular de anestesia para animais de pequeno porte foi desenvolvido por FONSECA e GOLDENBERG, em 1993. No presente estudo foram utilizados 20 coelhos brancos linhagem Nova Zelândia, submetidos ao sistema com isoflurane, distribuidos em dois grupos de 10 animais. O grupo I, controle, que foi apenas anestesiado e o grupo II foi submetido a raspagem periostal da parte proximal e medial da tíbia. Não foi administrada droga pré-anestésica. Após administração do propofol intravenoso (10mg/kg) procedeu-se a intubação endotraqueal. O isuflorane foi administrado lentamente no sistema circular de anestesia. Os animais respiravam espontaneamente. Houve efeitos cardiorespiratórios conseqüentes da anestesia, tais como, hipotensão, hipercapnia e acidose respiratória. O controle do plano anestésico foi realizado mediante os reflexos corneano e pelo pinçamento. O sistema desenvolvido por FONSECA e GOLDENBERG foi considerado satisfatório para anestesia em coelhos.
Anestesia; Anestesia; Coelhos
6 - ORIGINAL ARTICLE
AN EVALUATION OF NEW CIRCLE SYSTEM OF ANESTHESIA. QUANTITATIVE ANESTHESIA WITH ISOFLURANE IN NEW ZEALAND RABBITS1 1 Study developed in the Experimental Surgery of Federal University of São Paulo - Paulista School of Medicine, SP, Brazil. Presented as a Doctorate thesis. 2 Professor, Department of Surgery and Anesthesiology, Federal University of Uberlândia, MG, Brazil. 3 Professor of Experimental Surgery, Department of Surgery, Federal University of São Paulo - Paulista School of Medicine, SP, Brazil. 4 Professor, Department of Animal Medicine, Federal University of Uberlândia, MG, Brazil. 5 Professor of Statistic, Faculty of Medicine of Sorocaba - Catholic University of SP, Brazil.
Neuber M. Fonseca2 1 Study developed in the Experimental Surgery of Federal University of São Paulo - Paulista School of Medicine, SP, Brazil. Presented as a Doctorate thesis. 2 Professor, Department of Surgery and Anesthesiology, Federal University of Uberlândia, MG, Brazil. 3 Professor of Experimental Surgery, Department of Surgery, Federal University of São Paulo - Paulista School of Medicine, SP, Brazil. 4 Professor, Department of Animal Medicine, Federal University of Uberlândia, MG, Brazil. 5 Professor of Statistic, Faculty of Medicine of Sorocaba - Catholic University of SP, Brazil.
Saul Goldenberg3 1 Study developed in the Experimental Surgery of Federal University of São Paulo - Paulista School of Medicine, SP, Brazil. Presented as a Doctorate thesis. 2 Professor, Department of Surgery and Anesthesiology, Federal University of Uberlândia, MG, Brazil. 3 Professor of Experimental Surgery, Department of Surgery, Federal University of São Paulo - Paulista School of Medicine, SP, Brazil. 4 Professor, Department of Animal Medicine, Federal University of Uberlândia, MG, Brazil. 5 Professor of Statistic, Faculty of Medicine of Sorocaba - Catholic University of SP, Brazil.
Duvaldo Eurides4 1 Study developed in the Experimental Surgery of Federal University of São Paulo - Paulista School of Medicine, SP, Brazil. Presented as a Doctorate thesis. 2 Professor, Department of Surgery and Anesthesiology, Federal University of Uberlândia, MG, Brazil. 3 Professor of Experimental Surgery, Department of Surgery, Federal University of São Paulo - Paulista School of Medicine, SP, Brazil. 4 Professor, Department of Animal Medicine, Federal University of Uberlândia, MG, Brazil. 5 Professor of Statistic, Faculty of Medicine of Sorocaba - Catholic University of SP, Brazil.
