Abstracts
PURPOSE: To evaluate the influence of aerobic exercise on oxidative stress in mice. METHODS: The study included twenty female mice Mus musculus-Swiss divided into two groups: sedentary control (GA) and exercise (GB), each containing ten animals. All animals underwent an adaptation period of seven days isolated in individual boxes. After this period, the animals in the exercise group (GB) were trained in angled running wheel with circumference of 25 cm assembled on an articulated axle during five minutes for three consecutive days. On the fourth day, they underwent an exercise program of one session lasting 45 minutes. The evaluation of oxidative stress was performed by determining the levels of malondialhyde derived of lipid peroxidation by the TBA method. The samples were read in a spectrophotometer at 535 nm. RESULTS: No significant difference was observed in the intergroup comparison of MDA levels in the tissues evaluated. A significant difference was observed in the intragroup comparison of MDA levels in the control group (p = 0.0201).The Tukeys' post hoc test indicated significantly lower values of MDA in the smooth muscle in relation to plasma. In the analysis of variance in the exercise group, a significant difference between tissues (p = 0.0009), with significantly lower values in the smooth muscle in relation to plasma (p<0.001) and higher in striated muscle in relation to smooth muscle (p<0.05) was observed. CONCLUSION: There was no change in the analysis of oxidative stress in mice which were undergone a single session of aerobic exercise.
Oxidative Stress; Exercise; Lipids; Mice
OBJETIVO: Avaliar a influência do exercício físico aeróbico sobre o estresse oxidativo em camundongos. MÉTODOS: Participaram do estudo 20 camundongos (Swiss), distribuídos em dois grupos: controle-sedentário (GA) e exercício (GB) cada um contendo dez animais. Todos os animais passaram por um período de adaptação de sete dias. Após os animais do grupo (GB) receberam treinamento em roda giratória angulada montada sobre eixo articulado por cinco minutos durante três dias consecutivos. No quarto dia foram submetidos à única sessão de exercício por 45 minutos. A avaliação do estresse oxidativo foi realizada por meio dos níveis de malondiadeído pelo método do TBA. As amostras foram lidas em espectrofotômetro a 535nm. RESULTADOS: Não houve diferença significativa na comparação intergrupos nos tecidos avaliados. Diferença significativa foi observada na comparação intragrupo para o GA (p=0,0201). O post hoc test de Tukey apontou valores significantemente inferiores no músculo liso em relação ao plasma. A análise de variância do GB apontou diferença significativa entre os tecidos (p=0,0009), com valores menores no músculo liso em relação ao plasma (p<0,001), e maiores no músculo estriado em relação ao músculo liso (p<0,05). CONCLUSÃO: Não houve alteração nas análises de malondialdeído tecidual entre os grupos avaliados.
Estresse Oxidativo; Exercício; Lipídeos; Camundongos
5 ORIGINAL ARTICLE
MODELS, BIOLOGICAL
Evaluation of oxidative stress in mice subjected to aerobic exercise1 1 Research performed at Postgraduate Program in Health and Development, West Central Region of Brazil, Federal University of Mato Grosso do Sul (UFMS), Campo Grande-MS. Brazil. Part of Master thesis degree. Tutor: Guido Marks.
Avaliação do estresse oxidativo em camundongos submetidos ao exercício físico aeróbico
Mônica Cruvinel de LimaI; Guido MarksII; Iandara Schettert SilvaIII; Baldomero Antonio Kato da SilvaIV; Lourdes Zélia Zanoni CônsoloV; Gabriel Bogalho NogueiraVI
IFellow Master degree, Postgraduate Program in Health and Development, West Central Region, UFMS, Campo Grande-MS, Brazil. Main author. Responsible for the intellectual and scientific content of the study
IIPhD, Associate Professor, Postgraduate Program in Health and Development, West Central Region, UFMS, Campo Grande-MS, Brazil. Responsible for conception and critical revision
IIIPhD, Associate Professor, Postgraduate Program in Health and Development, West Central Region, UFMS, Campo Grande-MS, Brazil. Surgical procedures and critical revision
IVPhD, Associate Professor, Piaui Federal University, Piaui-PI, Brazil. Statistical analysis and critical revision
VPhD, Associate Professor, Postgraduate Program in Health and Development, West Central Region, UFMS, Campo Grande-MS, Brazil. Responsible for laboratory analysis
VIFellow Master degree, Postgraduate Program in Health and Development, West Central Region, UFMS, Campo Grande-MS, Brazil. Experimental procedures and critical revision
Correspondence Correspondence: Mônica Cruvinel de Lima Rua Silvio Romero, 420/203 79041-610 Campo Grande -MS Brasil Tel.: (55 67)92494133 monicacruvinel@hotmail.com
ABSTRACT
PURPOSE: To evaluate the influence of aerobic exercise on oxidative stress in mice.
