Abstract
Purpose:
To investigate the cellular response to injury, analyzing histopathologic changes associated with increased cellularity, degeneration and disorganization of collagen fibers.
Methods:
Thirty wistar rats were divided in two groups after partial Achilles tenotomy: the right hind paw were treated with the essential oil of Alpinia zerumbet (EOAz), diluted to 33% (0.3 mL kg-1), and the left hind paw received sunflower oil for 3, 14, 30 and 90 days. Statistical significance was determined using a Chi-square and Pearson Correlation qualitative variables test. Moreover, Mann-Whitney U-test test for comparison between different groups of the same cell, one-way ANOVA, and Tukey’s test of quantitative measurement.
Results:
A decrease hyperemia (p < 0.001) was observed in the acute phase of inflammatory cell number (p < 0.001), whereas sub-acute phase was marked by significant correlation with macrophages in fibroblasts (r = 0.17, p = 0.03), with probable induction a dense and modeled tissue. At chronic phase, it was found an increase in the number of fibroblasts and a higher percentage of type I collagen fibers (78%) compared with control collagen fibers (55%).
Conclusion:
Oil of Alpinia zerumbet stimulated the process of maturation, organization and tissue repair which gave it greater resistance.
Key words:
Achilles Tendon; Alpinia; Inflammation; Wound Healing; Phytotherapy; Rats.
Introduction
Urban society seeks more means of physical or recreational activity to reduce stress and avoid physical inactivity. As a result, a significant increase in tendon injuries been has observed over the past 50 years. Tendon injuries are caused by repetitive motion, heavy exercises, sports, degeneration or traumatic injury11 Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Appl Biomech. 2004 Jun;37(6):865-77. doi: 10.1016/j.jbiomech.2003.11.005.
https://doi.org/10.1016/j.jbiomech.2003....
. Achilles tendon injury have a high incidence and leads to functional limitations22 Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology. 2006 May;45(5):508-21. doi: 10.1093/rheumatology/ke1046.
https://doi.org/10.1093/rheumatology/ke1...
. Experimental models are used to reproduce human pathologies and conduct studies, many resources are being using for the treatment of parcial tenotomy as example ultrasound33 Yeung CK, Guo X, NG YF. Pulsed ultrasound treatment accelerates the repair of achilles tendon rupture in rats. J Orthop Res. 2006 Feb;24(2):193-201. doi: 10.1002/jor.20020.
https://doi.org/10.1002/jor.20020...
.
After the injury the body initiates a process of tissue repair that can be divided into three phases: inflammation, proliferation and remodeling11 Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Appl Biomech. 2004 Jun;37(6):865-77. doi: 10.1016/j.jbiomech.2003.11.005.
https://doi.org/10.1016/j.jbiomech.2003....
. Inflammation histamine-induced [Ca22 Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology. 2006 May;45(5):508-21. doi: 10.1093/rheumatology/ke1046.
https://doi.org/10.1093/rheumatology/ke1...
]i produces accumulation fibroblasts of human subcutaneous tissue, and is partially mediated by the release of ATP through the opening of Panx11 Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Appl Biomech. 2004 Jun;37(6):865-77. doi: 10.1016/j.jbiomech.2003.11.005.
https://doi.org/10.1016/j.jbiomech.2003....
hemi channels. Moreover, most studies have identified elevation of cytosolic Ca22 Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology. 2006 May;45(5):508-21. doi: 10.1093/rheumatology/ke1046.
https://doi.org/10.1093/rheumatology/ke1...
as an important regulator of ATP release in different cell models44 Fasciani I, Temperan A, Perez-Atencio LF, Escudero A, Martinez-Montero P, Molano J, Gomez-Hernandez JM, Paino CL, Gonzalez-Nieto D, Barrio LC. Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease. Neuropharmacology. 2013 Dec;75:479-90. doi: 10.1016/j.neuropharm.2013.03.040.
https://doi.org/10.1016/j.neuropharm.201...
.
Alpinia zerumbet, also called Alpinia speciosa, is an herbaceous plant from the Zingiberaceae family. Predominates in northeastern Brazil, where it is commonly known as “colony”55 Koh KJ, Pearce AL, Marshman G, Finlav-Jones JJ, Hart PH. Tea tree oil reduces histamine-induced skin inflammation. Br J Dermatol. 2002 Dec;147(6):1212-7. doi: 10.1046/j.1365-2133.2002.05034.x.
https://doi.org/10.1046/j.1365-2133.2002...
-66 Khalil Z, Pearce AL, Satkunanathan N, Storer E, Finlay-Jones JJ, Hart PH. Regulation of wheal and flare by tea tree oil: complementary human and rodent Studies. J Invest Dermatol. 2004 Oct;123(4):683-90. doi: 10.1111/j.0022-202X.2004.23407.x.
https://doi.org/10.1111/j.0022-202X.2004...