Neil F. Novo5 1 Study developed in the Experimental Surgery of Federal University of São Paulo - Paulista School of Medicine, SP, Brazil. Presented as a Doctorate thesis. 2 Professor, Department of Surgery and Anesthesiology, Federal University of Uberlândia, MG, Brazil. 3 Professor of Experimental Surgery, Department of Surgery, Federal University of São Paulo - Paulista School of Medicine, SP, Brazil. 4 Professor, Department of Animal Medicine, Federal University of Uberlândia, MG, Brazil. 5 Professor of Statistic, Faculty of Medicine of Sorocaba - Catholic University of SP, Brazil.
Cirilo A. P. Lima4 1 Study developed in the Experimental Surgery of Federal University of São Paulo - Paulista School of Medicine, SP, Brazil. Presented as a Doctorate thesis. 2 Professor, Department of Surgery and Anesthesiology, Federal University of Uberlândia, MG, Brazil. 3 Professor of Experimental Surgery, Department of Surgery, Federal University of São Paulo - Paulista School of Medicine, SP, Brazil. 4 Professor, Department of Animal Medicine, Federal University of Uberlândia, MG, Brazil. 5 Professor of Statistic, Faculty of Medicine of Sorocaba - Catholic University of SP, Brazil.
FONSECA, N.M. GOLDENBERG, S.; EURIDES, D.; NOVO, N.F.; LIMA, C.A.P. - An evaluation of new circle system of anesthesia: quantitative anesthesia with insoflurane in New Zealand rabbits. Acta Cir. Bras. 12(4): 240-5, 1997.
SUMMARY: A small circuit system of anesthesia was developed by Fonseca and Goldenberg in 1993. The authors used in this study New Zealand White (NZW) rabbits under closed system anesthetic regiment by insoflurane. Twenty male adult New Zealand rabbits were distributed in two groups of ten animals. No premedicant drugs were given. Endotraqueal intubation was made after intravenous administration of propofol (10mg/kg). Insoflurane was used to anesthesia management, administred by lowflow closed system technique with cooper kettle vaporizer, fixed by pre-calculated vaporizing flow in double times intervals. The group II underwent surgical periostal scratching in the medial tibial surface at the proximal shaft. Rabbits breathed spontaneously. Hypotensio, hypercapnia and respiratory acidosis were characteristic of the cardiopulmonary effects of the anesthesia. The corneal reflex and pinch reflex was useful as reliable indicators of anesthesic depth. Manual or mechanical ventilation should be considered as a way of improving alveolar ventilation and normalize blood-gas values. The system developed by Fonseca and Goldenberg was considered suitable for anesthesic management in rabbits.
SUBJECT HEADINGS: Anesthesia, animals. Anesthesia, equipment. Rabbits.
INTRODUCTION
Rabbits are used widely in biomedical research, but their use in surgery is limited due to problems encountered during anesthesia. Rabbits are often considered to be the most difficult laboratory animal to anesthetize successfully, in part due to the difficulty in endotracheal intubation, because of the anatomical conformation of the oral cavity that impede visualization of the larynx and also due to their susceptibility to respiratory arrest once adequate surgical anesthesia has been achieved.
Inhalation anesthesia has gained wide acceptance as a method of choice to provide moderate to long periods of anesthesia in rabbits. Inhalation agents has been traditionally administrated by use of relatively high calculated flow rate, on the basis of personal preference and habit and also difficult to anesthetic apparatus equipments.
Fonseca and Goldenberg8 in 1993 developed a circuit system to use in closed system of anesthesia (Fig 1). This study was designed to evaluate the use of this system in New Zealand White (NZW) Rabbits under close system anesthetic regimen with isoflurane.
- Anesthetic closed system by Fonseca and Goldenberg8. Cylindrical canister (1), two rubber breathing tubes (2), breathing bag (3), valves (4) and light rubber Y piece.