METHODS: The study included twenty female mice Mus musculus-Swiss divided into two groups: sedentary control (GA) and exercise (GB), each containing ten animals. All animals underwent an adaptation period of seven days isolated in individual boxes. After this period, the animals in the exercise group (GB) were trained in angled running wheel with circumference of 25 cm assembled on an articulated axle during five minutes for three consecutive days. On the fourth day, they underwent an exercise program of one session lasting 45 minutes. The evaluation of oxidative stress was performed by determining the levels of malondialhyde derived of lipid peroxidation by the TBA method. The samples were read in a spectrophotometer at 535 nm.
RESULTS: No significant difference was observed in the intergroup comparison of MDA levels in the tissues evaluated. A significant difference was observed in the intragroup comparison of MDA levels in the control group (p = 0.0201).The Tukeys' post hoc test indicated significantly lower values of MDA in the smooth muscle in relation to plasma. In the analysis of variance in the exercise group, a significant difference between tissues (p = 0.0009), with significantly lower values in the smooth muscle in relation to plasma (p<0.001) and higher in striated muscle in relation to smooth muscle (p<0.05) was observed.
CONCLUSION: There was no change in the analysis of oxidative stress in mice which were undergone a single session of aerobic exercise.
Key words: Oxidative Stress. Exercise. Lipids. Mice.
RESUMO
OBJETIVO: Avaliar a influência do exercício físico aeróbico sobre o estresse oxidativo em camundongos.
MÉTODOS: Participaram do estudo 20 camundongos (Swiss), distribuídos em dois grupos: controle-sedentário (GA) e exercício (GB) cada um contendo dez animais. Todos os animais passaram por um período de adaptação de sete dias. Após os animais do grupo (GB) receberam treinamento em roda giratória angulada montada sobre eixo articulado por cinco minutos durante três dias consecutivos. No quarto dia foram submetidos à única sessão de exercício por 45 minutos. A avaliação do estresse oxidativo foi realizada por meio dos níveis de malondiadeído pelo método do TBA. As amostras foram lidas em espectrofotômetro a 535nm.
RESULTADOS: Não houve diferença significativa na comparação intergrupos nos tecidos avaliados. Diferença significativa foi observada na comparação intragrupo para o GA (p=0,0201). O post hoc test de Tukey apontou valores significantemente inferiores no músculo liso em relação ao plasma. A análise de variância do GB apontou diferença significativa entre os tecidos (p=0,0009), com valores menores no músculo liso em relação ao plasma (p<0,001), e maiores no músculo estriado em relação ao músculo liso (p<0,05).
CONCLUSÃO: Não houve alteração nas análises de malondialdeído tecidual entre os grupos avaliados.
Descritores: Estresse Oxidativo. Exercício. Lipídeos. Camundongos.
Introduction
Oxygen is a fundamental molecule for the survival of aerobic organisms, used both for the production of energy through the electron transportation chain in the mitochondria of eukaryotes and in the cell membrane of several bacteria, it is also necessary for innumerous fundamental metabolic pathways. The oxygen consumed has the aerobic system as the main metabolic pathway, that is, the mitochondria. This system is responsible for 85 to 90% of the total oxygen consumption. The remaining 10 to 15% is used by oxidase and oxygenases enzymes and by chemical direct oxidation reactions1.
Simultaneously, the oxygen consumption generates intra and extra cellular toxic substances, creating the oxygen paradox, due to the existing balance among its advantages and disadvantages. These toxic substances are generated during the electron transportation, enzyme reaction, or even anti oxidation reactions and are commonly called Reactive Oxygen Species (ROS)2.