. Active principle of essential oil of Alpinia zerumbet (EOAz), are terpinen-4-ol and 1,8 cineole, have anti-inflammatory properties, inhibit histamine and modulate calcium channels type L. EOAz can influence tissue repair and terpenes present in principle active may improve transdermal drug absorption because they interact with lipids and keratin allowing for greater drug solubility77 Sapra B, Jain S, Tiwary AK. Percutaneous permeation enhancement by terpenes: mechanistic view. AAPS. 2008 Mar;10(1):120-32. doi: 10.1208/s12248-008-9012-0.
https://doi.org/10.1208/s12248-008-9012-...
.
EOAz toxicity showed by chromatographic analysis was 17.32% of terpinen-4-ol, 22.4% of 1,8 cineole, 11.42% y-terpinene, and 9.9% of sabinene, at concentrations of up to 300 µg/ mL or 400 mg/Kg doses, didn’t cause signs of mutagenicity in mouse leukocytes88 Cavalcanti BC, Ferreira JR, Cabral IO, Magalhães HI, Oliveira CC, Rodrigues FA, Rocha DD, Barros FW, Silva CR, Júnior HV, Canuto KM, Silveira ER, Pessoa C, Moraes MO. Genetic toxicology evaluation of essential oil of alpinia zerumbet and its chemoprotective effects against H(2)O(2)-induced DNA damage in cultured human leukocytes. Food Chem Toxicol. 2012 Nov;50(11):4051-61. doi: 10.1016/j.fct.2012.03.038.
https://doi.org/10.1016/j.fct.2012.03.03...
. Results of the toxicity EOAz used in this study were confirmed since they didin´t present toxicity in three types of rodents. A concentration of 1mg/ L is effective and safe to be used in endothelial cells in human umbilical vein99 Chen Y, Li D, Xu Y, Zhang Y, Tao L, Li S, Jiang Y, Shen X. Essential oils from fructus a. zerumbet protect human aortic endothelial cells from apoptosis induced by Ox-LDL in vitro. Evid Based Complement Alternat Med.?2014;2014:956824. doi: 10.1155/2014/956824.
https://doi.org/10.1155/2014/956824...
.
This study subjects to investigate the cellular response to injury, analyzing histopathologic changes associated with increased cellularity, degeneration and disorganization of collagen fibers.
Methods
This study was approved by the Ethical Committee for Animal Experimentation (approval 040911) according to Ethical principles of the COBEA (Brazilian College for Animal Experimentation) for experiments in animals.
Thirty animals were subjected to tenotomy of the calcaneal (Achilles) tendon. Prior to the surgical procedure, each animal was weighed and anesthetized with an intraperitoneal injection of ketamine (10%) and xylazine (2%) at a dosage of 0.1mg/100g. A superficial skin incision was done over the calcaneal tendon and a partial tenotomy was performed in the middle third of the tendon. Specifically, the lesion was between the musculotendinous junction at a distance of 0.5 cm from the calcaneal insertion. The incision transverse shear scalpel blade was 0.3 cm wide and 0.1 cm deep for partial tenotomy followed by suturing skin only (Figure 1)33 Yeung CK, Guo X, NG YF. Pulsed ultrasound treatment accelerates the repair of achilles tendon rupture in rats. J Orthop Res. 2006 Feb;24(2):193-201. doi: 10.1002/jor.20020.
https://doi.org/10.1002/jor.20020...
.
Experimental model in the tendon injuries (Yeung et al. 4). Private image of partial and transversal (0.3 cm) tenotomy the Achilles tendon of rats is located 0.5 cm from the insertion in the calcaneus.
The severed ends of the tendon were not sutured before the skin incision was closed. All surgical procedures were performed under aseptic conditions. In all experiments, data were collected using a blinded, randomized controlled design. Treatment group received daily topical tendon application of EOAz (rear right paw) at a concentration of 0.3 mL/Kg (solubilized in sunflower oil) and the control group used pure vegetable oil (rear left paw)1010 Hase E, Sato K, Yonekura D, Minamikawa T, Takahashi M, Yasui T. Evaluation of the histological and mechanical features of tendon healing in a rabbit model with the use of second-harmonic-generation imaging and tensile testing. Bone Joint Res. 2016;5(11):577-85. doi: 10.1302/2046-3758.511.BJR-2016-0162.R1.
https://doi.org/10.1302/2046-3758.511.BJ...
-1111 Jacobson E, Dart Aj, Mondori T, Horadogoda N, Jeffcott LB, Little CB, Smith MM. Correction: focal experimental injury leads to widespread gene expression and histologic changes in equine flexor tendons. PLoS One. 2016 Mar 1;11(3):e0150823. doi: 10.1371/journal.pone.0150823.
https://doi.org/10.1371/journal.pone.015...