METHOD
Twenty adult NZW rabbits, male, weighing 2.0 to 2.9 kg were used for this project. The rabbits were quarantined 2 to 7 days prior to the use to permit adaptation to environmental conditions, food and water, and to allow daily evaluation of health status. During quarantine they were housed singly in stainless steel cages measuring 80 x 80 x 30 cm . The animals were fed commercial rabbit diet and water provided ad libitum. The photoperiod consisted of light and darkness with no twilight.
To provide access for direct blood samples and arterial blood samples, an 20-gauge catheter was implanted in the left auricular caudal ear artery. The arterial catheter was filled with fresh heparinized saline. The 1 ml blood sample was withdrawn into a preheparinized syringe for blood gas analyzer prior to induction of anesthesia and also 10 and 20 minutes of closed system of anesthesia.
The baseline mean arterial blood pressure, heart and respiratory rates, temperature were determined for each rabbit. Baseline measurements were taken prior to administration of any pharmaceutical agents. Blood pressure was determined by attaching the exposed end of the left auricular caudal catheter to a pressure mercury manometer via saline filled polyethylene tubing .
Heart rates were determined by eletrocardiografic (ECG) and respiratory rates were measured by auscultation.
The left marginal ear vein were catheterized with 20-gauge catheter. The catheter were secured with adhesive tape, used to infuse lactat ringer in glucose 5% (5 ml/kg/minute) and for administration of the anesthetic agent.
After control measurements were taken, the rabbits were distributed in two groups of ten animals. Group I were only anesthesed and group II also underwent a surgery. All rabbits were anesthetic in same technique.
No premedicant drugs were given
Anesthesia was induced with propofol 0,5% (< 10 mg.kg) as intravenous infusion. The dosages were based on the sufficient dose to immobilize the rabbit.
Restraint of the animals was made in a wooden box with only the head exposed until administration of intravenous agent, when it was removed to animal board.
The animals tracheas were intubated with an oral tube (external diameter, 3 - 4,5 mm) chosen to fit the trachea using a pediatric laryngoscope of size 1 blade use to expose the larynx and the rabbits placed in a supine position with head hanging backwards a 45o angle to the body. The tube was connect to the system circle of anesthesia (Time "O") and started administration of inhalation agent anesthetic.
Isoflurane was used to anesthesia management, administrated by lowflow closed system technique with copper kettle vaporizer, fixed by pre-calculated vaporizing flow in double time intervals14 . Additional flow of oxygen was administrated by low flowmeter. The interval of anesthesia consisted of 0 to 36 minute.
Rabbits breathing was managed spontaneously or manual if apnea.
Group II was anesthesed and underwent surgical periostal scratching in the medial tibia surface at the proximal shaft after 16 minute of close system of anesthesia.
Heart rates were determined by EKG at 5 minute intervals until the end of the anesthesia.
Respiratory rate was determined by auscultation at 5 minute intervals until the end of the anesthesia.
The corneal reflex was tested by placing a moistened cotton on the cornea, and ranked as absent (-) or present (+) at 5 minute intervals until the end of the anesthesia.
Assessment of pedal withdrawal reflexes was performed by applying a compression force in a alligator clip that had the teeth dulled. The force of the clip was calculated and found to be 0,5 kgf (4,9 N). This was applied for up to 5 seconds between fourth and fifth digit on the left pedal. Any movement was considered to be a positive response and no movement was considered to be a negative response. Were monitored at 5 minute intervals until the end of the anesthesia.
Arterial blood pH, partial pressure of oxygen (PaO2) and partial pressure of carbon dioxide (PaCO2) were determined from arterial blood samples prior to induction of anesthesia and also 10 and 20 minutes of closed system of anesthesia. The blood gas values were corrected for the rabbits body temperature, obtained with an electronic thermometer inserted 4-5 cm in the rectum.
The body temperature was determined at 10 minute intervals following induction of anesthesia.
The cardiopulmonary parameters and reaction to noxious stimuli were monitored at 5 minute intervals until the end of the anesthesia.