The oxidative stress can be described as the imbalance among the formations of the reactive oxygen species and the antioxidant system, generating a potential oxidative damage to the structure of the cells. Accordingly, the balance among the pro oxidants and the antioxidants is essential for the maintenance of the homeostasis of the cells (redox balance)3.
Reactive oxygen species, if in lethal sub-doses, act as intracellular expression gene modifiers, and can mediate the processes of cells proliferation and differentiation. In higher levels, these substances can start or execute the death of the cell by apoptosis4.
Inappropriate life habits such as alcohol ingestion, smoke, inadequate diet, inappropriate environment conditions (such as exposure to non-ionizing UV radiation and short waves, pollution, high relative humidity and high temperature), psychological states (which cause emotional stress), ageing and extreme physical exercise training are also related to oxidative stress5.
The amount of species produced during the exercise is directly related to the speed of the aerobic metabolism. The exercise increases the muscles oxidative processes, leading to a general increase in ROS generation and of its sub products in the human body6.
The exercises practiced excessively by athletes can promote ROS exposion which can exceed the protection effect of any adaptable response achieved during tanning. Other factors that can overcome the exercise inducing the oxidative damage, for instance, is the anti oxidative nutritional deficiency. During the execution of the low intensity and duration exercise protocols, antioxidant defenses seem to be enough against the ROS production, but in high intensity and duration levels of exercise, these defenses are no longer enough, resulting in oxidative damage to the tissue7.
The random nature of the attacks carried out by ROS causes difficulties to the characterization of its products reaction, but every biological molecule is susceptible to its oxidative lesions. The polyunsaturated lipid oxidation in the cells break through the biological membrane structures, and the oxidative lesions on the DNA can suffer mutations. When the free radicals escape from the antioxidant system and attack the unsaturated fatty acids located in the double bond between some carbon atoms, a chain reaction starts and alters the membrane structure. This reaction is named lipid peroxidation which is intensively deleterious to the organism8.
The lipid peroxidation is one main consequence of the oxidative stress and one of the most used reactions to evaluate it, being considered an indirect indicator of free radicals action9.
Physical exercise can induce the lipid peroxidation resulting in problems such as the inactivation of the enzymes of cell membranes, decrease of the immune system effectiveness, and the progression of chronic-degenerative diseases such as cancer and cardiovascular ilnesses10.
Between the aldehydes originated from lipid peroxidation in the membrane is the malondialdehyde (MDA) which has been the object of great interest despite its complex and not yet fully understood origin. The MDA has been widely used as an oxidative stress marker11.
Studies have shown that intense physical exercise causes oxidative stress in animals and humans, being possibly related, for instance, to fatigue and tissue lesions. The factors related to the increase of lipid peroxidation from physical exercise are intensity, level of physical fitness level, antioxidant status, the tissue, the diet and recovery10.
Although there are numerous studies addressing this topic, in many consulted articles, it was possible to notice gaps related to the determination of a training protocol for the lipid peroxidation analysis via the MDA in different body tissues in animal model. Accordingly, this study's aim was to evaluate the influence of aerobic physical exercise over the oxidative stress in mice.
Methods
This study was conducted at the animal research center of the de Mato Grosso do Sul Federal University, Campo Grande MS, during the period between november and december of 2011.
The sample consisted of twenty female mice (Mus musculus) Swiss, consistent with the conventional sanitary standard coming from the animal colony of the Mato Grosso do Sul Federal University (UFMS). All animals were about four-week old, weighing between 19 and 24 grams.
All procedures were done according to the international rules for animal experimentation. The study was submitted to the analysis of the Ethics Committee for Animal Usage/CEUA/UFMS and approved (310/2011 protocol).
The animals were confined in individual polypropylene cages of the following dimensions: 19.3 cm wide, 30.3 cm deep and height of 12.6 cm. The lid and the bars were made of stainless steel and the cage contained a place for food and water Brasholanda. The temperarature was kept between 21˚C and 23˚C, in a photoperiod of 12 hours on a ventilated shelf. The place of accommodation and experimentation were isolated from loud noises in order to avoid behavioral and physiological disruption.
The feeding/hydration of the animals were kept ad libitum replaced every three days. The food offered was of the brand Nuvilab CR1 Nuvital nutrientes®.
The animals were randomly divided into two distinct groups according with the time of adjustment, physical exercise and euthanasia being each group sub divided into group GA: (Control-Sedentary = 10) and group GB (Exercise = 10).