. At the end of each experiment, on postoperative days 3, 14, 30 and 90, tissue samples were removed to prepare histomorphological slides. After a designated postoperative period, the animals were euthanized. The two-calcaneus tendons were excised from animals by dissecting them from the musculotendinous junction in the calcaneal attachment. The excised tendons immediately were placed in formaldehyde 10% and later embedded in paraffin. Serial histological sections (5 µm) for further analysis were prepared using routine laboratory techniques.
Histological analysis of the inflammatory infiltrate
To evaluate the intensity of the inflammatory response were analyzed the Hyperemia of the histological sections. After fixation for 48h, the pieces were cross-sectioned, dehydrated in alcohol, diaphanized in xylol and embedded in paraffin in the form of blocks. The blocks were mounted on the microtome and cut 5 μm thick with 6 serial histological sections of each block that were obtained from each anatomical segment of the sample and then stained with Hematoxylin / Eosin (HE) and Picrossirius. The images were captured from the slides using Olympus C-7070 camcorder, coupled to Olympus CX31 microscope and blindly evaluated. The histological sections stained were with hematoxylin-eosin (HE). The intensity of inflammation and hyperemia, infiltration type and the inflammatory process were classified as acute when polymorphonuclear cells predominated; or chronic when mononuclear cells predominated. The classification was further graded as scarse, moderate, or intense (Table 1). The inflammatory reaction was classified based on the predominate leukocyte type1212 Albuquerque-Júnior RLC, Barreto ALS, Pires AJ, Reis FP, Lima SO, Ribeiro MAG, Cardoso JC. Effect of bovine type-I collagen-based films containing red propolis on dermal wound healing in rodent model. Int J Morphol. 2009;27(4):1105-10. doi: 10.4067/S0717-95022009000400025.
https://doi.org/10.4067/S0717-9502200900...
.
Scores of intensity of hyperemia and hemosiderosis, adapted from Albuquerque-Júnior et al. 12.
To evaluate the intensity of inflammation used scale which measures in five scores this inflammation. The description of the form Semiquantitative are: absent, mild, moderate, and intense (Table 2).
Description of the parameters for histological assessment of the intensity of the inflammatory response in the wounded areas.
Quantitative analysis of stromal cells (fibroblasts and tenocytes)
Stromal cell counts were selected from cells having a morphology consistent with fusiform and either oval, with large nucleus and finely dispersed chromatin, which were interpreted as active fibroblasts and spindle cells with slender elongated nucleus, compact and densely arranged chromatin, which were interpreted as tenocytes1212 Albuquerque-Júnior RLC, Barreto ALS, Pires AJ, Reis FP, Lima SO, Ribeiro MAG, Cardoso JC. Effect of bovine type-I collagen-based films containing red propolis on dermal wound healing in rodent model. Int J Morphol. 2009;27(4):1105-10. doi: 10.4067/S0717-95022009000400025.
https://doi.org/10.4067/S0717-9502200900...
. The counting was performed in the Power point program from the photos of the selected areas, were divided into four to zoom in and placed a table over 10x10 squares, in quality were counted as cells that program within the squares excluding as they sloped in the rows of the table. The average number of stromal cells was determined by counting each of these morphological structures in each histological field. The average was determined from the mean of the ratio between the sum of structures and the number of analyzed fields.
Analysis of the architectural organization of the collagen fibers in tendons
The tendon organization was analyzed by using the deposition pattern of collagen fibers. The fibers were categorized according to their layout and appearance: in loose connective tissue (loose tissue network), thick and unshaped tissue (collagen fibers with random/varied architectural guidance), and densely modeled tissue (fibers arranged in the same direction with parallel disposition freely along the tendon axis)1212 Albuquerque-Júnior RLC, Barreto ALS, Pires AJ, Reis FP, Lima SO, Ribeiro MAG, Cardoso JC. Effect of bovine type-I collagen-based films containing red propolis on dermal wound healing in rodent model. Int J Morphol. 2009;27(4):1105-10. doi: 10.4067/S0717-95022009000400025.
https://doi.org/10.4067/S0717-9502200900...
.
Assessment of collagen deposition patterns
For the assessment of the collagen deposition, histological sections stained in Sirius Red and analyzed under polarized light were used to the descriptive analysis. Collagen fibers were analyzed according their birefringence pattern (greenish/yellow-greenish or orange, orange-reddish), morphological appearance (wavy or stretched, thin or thick, short or long), and architectural arrangement (reticular, parallel or interlaced)1212 Albuquerque-Júnior RLC, Barreto ALS, Pires AJ, Reis FP, Lima SO, Ribeiro MAG, Cardoso JC. Effect of bovine type-I collagen-based films containing red propolis on dermal wound healing in rodent model. Int J Morphol. 2009;27(4):1105-10. doi: 10.4067/S0717-95022009000400025.
https://doi.org/10.4067/S0717-9502200900...