The time required for the return of the corneal and pedal reflexes, extubantion and spontaneous jumping were recorded.
Data for mean arterial blood pressure, body temperature, respiratory and heart rates were analyzed using two-way analysis of variance (ANOVA). The single t-test for paired differences was used to compare mean arterial blood pressure, body temperature, respiratory and heart rates. Data of administration and consume of inalatory agent, arterial blood pH, partial pressure of oxygen (PaO2) and partial pressure of carbon dioxide (PaCO2) by Freedman Test. To compare the same data between groups, Mann-Whitney Test was used. The Fishers Exact Test were used to compare the presence of corneal and pedal withdrawal reflex between groups. The G Cochran test was used to compare the reaction to noxious stimuli between groups For all of the statistical analyses, a p value of less than of 0,05 was considerated significant.
RESULTS
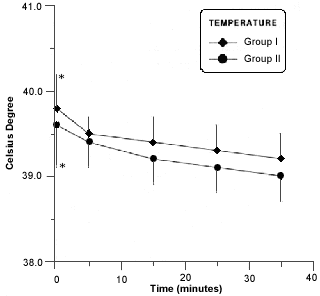
The degree of temperature was observed during anesthetic procedure (Fig. 2).
- Body temperature in rabitts of group I and II during anesthesia procedure. Asterisk (*) indicates a significant difference (p £ 0,05) in values of the same group.
There was a marked and statistically significant decrease in respiratory rate in all rabbits in both groups that began at 5 minutes of anesthesia and persisted during all procedure (Fig 3).
- Mean respiratory rates in rabitts of group I and II before, during and after anesthesia procedure. Asterisk (*) indicates a significant difference (p £ 0,05) in values of the same group.
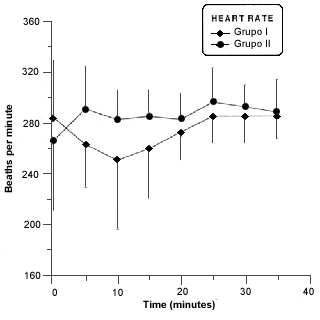
There wasnt a statistically significant difference in heart rate (Fig. 4).
- Mean heart rates in rabitts of group I and II before and during anesthesia procedure. Asterisk (*) indicates a significant difference (p £ 0,05) in values of the same group.
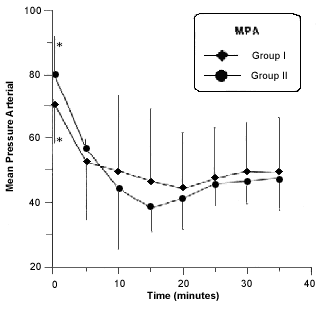
There was a significant decrease in MAP after 10 minutes of anesthesia in both groups. The hypotensive state continued throughout testing (Fig. 5).
- Mean bood pressure in rabitts of group I and II before and during anesthesia procedure. Asterisk (*) indicates a significant difference (p £ 0,05) in values of the same group.
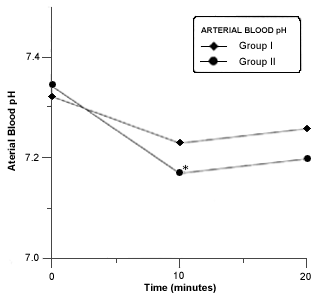
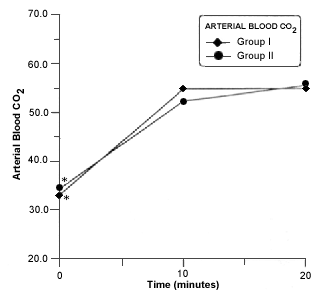
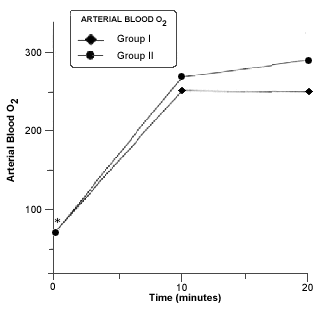
Respiratory acidosis occurred in all rabbits in closed system of anesthesia. A significant decrease in blood pH was seen in group II at 10 minutes of closed system anesthesia (Fig. 6).The PaCO2 was significantly increased at 10 and 20 minutes in both groups (Fig.7). A Marked and significant increased in PaO2 was seen in all groups (Fig. 8).