Physical exercise
Every subject underwent an equal adaptation period of seven days isolated in individual cages. Afterwards, the animals of the exercise group (GB) received a training on an angled running wheel with circumference of 25 cm assembled on an articulated axle (brand Biosery), during five minutes for three consecutive days. On the fourth day the animals in the GB group were subjected to an exercise program of one forty-five minute single session. The animals which refused to run were encouraged by light back pats. The ones which still refused to run were excluded from the sample. Each animal ran for forty five (45) continuous minutes, then they were submitted to euthanasia for posterior analysis. The sedentary animals were kept in the cages for the entire experiment period and the feed was removed during group GB exercise sessions.
The animals' weight was measured by an accurate scale of the brand Shimadzu (variation: one hundredth of a gram). During the study every animal was weighed (at the beginning for the adaptation period and at the end of the exercise period) resulting in an individual record form.
Sample collection and storage
After the end of the physical exercise, the animals were anesthetized with a single intra peritoneal injection containing ketamine (10%) and xylazine (2%), in the ration of 1ml of ketamine (100mg) for each 1ml of xilazina (20mg). It was administered 0.1mL of the mixture for each 100g of live weight of the animals. The blood samples were obtained by cardiac puncture and stored into microtubes containing anticoagulants. The plasma was separated by centrifugation at 3500 rpm for five minutes. Then it was stored for later analysis. After the blood samples were collected, the animals were submitted to euthanasia by a lethal dose of sodium thiopental (150mg/Kg), to collect the other tissues. Afterwards, the trichotomy of the thoracic region was performed, then, the region was opened by a median trassternal incision and the heart was dissected. Subsequently the trichotomy of the lower limb was performed in the posterior part of the animal's paw, followed by a caudal access to the gastrocnemius and soleus muscle for the muscle dissection. Immediately after the tissue collection, they were washed in potassium chloride (KCL) solution 1.15%, stored in Eppendorf, identified and frozen in liquid nitrogen for later analysis.
Evaluation of the lipid peroxidation
The evaluation of the oxidative stress was performed through determination of the malondialdehyde (MDA) levels, resulted from the lipid peroxidation by means of the TBA.
Quantification of the plasmatic malondialdehyde by the method of thiobarbituric acid (TBA) or test
Plasma seric samples were thawed at room temperature and added to identified test tubes. 250 micro liters of TBA were added for each 125 micro liters of plasma. After the pipetting of the sample all tubes were closed with a screw cap and heated in a water-bath at 94°C for one hour. Subsequently the samples were cooled by resting over a counter at room temperature for 15 minutes. After the cooling process, 1ml of n-Butyl Alcohol reagent was added to each test tube. Then, each tube was individually mixed in the vortex agitator, in order to reach the maximum MDA extraction to the organic phase. Lastly the tubes were centrifuged at 3500 rpm for ten minutes and divided into two phases. About 3ml of the colored supernatant was placed in cuvettes for spectrophotometer reading at 535nm.
Quantification of the tissue malondialdehyde by the method of thiobarbituric acid (TBA) or test
The homogenization process of the tissue started with the thawing of the sample at room temperature, followed by washing it in the same KCl 1.15% solution. The sample was, then, removed from the washing solution, carefully dried with gases and weighed on an analytical scale. Using a disposable pipette, the KCl 1.15% solution was added in a ratio of 10:1 mass. The sample immersed in KCl 1.15% solution was cut in small pieces (as small as possible), in order to facilitate the homogenization. Then, the becker containing the material was taken to the grinder (Turratec TE-102) for homogenization. The time of stay of the sample in the machine and its power were adjusted individually for each type of tissue. At the end, the homogenate material was placed in a test tube. Subsequently, it was centrifuged at 2500 rpm for ten minutes. 500 micro liters of supernatant were pipetted and 1ml of TBA was added, then it was heated in water-bath for one hour. Afterwards the samples were cooled at room temperature for 15 minutes. After the cooling, it was added to each test tube 1ml of n-Butyl alcohol reagent. Then, each tube was individually mixed in the vortex agitator (QL-901) for 40 seconds and centrifuged at 3.500 rpm for 10 minutes. About 3ml of the colored supernatant was placed in cuvettes for spectrophotometer reading at 535nm. The concentration of MDA in each cuvette was expressed in nmol of MDA/mg of protein.