. All readings were performed by blinded investigators.
Statistical analysis
All data were first subjected to analysis of Gaussian distribution. The program GraphPad Prism 6.01 was used for all statistical analyses. Statistically significant differences between mean values presenting Gaussian distribution were analyzed using Chi-square and Pearson Correlation qualitative variables test. Non-gaussian data were analyzed using Mann-Whitney U-test to comparison between different groups of the same cell, one-way ANOVA, and Tukey’s test for quantitative measurement. Each time point was analyzed separately. Differences between means were considered significant when p<0.05.
Results
The variables observed were: inflammatory infiltrate, hyperemia, stromal cells, architectural organization and assessment of the collagen fibers as described in Figure 2.
Histological sections the group Control (A1) and experimental treatment EOAz (A2) in 3-day, showing hyperemia and intense inflammatory infiltrate that is rich in macrophages (thick dark arrow), lymphocytes (thick arrow clear). (B1) Control group and (B2) in 14-day treatment group, showing remission of inflammatory infiltration and proliferation of spindle cells with nuclei that are sometimes spherical and clear [active fibroblasts (thick dotted arrow)] and sometimes dark and flattened [consistent with tenocytes (thin arrow crowded)]. (C1) Control group and (C2) 30-day treatment group, showing a lesser cell proportion in both groups compared in 14-day with (HE, x400).
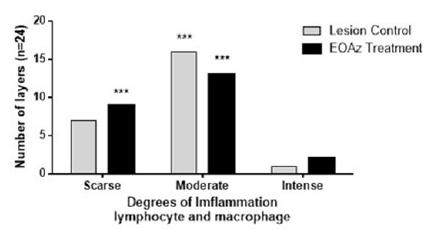
Three days after the tenotomy, the control and treated group specimens showed mainly lymphocytes. At control group, the number of lymphocytes was significantly higher than the monocytes, plasmocytes, neutrophils (p < 0.001 for all) and macrophages (p < 0.05). At treated group, the number of lymphocytes was significantly higher than monocytes, plasmocytes (p < 0.001 for both), neutrophils, and macrophages (p < 0.01; Figure 3). The slide of 3 days treatment of both groups showed a less significant inflammatory response than control group of lymphocytes and macrophages. The classification was chronic nonspecific inflammation at both groups. Control group showed an inflammatory moderate, and group EOAz was moderate and scarse response (Figure 4). Samples from the group treated with EOAz for 3 days after partial tenotomy of the Achilles tendon exhibited less significant hyperemia response than control group (Figure 5).
Mean±SEM number of cells found (lymphocytes, monocytes, plasmocytes, neutrophils and macrophage) in the tissue 3 days after partial tenotomy in the control and EOAz (33%)-treated rats (n=06). Lymphocyte and macrophage was significantly different (p<0.05; ANOVA ONE-WAY test and Tukey pos-test).
Selected were four layers per rats (n=6; layers =24). Intensity expressed as percent of degrees of imflammation response (less than 10% (Scarse); from 10 to 50% (Moderate); and higher than 50% (Intense) in the control and treated rats, 3 days after partial tenotomy controls were treated with vegetable oil while the test rats were treated with EOAz (33%). Effect response was significantly (***p<0.001, Chi-squared) between intensity express classification eat group.
Selected were four layers per rats (n=6; layers n=24). Intensity expressed as percent of degrees of hyperemia response (less than 10% (Scarse); from 10 to 50% (Moderate); and higher than 50% (Intense) in the control and treated rats, 3 days after partial tenotomy controls were treated with vegetable oil while the test rats were treated with EOAz (33%). Effect response was significantly (***p<0.001, Chi-squared) between groups.
At chronic and sub-acute inflammatory phases (14 and 30 days), were observed significant difference (p < 0.001 and p < 0.01) at the mean number of tenocytes and fibroblasts (Figure 6), between the treated and control groups.
Mean±SEM number of cells found (tenocytes and fibroblasts; n=6) in the tissue 14 days and 30 days after partial tenotomy in the control and EOAz- treated rats. Fibroblast control and EOAz cells was significantly different (p<0.05; ANOVA ONE-WAY test and Tukey pos-test).
Fourteen days after the tenotomy, was observed a insignificant (weak) correlation between the mean number of macrophages and a decrease in the number of tenocytes (34.90 ± 17.12; r, -0.17; p = 0.77) at control group. A similar but positive significant correlation was observed between the number of macrophages and an increase in the number of fibroblasts (30.85 ± 20.77; r, 0.17; p = 0.03). Treated group presented a decrease in the number of tenocytes (98.92 ± 17.63 r = -0.51; p=0.02) and fibroblasts (34.00 ± 6.46 r = - 0.64; p=0.003) in the tissue after partial tenotomy. These results were illustrated in Figure 7A and B.