- Mean arterial bood pH in rabitts of group I and II before and during anesthesia procedure. Asterisk (*) indicates a significant difference (p £ 0,05) in values in values of the same group.
- Mean arterial bood pO2 in rabitts of group I and II before and during anesthesia procedure. Asterisk (*) indicates a significant difference (p £ 0,05) in values of the same group.
There was a complete loss of corneal and pedal withdrawal reflexes (Table I) after 5 minutes of anesthesia. No significant diference was observed between the groups.
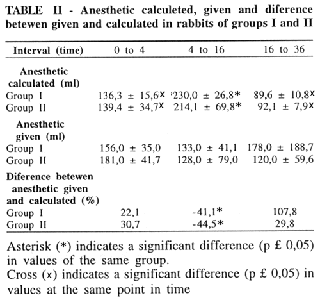
The consume of anesthetic was similar in both groups (Table II).
The time required for the return of the corneal and pedal reflexes, extubantion and spontaneous jumping were similar in both groups (Table III ).
DISCUSSION
This study shows that intravenous agent used to induced anesthesia in rabbit is feasible and permitted a rapid induction time. The dose was similar to reported by FLECKNELL6. Although there was no use of muscular relaxant drug, the trachea intubation of the rabbits was fast, easy and reliable. A pediatric laryngoscope was used to permitted the view of the vocal cords and introduction the tube into traquea as used by differents autors1,4,12,15.
Apnea has been reported as a common problem in anesthetize rabbits6,17 but didnt occur in this study, maybe by the fact of propofol had been injected slowly.
A closed system using fresh gas (oxygen plus anesthetic gases) exactly equal to the animals uptake permit conservation of heat moisture, as also wasting less oxygen and anesthetic gases. It was possible with the use of closed system of anesthesia as used in this study. Isoflurane was used to anesthesia management in the rabbits, administrated by lowflow closed system technique with copper kettle vaporizer, fixed by pre-calculated vaporizing flow in double time intervals as proposed by LEÃO et al14 as modification of the original technique of LOWE e ERNEST16. The excess of anesthetic given in the begging of the anesthetic procedure happened by manual error and corresponded with the decrease in mean arterial pressure.
The ECG in standard technique was used to monitor the rabbits. The choice was intentional because of monitoring devices for use in man often have a detection limit of 250 beat min-1, and this rate is exceeded in rabbits7.
Although decrease in body temperature observed in figure 2, none of the rabbits had temperature lower than the normal values7 , but for longer procedure is suggested better control of body temperature degree17.
Beats of heart didnt change during procedure. The values were considered normal concerned to some authors2,7. This normal values to be out of harmony of phisiological paraments because high frequency observed should be first by stress of animals6,17 and maybe have been continued by pharmacological effect on cardiovascular system of isoflurane5,9,19,20.
The marked decrease in respiratory rate in all the rabbits may have been in part due to restraint related hyerventilation prior to anesthesia. This hyperventilation also could have increased a little in the baseline pH values and also a bit decrease in the baseline PaCO2 values. The mechanism of action of respiratory depression during anesthesia involves a decreased responsiveness by peripheral chemoreceptors an the central respiratory center to anesthetics that may be responsible to acidosis and hipercapnia during anesthetic procedure5,9,13,. Elevation on PaO2 tension occurred by FiO2 1,016.
Hypercapnia occurred among the subjects in both groups. Decreased alveolar ventilation was the most likely cause of increased CO2 tension. Isoflurane induced central nervous system depression 5,9,13.