Before starting the sample reading, the spectrophotometer was calibrated and adjusted for a reading at 535nm. Then, the equipment was zeroed with a control solution (white), and the control standard reading for MDA was performed.
For the concentration computation of MDA it was used an equation obtained from the standard curve of the absorbance generated by concentrations known as standard.
Statistical analysis
The numerical variable measures were expressed in means ± standard deviation. For the intergroup comparison it was performed an analysis of variance (ANOVA) followed by the post-hoc Tukey test. The normality of the groups was analysed according to their distribution through Shapiro Wilk's test. The significance level considered for this study was p≤0.05.
The software Microsoft Office Excel 2007 was used for the tabulation of the results, and the Bioestat 5.0 program was used to calculate normality. The comparison of the data and the making of the graphs were done using the GraphPad Prism 4.0 program.
Results
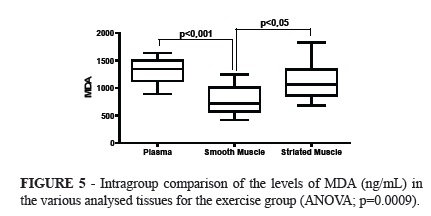
No significant difference was observed in the intergroup comparison of MDA levels in the evaluated tissues. Significant difference was observed when comparing the levels of MDA between the exercise group and the control group (ANOVA; p=0.0201). The Tukey's post hoc test showed significantly lower amounts of MDA in smooth muscle than in the plasma.
The analysis of variance in the exercise group showed significant differences when comparing the tissues (p=0.0009), with significantly lower amounts in the smooth muscle than in the plasma (Tukey; p<0.001), and higher in the striated muscle than in the smooth muscles (Tukey; p<0.05).
Discussion
This study aim was to verify the effects of aerobic physical exercise on the biomarkers of oxidative stress through the lipid peroxidation in mice. Physical activity is known to promote health and welfare. The exercise is also responsible for raising the production of reactive oxygen species (ROS) through the addition of mitochondrial oxygen consumption by the tissues. The imbalance between the ROS production and the oxidant defenses of the tissues can result in oxidative damages to proteins, lipids and DNA. The oxidative damage to lipoprotein, particularly, low density lipoprotein (LDL), is know to have an important role in a number of age related diseases such as cardiovascular diseases, cancer and dementia12.
Recent studies demonstrate that the growth of reactive oxygen species causes damages to lipoproteins, lipids, DNA and proteins. The oxidative stress induced by the modifications of these molecules has been known to be involved in diseases by many ways. One disease, suggested to be associated with high oxidative stress is the atherosclerotic cardiovascular disease (ASVD). The oxidative stress is believed to have a significant role in the initiation and progression of atherosclerosis13.
In this study the level of MDA was measured in order to observe the influence of aerobic exercise on the oxidative stress formation in various tissues. The evaluation method used was chosen for being mentioned in several articles, being considered a fast and economical means of studying the final products of lipid peroxidation. The malondialdehyde (MDA) is one of the important final products of the tissues lipid peroxidation and is usually accepted as a peroxidation intensity index. The MDA content in the plasma confirms that physical exercise is capable of generating free radicals that, in turn, causes lipid peroxidation.The effect is related to the intensity of the exercise2.
Historically, the most common method for measuring lipid peroxidation through malondialdehyde has been the trial run with reactive substances of the tiobarbituric acid (TBA). The malondialdehyde is a stable decomposed aldehyde and subproduct of the lipid peroxidation and can be measured directly through the HPLC, gas chromatography or spectrophotometry14.
In his study, the samples were collected for analysis immediately after the end of the exercise sessions. However, due to the recovery of the kidney blood flow, it was necessary to wait for a longer period in order to evaluate these biomarkers in the animals submitted to intense exercise. Lipoperoxides can be transferred from one organ or tissue to another and be metabolized by those with higher oxidant capability. This is particularly important during intense exercise because the splendid region has its blood flow greatly reduced under these conditions, while it is increased in the skeletal muscles. Thereby, besides the possibility of the lipoperoxides being metabolized by organs from the splendid region, hemodinamic factors can hinder the blood flow in this region, increasing its concentration in the plasma and other tissues where the lipoperoxydes are produced during intense physical exercise15. These findings are supported by a study conducted by Maugham et. al.16, which demonstrates that lipoperoxydes have their concentration increased in the plasma up to six hours after intense exercise.