Morphological analysis performed 90 days after tenotomy revealed a greater prevalence of modeled tissue (40%) in the treated rats than in the controls (18%), with the treated rats showing more organized tissue with fibers arranged in parallel (Figure 8).
Fourteen days after the tenotomy, was observed an insignificant (weak) correlation between the mean number of macrophages and a decrease in the number of tenocytes (34.90 ± 17.12; r, -0.17; p = 0.77) at control group. A similar but positive significant correlation was observed between the number of macrophages and an increase in the number of fibroblasts (30.85 ± 20.77; r, 0.17; p = 0.03). Treated group presented a decrease in the number of tenocytes (98.92 ± 17.63 r = -0.51; p=0.02) and fibroblasts (34.00 ± 6.46 r = - 0.64; p=0.003) in the tissue after partial tenotomy. These results were illustrated in Figure 7A and B.
Relative frequency of the appearance and arrangement of modeled and no modeled tissue according to the percentage measured at 90 days after EOAz - 33%, treatment after partial tenotomy. Effect response was significantly (***p<0.001, Chi-squared) between groups.
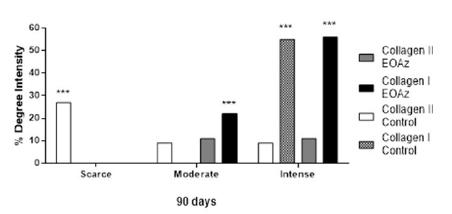
Arrangement analysis, appearance, and type of collagen after EOAz treatment for 90 days (Figures 9 and 10) revealed a higher proportion of type I collagen fibers (78%) in the treated rats. This finding indicated tissue with greater maturity in the EOAz-treated rats. The percentage of thick fibers (57%) was higher at EOAz-treated group than control group (45%).
Absolute and relative frequency of the appearance and arrangement of collagen type I and III fibers according to the percentage measured at 90 days after EOAz treatment after partial tenotomy. Effect response was significantly (***p<0.001, Chi-squared) between group.
Histological sections examined under polarized light areas of accumulation of collagen type III fibers (A), not modeled collagen type I fibers (B), and modeled collagen type I fibers (C) in the control (1) and treated (2) groups after 90 days (Picrossirius, x400).
Discussion
Investigate the degeneration and disorganization of collagen fibers, cellular response to injury, analyzing histopathologic changes associated with increased cellularity have been studied for atual researches. The investigation of the composition of EOAz and its potential biological activities have been extensively reported during the last 30 years, it is still a current topic. EOAz is still a promising raw material in terms of beneficial biological activities for several applications1313 Ghosh S, Rangan L. Alpinia: the gold mine of future therapeutics. Biotech. 2013 Jun;3(3):173-85. doi: 10.1007/s13205-012-0089-x.
https://doi.org/10.1007/s13205-012-0089-...
.
Traditional treatment aimed at modular inflammation type has had limited success in treating chronic, painful conditions arising from overuse of tendons. The acute inflammatory phase is characterized by polymorphonuclear cells; the sub-acute phase by mononuclear cells and polymorphonuclear; the chronic nonspecific phase by lymphocyte cells; and chronic inflammation macrophages-gigantocites specific. There is presence of inflammatory infiltrate with lymphocytes and macrophages in the synovial sheath of the tendon during healing1414 Hefti F, Stoll TM. Healing of ligaments and tendons. Orthopade. 1995 Jun;24(3):237-45. PMID: 7617380.. Clinical research1515 Brand C, Townley SL, Finlay-Jones JJ, Hart PF. Tea tree oil reduces histamine-induced oedema in murine ears. Inflamm Res. 2002 Jun;51(6):283-9. PMID: 12088268.
16 Wojciak B, Crossan JF.The accumulation of inflammatory cells in synovial sheath and epitenon during adhesion formation in healing rat flexor tendons. Clin Exp Immunol. 1993 Jul;93(1):108-14. doi: 10.1111/j.1365- 2249.1993.tb06505.x.
https://doi.org/10.1111/j.1365-...
17 Bastos JLN, Lizarelli RFZ, Parizotto NA. Comparativive study of laser and LED systems of low intensity applied to tendo healing. Laser Physics. 2009 July;19(9):1925-31. doi: 10.1134/S1054660X09170022.
https://doi.org/10.1134/S1054660X0917002...
18 Pagnano LO, Baraldi-Artoni SM, Pacheco MR, Santos E, Oliveira D, Lui JF. Moformetria de fibroblastos e fibrócitos durante o processo cicatricial na pele de coelhos da raça Nova Zelândia Branco tratados com calêndula. Ciênc Rural. 2008 Set;38(6):1662-6. doi: 10.1590/S0103-84782008000600026.
https://doi.org/10.1590/S0103-8478200800...