The arterial blood pressure was monitored by introduction the cannula into auricular caudal ear artery as GUERREIRO e PAGE10 and WYATT et all22 . It was fast, easy and reliable technique.
The decrease in mean arterial pressure was probably due mainly to the use of isoflurane. The mechanism of action of isoflurane on the cardiovascular system is decreasing the peripheral circulatory system5,18.
The wide variability in corneal reflex data obseved by various subjects and not considered as reliable indicators of anesthetic depth anesthesia in rabbit3,11,21. This observation was not supported in this study because there was a complete loss of corneal, similar with pedal withdrawal reflexes of anesthesia. Of the noxious stimuli used in this study, the pedal withdrawal reflex appeared to be the most sensitive indicator of analgesia. The true consistency of the corneal reflex limit its usefulness as reliable indicator of adequate anesthesia18.
The surgery stimulation in group II was used to compare with group I if the same stage of anesthesia were similar with the pedal withdrawal reflexes performed. This observation shows the correspondence of the stimulus17.
The alterations in arterial blood gas tensions were quite dramatic, however, no post-recovery sequelae were seen. Mechanical ventilation should be considered as a way to improve alveolar ventilation and normalize blood gas values. These method require further investigation before specific recommendations can be made.
FONSECA, N. M.; GOLDENBERG, S, EURIDES, D.; NOVO, N.F.; LIMA, C.A.P. - Avaliação de um novo sistema circular de anestesia: anestesia quantitativa com isuflorane em coelhos. Acta. Cir. Bras., 12(4):240-5, 1997.
RESUMO: Um sistema circular de anestesia para animais de pequeno porte foi desenvolvido por FONSECA e GOLDENBERG, em 1993. No presente estudo foram utilizados 20 coelhos brancos linhagem Nova Zelândia, submetidos ao sistema com isoflurane, distribuidos em dois grupos de 10 animais. O grupo I, controle, que foi apenas anestesiado e o grupo II foi submetido a raspagem periostal da parte proximal e medial da tíbia. Não foi administrada droga pré-anestésica. Após administração do propofol intravenoso (10mg/kg) procedeu-se a intubação endotraqueal. O isuflorane foi administrado lentamente no sistema circular de anestesia. Os animais respiravam espontaneamente. Houve efeitos cardiorespiratórios conseqüentes da anestesia, tais como, hipotensão, hipercapnia e acidose respiratória. O controle do plano anestésico foi realizado mediante os reflexos corneano e pelo pinçamento. O sistema desenvolvido por FONSECA e GOLDENBERG foi considerado satisfatório para anestesia em coelhos.
DESCRITORES: Anestesia, animais. Anestesia, equipamentos. Coelhos.
Address for correspondence:
Neuber Martins Fonseca
Rua José Andraus, 888 - Ap. 101/2
CEP: 38401-075 - UBERLÂNDIA - MG
Accept for publication on June, 1997
- 1. BECHTOLD, S.V. & ABRUTYN, D. - An improved method of endotracheal intubation in rabbits. Lab. Anim. Sci., 41:630-1, 1991.
- 2. BJELLIN, L.& CARTER, A.M. - Circulatory adjustaments to pregnancy in the rabbit. Biol. Reprod., 16:112-6, 1977.
- 3. BORKOWSKI, G.L.; DANNEMAN, P.J.; RUSSEL, G.B; LANG, C.M. - An evaluation of theree intravenous anesthesic regiments in Nes Zealand Rabbits. Lab. Anim. Sci., 40:270-6, 1990.
- 4. DAVIS, N. L. & MALININ, T. - Rabbit intubation and halothane anesthesia. Lab. Anim. Sci., 24:617-21, 1974.
- 5. EGER, E.I. - Insoflurane: a review. Anesthesiology, 55:559-76, 1981.