The aerobic exercise used for this study was not able to induce a significant growth in the MDA levels in mice, comparing with the sedentary groups, based on the assessed tissue. In the study conducted by Child et al.17, trained individuals were submitted to a simulation of a half marathon test; it was possible to observe, by measuring the total antioxidant capability and the uric acid level, a higher scavenger ability (the ability of neutralizing free radicals producing a less reactive compound) over the free radicals of the serum. Even so, the exercise induced higher concentrations of malondialdehyde, suggests that these responses were insufficient to prevent lipoperoxidation by exercise.
Nikolaidis et al.18, put physically active men through two exhaustive exercise protocols, on a conveyor belt, being one long and the other short duration. They verified that both exercise protocols induced the increase in the MDA plasmatic concentration. In athlets submitted to other types of long lasting run, Machefer et al.19 observed the raise in the MDA plasmatic concentration, up to 72 hours after the exercise.
This study assessed the MDA patterns in mice in comparison with the control group value as reference. The reference value for the levels of total MDA reported in literature show great variability, being related to the experiment conditions used in different studies. Thus, the MDA plasmatic value determined for healthy individuals by Steghens et al.20, is 0.138 ± 0.028 μM, while for Pilz, Meineke and Gleiter21, is 2.16 ± 0.29 μM, contrasting with the values obtained by Sim et al.22, 13.8 ± 1.32 μM, Londero and Greco 23, 0.85 ± 0.25 μM and Mao et al.24, 0.426 ± 0.029 μM. Considering the variations between values in the mentioned reference, it becomes necessary to determine an interlude as individual reference for each designed method.
In the present study it was not observed significant differences when comparing the intergroup levels of MDA in the cardiac tissues analised after the training in the running wheel. The cardiac muscle has a high consumption of oxygen in resting conditions. The coronary blood flow increases the oxygen absorption by up to four times during exercise. Although, it is essential for the organism aerobic metabolism, high oxygen metabolism can lead to an increase in oxidative stress in the heart during physical exercise25.
Gul et al.26 studied an eight-week training protocol in conveyor-belt for the production of antioxidants enzymes and decrease of the lipid peroxidation in the cardiac tissues in mice. The effects of exhaustive exercise were also investigated. The resistance training consisted of running in conveyor belt, 1.30h for five days per week during 8 weeks. For the exhaustive exercise, the belt was graduated so the speed was increased gradually to 2.1km/h at 95 minutes, and kept constant until the mice were exhausted. The malondialdehyde level in cardiac tissue was not affected by exhaustive exercise in sedentary and exercised mice.
For this study the exercise performed by the mice lasted 45 minutes, and the results differ from the mentioned above, with significantly higher lipoperidation in striated muscle than in smooth muscle. The cardiac and skeletal muscles are different functioning tissues. Arévalo et al.27 determined the activities of TBARS, total SOD, Cu-Zn-SOD and MnSOD, and the isoenzyme pattern of the SOD in the skeletal and cardiac muscle in male, young and old Wistar mice, in rest and after exhaustive exercise. The exhaustive exercise training was executed on a conveyor-belt, at different speeds and inclination. The average time of exhaustion for young mice was 55 minutes. There was no differences in lipoperoxidation levels after exercise in either tissue.
Chiaradia et al.28, conducted a study to evaluate the lipid peroxidation in plasma and its effects on skeletal muscle lesion in horses. The animals were submitted to physical training for three months, 30 minutes per day, six days a week and the relative intensity of the exercise was gradually increased. The conclusion was that the exercise was insufficient because of the significant lysis of the skeletal muscle cells. However, the results obtained indicated that the physical exercise adopted for the study was enough to modify MDA and gluathione in the blood content.
No significant data was found regarding the activity of the levels of MDA in the analysed tissues when comparing the control and the exercise groups. However, the striated muscle showed higher sensitivety to the action of lipoperoxidation in comparison with smooth muscle.
There are not many studies regarding the strength training and oxidative stress. In one of them it was established by measuring the blood lipoperoxyde that, when the isometric contraction predominates in the strength training, oxidative lesions occur in the biomelecule15.