19 Mendonça RJ, Coutinho-Netto J. Aspectos celulares da cicatrização. An Bras Dermatol. 2009 July;84(3):257-62. doi: 10.1590/S0365-05962009000300007.
10.1590/S0365-05962009000300007...
20 Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009 Jan;15(1):42-9. doi: 10.1038/nm.1905.
https://doi.org/10.1038/nm.1905...
21 Manning CN, Martel C, Sakiyama-Elbert SE, Silva MJ, Shah S, Gelberman RH, Thomopoulos S. Adipose-derived mesenchymal stromal cells modulate tendon fibroblast responses to macrophage-induced inflammation in vitro. Stem Cell Res Ther. 2015 Apr;16;6:74. doi: 10.1186/s13287-015-0059-4.
https://doi.org/10.1186/s13287-015-0059-...
-66 Khalil Z, Pearce AL, Satkunanathan N, Storer E, Finlay-Jones JJ, Hart PH. Regulation of wheal and flare by tea tree oil: complementary human and rodent Studies. J Invest Dermatol. 2004 Oct;123(4):683-90. doi: 10.1111/j.0022-202X.2004.23407.x.
https://doi.org/10.1111/j.0022-202X.2004...
reported that terpinen-4-ol and 1,8 cineole are the main monoterpenes found in Alpinia zerumbet. These compounds inhibit the histamine-induced inflammation and increase capillary and venular permeability, which is essential for cellular infiltration. Histamine-induced related swelling in murine ears suppressed was per terpinen-4-ol1616 Wojciak B, Crossan JF.The accumulation of inflammatory cells in synovial sheath and epitenon during adhesion formation in healing rat flexor tendons. Clin Exp Immunol. 1993 Jul;93(1):108-14. doi: 10.1111/j.1365- 2249.1993.tb06505.x.
https://doi.org/10.1111/j.1365-...
. According to these studies, EOAz treatment was effective in decreasing the inflammatory process, suggesting that monoterpenes inhibited the formation of inflammatory process mediators.
The data found in the current study corroborate the authors, presenting significant difference between groups with respect to intensity of hyperemia in 3 days.
Recovery after tendon injury includes infiltration of inflammatory cells, fibroblast proliferation and growth factor release1717 Bastos JLN, Lizarelli RFZ, Parizotto NA. Comparativive study of laser and LED systems of low intensity applied to tendo healing. Laser Physics. 2009 July;19(9):1925-31. doi: 10.1134/S1054660X09170022.
https://doi.org/10.1134/S1054660X0917002...
. Results of this study are in accordance with the authors, considering that the observations included inflammatory cell proliferation with predominance of lymphocytes in the control and treated groups 3 days after tenotomy. In addition, rats treated with EOAz for 14 days showed a significant increase in fibroblast count, which was promoted by macrophages involved in immune activity, phagocytic activity and chemotactic activity that preceded tissue repair.
Results demonstrated that the inflammatory response to tendon injury was lower when treated with EOAz than with vegetable oil treatment. The terpenoid 1,8-cineole has anti-inflammatory properties, being a potent inhibitor of Tumor Necrosis Factor alpha (TNF-α) and interleukin (IL) 1b production by monocytes and lymphocytes; it also inhibits IL-8 and IL518. The anti-inflammatory action of terpene-4-ol suppressed TNF-α, IL-1b, IL-8, IL-10 and PGE2 production by lipoproteins activated by monocytes. The present results (Figure 2) corroborate these authors and show that EOAz depressed the inflammatory response, increased lymphocyte and macrophage concentration and decreased the hyperemia response.
After the inflammatory phase, fibroblasts are recruited to initiate collagen synthesis that promotes repair1212 Albuquerque-Júnior RLC, Barreto ALS, Pires AJ, Reis FP, Lima SO, Ribeiro MAG, Cardoso JC. Effect of bovine type-I collagen-based films containing red propolis on dermal wound healing in rodent model. Int J Morphol. 2009;27(4):1105-10. doi: 10.4067/S0717-95022009000400025.
https://doi.org/10.4067/S0717-9502200900...
. The mitogenic effect of the beta transformer growth factor (BTGF) can improve tendon regeneration by increasing the number of necessary cells available for subsequent extracellular matrix synthesis. This pattern probably occurred in the present study, considering that it was observed an increase in macrophages and a significant increase (p < 0.01) in fibroblasts in EOAz-treated rats compared with controls.
The primary function of tenocytes and fibroblasts is the maintenance of tissue integrity1919 Mendonça RJ, Coutinho-Netto J. Aspectos celulares da cicatrização. An Bras Dermatol. 2009 July;84(3):257-62. doi: 10.1590/S0365-05962009000300007.
10.1590/S0365-05962009000300007...