- 6. FLECKNELL, P.A. - Laboratory animal anesthesia ed. London, Academic Press, 1992. 156p.
- 7. FLECKNELL, P.A. - Anesthesia of animals for biomedical reserach. Br. J. Anaesth., 71:885-94, 1993.
- 8. FONSECA, N.M. & GOLDENBERG, S. - Sistema circular de anestesia para animais de pequeno porte: estudo das resistęncias ao fluxo de ar. Rev. Bras. Anestesiol., 43:303-11, 1993.
- 9. FOURCAD, H.E.; STEVENS, W.C.; LARSON, P.; CROMWELL, T.H.; BALHMAN, S.H.; HICKEY, R.F.; HALSEY, M.J.; EGER, E.I. - The ventilatory effects of forane a new inhaled anesthesic. Anesthesiology, 35:26-31, 1971.
- 10. GUERREIRO, O. & PAGE, C.P. - The effect of neuroleptanalgesia on some cardiorespiratory variables in rabbit. Lab. Anim. Care, 21:205-9, 1987.
- 11. HODESSON, S.; RICH, S.; WASHINGTON, J.; APT, L. - Anesthesia of the rabbit with Equi-thesin following the administration of preanesthetics. Lab. Anim. Care, 15:336-44, 1965.
- 12. HOGE, R.S.; HODESSON, S.; SNOW, I.B.; WOOD, A.I. - Intubation technique and methoxyflurane administration in rabbits. Lab. Anim. Care, 19:593-5, 1969.
- 13. KIERASZEWICS, H.; KNILL, R.L.; CLEMENT, J.L. - Chemical regulation of ventilation during isoflurane sedation and anesthesa. Can. Anaesth. Soc. J., 28:544-9, 1981.
- 14. LEĂO, D.G.; VIEIRA, Z.E.G.; SARAIVA, R.A. - Anestesia com baixo fluxo de gases: Uso de vaporizador tipo "kettle" com novos intervalos. Rev. Bras. Anestesiol., 37:89-95, 1987.
- 15. LINQUIST, P.A. - Induction of methoxyflurane anesthesia in the rabbit after ketamine hydrochloride and endotracheal intubation. Lab. Anim. Sci., 22:898-9, 1972.
- 16. LOWE, H.J. & ERNEST, E.A.. - The quantitative practice of anaesthesia: use of closed circuit. London, Williams and Wilkins, 1981. 234p.
- 17. MURDOCK, H.R. - anesthesia in the rabbit. Fed. Proc., 28:1510-16, 1969.
- 18. NOCITE, J.R. - Isoflurano, vantagens e desvantagens. Rev. Bras. Anestesiol., 37:253-9, 1987.
- 19. SEAGARD, J.L..; ELEGBE, E.O.; HOPP, F.A.; BOSNJAK, Z.J.; VON COLDITZ, J.H. KALBFLISCH, J.H.; KAMPINE, J.P. - Effects of isoflurane on the baroreceptor reflex. Anesthesiology, 59:511-20, 1983.
- 20. SKOVSTED, P & SAPTHAVICHAILUL, S. - The effects of isoflurane on arterial pressure, pulse rate, autonomic nervous activy, and barostatic reflexes. Canad. Anaesth. Soc. J., 24:304-14, 1977.
- 21. WHITE, W.J. & FIELD, K.J. - Anesthesia and surgery of laboratory animals. In: Veterinary Clinics of North America: small animal practice Philadelphia, W.B. Sauders. 17:989-1017, 1987.
- 22. WYATT, J. D.; SCOTT, R.A.W.; RICHARDSON, M.E. - The effects of prolonged ketamine-xylazine intravenous infusion on arterial blood pH, blood gases, rectal temperature and reflexus in the rabbit. Lab. Anim. Sci., 39:411-6, 1989.
Publication Dates
-
Publication in this collection
31 May 2001 -
Date of issue
Dec 1997
History
-
Accepted
June 1997