Radák et al.29, after submitting mice to a nine-week swimming training, 60 minutes long in the first six weeks and three weeks being 90 minutes long, did not verify significant changes in MDA in gastrocnemius muscle 24 hours after the last training session. On the other hand, Liu et al.30 verified, after eight weeks of training on a conveyor-belt, higher levels of MDA in the cardiac and vatus lateralis muscles, after 48 hours of the last training session in female mice. However, the same result was not observed after exhaustive exercise. Alessio and Goldfarb31, in turn, after submitting mice to a eighteen-week training on conveyor-belt, verified lower levels of MDA at rest, in the liver and in the deeper portion of the vastus lateralis muscle, when compared to the values of sedentary individuals.
Ji32, observed that an isolated exhaustive work load produced an increase of the LPO in skeletal muscles and that the activity of the antioxidant glutathione reductase enzymes, Gpx, SOD and CAT were significantly higher.
It is known that free radicals can attack every main class of bio-molecule, being the lipids the most susceptible. The oxidative destruction of the polyunsaturated fatty acids, known as the lipid peroxidation, is considerably harmful for being a reaction of self-propagation in the membrane33. The disputes about the effects of exercise over the lipid peroxidation are innumerous, probably, due to differences in intensity and duration of the exercise protocols31. In this work there was no change in the analysis of the oxidative stress in the mice that underwent one single session of aerobic exercise.
Conclusions
There was no change in the analysis of the tissue malondialdehyde between the evaluated groups. In the exercise group it was observed that the smooth muscle was less sensitive to lipid peroxidation when compared to the striated muscle and to the plasma.
Received: March 12, 2012
Review: May 14, 2012
Accepted: June 11, 2012
Conflict of interest: none
Financial source: none
- 1. Schneider CDE, Oliveira AR. Radicais livres de oxigênio e exercício: mecanismos de formação e adaptação ao treinamento físico. Rev Bras Med Esporte. 2004;10:87-90.
- 2. Halliewll B, Gutteridge, J. Free radicals in biology and medicine. Nova York: Oxford University Press; 2007.
- 3. Sies H, Cadenas E. Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci. 1985;311(1152):617-31.
- 4. Bloomer RJ, Goldfarb AH, Wideman L, Mckenzie MJ, Consitt LA. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stres. J Strength Cond Res. 2005;19 (2):276-85.
- 5. Elsayed NM. Antioxidant mobilization in response to oxidative stress: a dynamic environmental-nutritional interaction. Nutrition. 2001;17(10):828-34.
- 6. Wolinsky I, Hickson JR, JF. Nutrição no exercício e no esporte. 2ed. São Paulo: Roca; 1996.
- 7. Longo UG, Oliva F, Denaro V. Maffulli N. Oxygen species and overuse tendinopathy in athletes. Disabil Rehabil. 2008;30 (20-22):156371.
- 8. Auroma OI. Nutrition and health aspects of free radicals and antioxidants. Food Chem Toxicol. 1994;32(7):671-83.
- 9. Halliwell B, Gutteridge JMC. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1-85.
- 10. Miyazaki H, Oh-Ishi S, Ookawara T, Kiazaki, T.; Toshinai K, Ha S, Hagas, Ji LI, Ohno H. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physiol. 2001;84:1-6.
- 11. Draper H, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421-31.
- 12. Aldred S, Manjit, R. A moderate intensity exercise program did not increase the oxidative stress in older adults. Arch Gerontol Geriatr. 2011;53:3503.
- 13. Strobel NA, Fassett RG, Marsh SA, Coombes JS. Oxidative stress biomarkers as predictors of cardiovascular disease. Int J Cardiol. 2011;147:191201.
- 14. Knight JA, Pieper RK, Mc Clellan L. Specificity of the thiobarbituric acid reaction: its use in studies of lipid peroxidation. Clin Chem. 1988;34:24338.
- 15. Souza Jr TP, Oliveira PR, Pereira B. Exercício físico e estresse oxidativo. Rev Bras Med Esporte. 2005;11(1):91-6.
- 16. Maughan RJ, Donnelly AR, Gleeson M, Whitin PH, Walker KA. Delayed-onset muscle damage and lipid peroxidation in man after a downhill run. Muscle Nerve. 1989;12:332-6.