. The results indicated that the highest values for tenocytes and fibroblasts was on day 14, indicating strong involvement of these cells in the proliferative phase. Nevertheless, it was found that the cells decreased over time, particularly in rats treated for 30 days. These results are in agreement with those from a previous paper that described the healing process as having three continuous phases in which the process of maturation and remodeling implies on the disappearance of inflammatory cells, leading to scarring or regeneration2020 Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009 Jan;15(1):42-9. doi: 10.1038/nm.1905.
https://doi.org/10.1038/nm.1905...
.
Mesenchymal application of stromal cells, as effect modulate on inflammatory environment may improve the healing response. Mesenchymal adipose derived stromal cells mediated modulation of the post-operative inflammatory response may be beneficial for tendon injury. A significant increase in fibroblasts was observed after 14 days compared with fibroblasts after 30 days (p < 0.001)2121 Manning CN, Martel C, Sakiyama-Elbert SE, Silva MJ, Shah S, Gelberman RH, Thomopoulos S. Adipose-derived mesenchymal stromal cells modulate tendon fibroblast responses to macrophage-induced inflammation in vitro. Stem Cell Res Ther. 2015 Apr;16;6:74. doi: 10.1186/s13287-015-0059-4.
https://doi.org/10.1186/s13287-015-0059-...
.
Histological results showed that EOAz promoted better organization and orientation of collagen fibers (fibers stretched) and the formation of thicker collagen fibers, which indicate the presence of repaired tissue with an improved ability to support tensional load. However, it was affirmed that injured tendons contain a higher proportion of collagen type III fibers, which are weaker because of fewer cross-links and inter- and intra-unities.
Therefore, the EOAz-treated group showed the opposite effect because the results revealed a higher proportion of type I collagen fibers (78%) compared with type III fibers (22%). Type I collagen is characterized by large tensile strengths and is capable of withstanding large loads.
Conclusions
Alpinia zerumbet administered after partial tenotomy was able to producing an appropriate sequence of events from the acute phase (hyperemia decrease) to the remodeling tissue phase. EOAz induced an increased number of fibroblasts within 14 days, which decreased after 30 days. After 90 days of EOAz treatment, dense tissue maturation characterized by a pattern of modeled, thick fiber, and rich type I collagen tissue.
References
-
1Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Appl Biomech. 2004 Jun;37(6):865-77. doi: 10.1016/j.jbiomech.2003.11.005.
» https://doi.org/10.1016/j.jbiomech.2003.11.005 -
2Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology. 2006 May;45(5):508-21. doi: 10.1093/rheumatology/ke1046.
» https://doi.org/10.1093/rheumatology/ke1046 -
3Yeung CK, Guo X, NG YF. Pulsed ultrasound treatment accelerates the repair of achilles tendon rupture in rats. J Orthop Res. 2006 Feb;24(2):193-201. doi: 10.1002/jor.20020.
» https://doi.org/10.1002/jor.20020 -
4Fasciani I, Temperan A, Perez-Atencio LF, Escudero A, Martinez-Montero P, Molano J, Gomez-Hernandez JM, Paino CL, Gonzalez-Nieto D, Barrio LC. Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease. Neuropharmacology. 2013 Dec;75:479-90. doi: 10.1016/j.neuropharm.2013.03.040.
» https://doi.org/10.1016/j.neuropharm.2013.03.040 -
5Koh KJ, Pearce AL, Marshman G, Finlav-Jones JJ, Hart PH. Tea tree oil reduces histamine-induced skin inflammation. Br J Dermatol. 2002 Dec;147(6):1212-7. doi: 10.1046/j.1365-2133.2002.05034.x.
» https://doi.org/10.1046/j.1365-2133.2002.05034.x -
6Khalil Z, Pearce AL, Satkunanathan N, Storer E, Finlay-Jones JJ, Hart PH. Regulation of wheal and flare by tea tree oil: complementary human and rodent Studies. J Invest Dermatol. 2004 Oct;123(4):683-90. doi: 10.1111/j.0022-202X.2004.23407.x.
» https://doi.org/10.1111/j.0022-202X.2004.23407.x -
7Sapra B, Jain S, Tiwary AK. Percutaneous permeation enhancement by terpenes: mechanistic view. AAPS. 2008 Mar;10(1):120-32. doi: 10.1208/s12248-008-9012-0.
» https://doi.org/10.1208/s12248-008-9012-0 -
8Cavalcanti BC, Ferreira JR, Cabral IO, Magalhães HI, Oliveira CC, Rodrigues FA, Rocha DD, Barros FW, Silva CR, Júnior HV, Canuto KM, Silveira ER, Pessoa C, Moraes MO. Genetic toxicology evaluation of essential oil of alpinia zerumbet and its chemoprotective effects against H(2)O(2)-induced DNA damage in cultured human leukocytes. Food Chem Toxicol. 2012 Nov;50(11):4051-61. doi: 10.1016/j.fct.2012.03.038.