- 17. Child RB, Wilkinson DM, Fallowfield JL, Donnely AE. Elevated serum antioxidant capacity and plasma malondialdehyde concentration in response to a simulated half-marathon run. Med Sci Sports Exerc. 1998;30:1603-7.
- 18. Nikolaidis MG, Jamurtas AZ, Paschalis V, Kostaropoulos IA, KladiI-Skandali A, Balamitsi V, Koutedakis Y, Kouretas D. Exercise-induced oxidative stress in G6PD-deficient individuals. Med Scie Sports Exerc. 2006;38:1443-50.
- 19. Machefer G, Groussard C, Zouhal H, Vincent S, Youssef H, Faure H, Malardé L, Grastas-Delamarche A. Nutritional and plasmatic antioxidant vitamins status of ultra endurance athletes. J Am Coll Nutr. 2007;26:311-6.
- 20. Steghens JP, Kappel ALV, Denis I, Collmbel C. Diaminonaphtalene, a new highly specific reagent for HPLC-UV measurement of total and free malondialdehyde in human plasma or serum. Free Radical Biol Med. 2001;31(2):242-9.
- 21. Pilz J, Meineke I, Gleiter C. Measurement of free and bound malondialdehyde in plasma by high-performance liquid chromatography as the 2,4-dinitrophenylhydrazine derivative. J Chromatogr B Biomed Sci Appl. 2000;742:315-25.
- 22. Sim AS, Salonikas C, Naidoo D, Wilcken DEL. Improved method for plasma malondialdehyde measurement by high-performance liquid chromatography using methyl malondialdehyde as an internal standard. J Chromatogr B Biomed Sci Appl. 2003;785:337-44.
- 23. Londero D, Lo Greco P. Automated high-performance liquid chromatographic separation with spectrofluorometric detection of a malondialdehyde-thiobarbituric acid adduct in plasma. J Chromatogr A. 1996;729:207-10.
- 24. Mao J, Zhang H, Luo J, Li L, Zhao R, Zhang R, Liu G. New method for HPLC separation and fluorescence detection of malonaldehyde in normal human plasma. J Chromatogr B Biomed Sci Appl. 2006;832(1):103-8.
- 25. Frankiewicz-Jozko A, Faff J, Sieradzan-Gabelska B. Changes in concentrations of tissue free radical marker and serum creatine kinase during the post-exercise period in rats. Eur J Appl Physiol. 1996;74:4704.
- 26. Gul M, Demircan B, Taysi S, Oztasan N, Gumustekin F, Siktar E, Polat M F, Akar S, Akcay F, Dane S. Effects of endurance training and acute exhaustive exercise on antioxidant defense mechanisms in rat heart. Comp Biochem Physiol A Mol Integr Physiol. 2006;143:23945.
- 27. Arévalo AN, Canavate C, Del-Pino MJS. Myocardial and skeletal muscle aging and changes in oxidative stress in relationship to rigorous exercise training. Aging Res Rev. 1999;108:20717.
- 28. Chiaradia E, Avellini L, Rueca F, Spaterna A. Porciello F. Antonioni MT, Gaiti A. Physical exercise, oxidative stress and muscle damage in racehorses. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:8336.
- 29. Radák Z, Asano K, Inoue M, Kiazaki T, Oh-Ishi S, Suzuki K, Taniguchi N, Ohno H. Superoxide dismutase derivative reduces oxidative damage in skeletal muscle of rats during exhaustive exercise. J Appl Physiol. 1995;79(1):129-35.
- 30. Liu J, Yeo HC, Övervik-Douki E, Hagen T, Doniger SJ, Chu DW, Brooks GA, Ames BN. Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol. 2000;89(1):21-8.
- 31. Alessio HM, Goldfarb AH. Lipid peroxidation and scavenger enzymes during exercise: adaptative response to training. J Appl Physiol. 1988;64:1333-6.
- 32. Ji LL. Exercise-induced modulation of antioxidant defense. Ann N Y Acad Sci. 2002;959:82-92.
- 33. Halliwell B, Gutteridge JMC. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1-85.
Correspondence:
Publication Dates
-
Publication in this collection
25 July 2012 -
Date of issue
Aug 2012
History
-
Received
12 Mar 2012 -
Accepted
11 June 2012 -
Reviewed
14 May 2012