» https://doi.org/10.1016/j.fct.2012.03.038 -
9Chen Y, Li D, Xu Y, Zhang Y, Tao L, Li S, Jiang Y, Shen X. Essential oils from fructus a. zerumbet protect human aortic endothelial cells from apoptosis induced by Ox-LDL in vitro. Evid Based Complement Alternat Med.?2014;2014:956824. doi: 10.1155/2014/956824.
» https://doi.org/10.1155/2014/956824 -
10Hase E, Sato K, Yonekura D, Minamikawa T, Takahashi M, Yasui T. Evaluation of the histological and mechanical features of tendon healing in a rabbit model with the use of second-harmonic-generation imaging and tensile testing. Bone Joint Res. 2016;5(11):577-85. doi: 10.1302/2046-3758.511.BJR-2016-0162.R1.
» https://doi.org/10.1302/2046-3758.511.BJR-2016-0162.R1 -
11Jacobson E, Dart Aj, Mondori T, Horadogoda N, Jeffcott LB, Little CB, Smith MM. Correction: focal experimental injury leads to widespread gene expression and histologic changes in equine flexor tendons. PLoS One. 2016 Mar 1;11(3):e0150823. doi: 10.1371/journal.pone.0150823.
» https://doi.org/10.1371/journal.pone.0150823 -
12Albuquerque-Júnior RLC, Barreto ALS, Pires AJ, Reis FP, Lima SO, Ribeiro MAG, Cardoso JC. Effect of bovine type-I collagen-based films containing red propolis on dermal wound healing in rodent model. Int J Morphol. 2009;27(4):1105-10. doi: 10.4067/S0717-95022009000400025.
» https://doi.org/10.4067/S0717-95022009000400025 -
13Ghosh S, Rangan L. Alpinia: the gold mine of future therapeutics. Biotech. 2013 Jun;3(3):173-85. doi: 10.1007/s13205-012-0089-x.
» https://doi.org/10.1007/s13205-012-0089-x -
14Hefti F, Stoll TM. Healing of ligaments and tendons. Orthopade. 1995 Jun;24(3):237-45. PMID: 7617380.
-
15Brand C, Townley SL, Finlay-Jones JJ, Hart PF. Tea tree oil reduces histamine-induced oedema in murine ears. Inflamm Res. 2002 Jun;51(6):283-9. PMID: 12088268.
-
16Wojciak B, Crossan JF.The accumulation of inflammatory cells in synovial sheath and epitenon during adhesion formation in healing rat flexor tendons. Clin Exp Immunol. 1993 Jul;93(1):108-14. doi: 10.1111/j.1365- 2249.1993.tb06505.x.
» https://doi.org/10.1111/j.1365- -
17Bastos JLN, Lizarelli RFZ, Parizotto NA. Comparativive study of laser and LED systems of low intensity applied to tendo healing. Laser Physics. 2009 July;19(9):1925-31. doi: 10.1134/S1054660X09170022.
» https://doi.org/10.1134/S1054660X09170022 -
18Pagnano LO, Baraldi-Artoni SM, Pacheco MR, Santos E, Oliveira D, Lui JF. Moformetria de fibroblastos e fibrócitos durante o processo cicatricial na pele de coelhos da raça Nova Zelândia Branco tratados com calêndula. Ciênc Rural. 2008 Set;38(6):1662-6. doi: 10.1590/S0103-84782008000600026.
» https://doi.org/10.1590/S0103-84782008000600026 -
19Mendonça RJ, Coutinho-Netto J. Aspectos celulares da cicatrização. An Bras Dermatol. 2009 July;84(3):257-62. doi: 10.1590/S0365-05962009000300007.
» 10.1590/S0365-05962009000300007 -
20Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009 Jan;15(1):42-9. doi: 10.1038/nm.1905.
» https://doi.org/10.1038/nm.1905 -
21Manning CN, Martel C, Sakiyama-Elbert SE, Silva MJ, Shah S, Gelberman RH, Thomopoulos S. Adipose-derived mesenchymal stromal cells modulate tendon fibroblast responses to macrophage-induced inflammation in vitro. Stem Cell Res Ther. 2015 Apr;16;6:74. doi: 10.1186/s13287-015-0059-4.
» https://doi.org/10.1186/s13287-015-0059-4
-
Financial source:
none
-
1
Research performed at Bio Laboratory of Morphology and Structural Biology (LMBE), Laboratory of Biological Studies and Natural Products, Research and Technology Institute - ITP, Universidade Tiradentes (UNIT), Aracaju-SE, Brazil.
Publication Dates
-
Publication in this collection
June 2017
History
-
Received
23 Feb 2017 -
Reviewed
25 Apr 2017 -
Accepted
24 May 2017