Abstract
PURPOSE: To investigate the hemopreventive effect of defatted flaxseed meal in C57BL/6 mice after induction of precancerous colon lesions with 1.2-dimethylhydrazine (DMH). METHODS: Thirty-six 12-week-old C57BL/6 mice were divided into three treatment groups(n=12 in each group): (1) diet with 10% defatted flaxseed meal; (2) diet with defatted flaxseed meal and precancerous colon lesions induced by DMH; and (3) precancerous colon lesions induced by DMH, without defatted flaxseed meal. The incidence of aberrant crypt foci (ACF), oxidative processes, expression of tumor suppressor proteins and cyclins, as well as the profile of short-chain fatty acids (SCFA) in animal feces were investigated in the presence and absence of DMH. RESULTS: The rats consuming defatted flaxseed meals showed lesions with lower multiplicity and a reduced incidence of lesions. No changes in the expression of tumor suppressor proteins and those involved in cell cycle control were detected. CONCLUSION: Defatted flaxseed meal protected the distal colon of mice from precancerous lesions.
Precancerous Conditions; Cell Cycle; Colorectal Neoplasms; Mice
9 - ORIGINAL ARTICLE
EXPERIMENTAL ONCOLOGY
Use of defatted flaxseed meal reduces precancerous colon lesions in C57BL/6 mice1 1 Research performed at Department of General Biology, Federal University of Viçosa (UFV), Minas Gerais, Brazil. Part of PhD degree thesis, Postgraduate Program in Department of General Biology. Tutor: Sérgio Oliveira de Paula.
Antônio Frederico de Freitas GomidesI; Sérgio Oliveira de PaulaII; Damiana Diniz RosaIII; Leandro Licusi de OliveiraII; Débora Silva ComastriIV; Maria do Carmo Gouveia PeluzioV
IPhD, Associate Professor, Department of Nutrition, Federal University of Juiz de Fora (UFJF), Minas Gerais, Brazil. Conception and design of the study, acquisition and interpretation of data, statistical analysis, drafting the article, critical revision, approving the final version
IIPhD, Associate Professor, Department of General Biology, Federal University of Viçosa (UFV), Viçosa-MG, Brazil. Conception and design of the study, drafting the article, critical revision, approving the final version
IIIFellow PhD degree, Department of Nutrition and Health, UFV, Viçosa-MG, Brazil. Interpretation of data, statistical analysis, critical revision, approving the final version
IVNutritionist, Department of Nutrition and Health, UFV, Viçosa-MG, Brazil. Interpretation of data, drafting the article, critical revision, approving the final version
VPhD, Associate Professor, Department of Nutrition and Health, UFV, Viçosa-MG, Brazil. Conception and design of the study, interpretation of data, critical revision, approving the final version
Correspondence Correspondence: Maria do Carmo Gouveia Peluzio Departamento de Nutrição e Saúde Universidade Federal de Viçosa Avenida Peter Henri Rolfs, s/n, Campus Universitário 36570-000 Viçosa MG Brasil Tel.: (55 31)3899-1275 Fax: (55 31)3899-2541 mpeluzio@ufv.br
ABSTRACT
PURPOSE: To investigate the hemopreventive effect of defatted flaxseed meal in C57BL/6 mice after induction of precancerous colon lesions with 1.2-dimethylhydrazine (DMH).
METHODS: Thirty-six 12-week-old C57BL/6 mice were divided into three treatment groups(n=12 in each group): (1) diet with 10% defatted flaxseed meal; (2) diet with defatted flaxseed meal and precancerous colon lesions induced by DMH; and (3) precancerous colon lesions induced by DMH, without defatted flaxseed meal. The incidence of aberrant crypt foci (ACF), oxidative processes, expression of tumor suppressor proteins and cyclins, as well as the profile of short-chain fatty acids (SCFA) in animal feces were investigated in the presence and absence of DMH.
RESULTS: The rats consuming defatted flaxseed meals showed lesions with lower multiplicity and a reduced incidence of lesions. No changes in the expression of tumor suppressor proteins and those involved in cell cycle control were detected.
CONCLUSION: Defatted flaxseed meal protected the distal colon of mice from precancerous lesions.
Key words: Precancerous Conditions. Cell Cycle. Colorectal Neoplasms. Mice.
Introduction
Cancer is a major public health problem in the United States and many other parts of the world. Among the different types of cancer, colorectal cancer (CRC) is the third most common type worldwide in both sexes1. CRC is characterized by uncontrolled growth of malignant cells arising from a series of genetic errors that accumulate over the years, which have invasive potential and may spread to other regions2.
Evidence from ecological, migrant, and secular trend studies suggests that environmental risk factors are of major importance in the cause of colorectal cancer3, including smoking, obesity, sedentary lifestyle, aging, and eating habits4. The World Cancer Research Fund and the American Institute for Cancer Research5 concluded that colorectal cancer is mostly preventable by appropriate diets and associated factors.
Diet is the most important exogenous risk factor in the etiology of CRC6. Foods containing dietary fiber protect against this cancer7. Several plausible mechanisms have been proposed to explain this, including increased stool bulk and dilution of carcinogens in the colonic lumen, a reduced transit time, and bacterial fermentation of fiber into short-chain fatty acids (SCFA)8, which can control gene expression in colonocytes, thus working as a shield against CRC9,10.
Flaxseed meals have been used as food in many countries around the world. Flaxseed has three major components that make it beneficial in human nutrition: a very high content of alpha linolenic acid (omega-3 fatty acid); a high percentage of dietary fiber, both soluble and insoluble; and the highest content of plant ''lignans''11. Flaxseed provides as much as 75800 times the amount of lignans compared to other oil seeds, cereals, legumes, fruits and vegetables12. It is also a particularly rich source of the lignan secoisolariciresinol diglycoside (SDG). SDG is a plant lignan that is converted into mammalian lignans by bacteria in the colon of humans and other animals. They are biologically active phytochemicals with apparent anticancer and antioxidant potential13. When consumed, SDG is converted by intestinal microflora into two lignan metabolites, enterodiol and enterolactone. Lignan metabolites act as antioxidants and free radical scavengers11, which may decrease the risk of carcinogenesis, protecting the cell from free radicals and DNA damage14.
Carcinogenesis involves multiple steps, which includes carcinogens causing mutations in DNA that may or may not be corrected by DNA repair genes. When DNA damage is intense and the repair is insufficient, the mechanism of apoptosis is activated and the cell is eliminated. The activation of repair and apoptosis may occur through the action of p53, which stops the cell cycle, checks for possible DNA damage and, if necessary, induces repair or triggers apoptosis. However, when a mutation occurs in the p53 gene itself, the p53 protein becomes non-functional, leading to genetic instability and malignant transformation. In general, mutations in tumorigenesis may cause activation of oncogenes, inactivation of tumor suppressor genes and alterations in DNA repair genes. The proteins p16, p21 and p53 are inhibitors of the cyclin-dependent kinase (CDK) 4 and 6, besides cyclin D1, which is responsible for the phosphorylation of the pRB repressor protein, leading to cell cycle arrest in G115.
The purpose of this investigation was to evaluate the chemopreventive effect of defatted flaxseed meal in C57BL/6 mice after the induction of precancerous colon lesions with DMH. The effects of this meal on the incidence of ACF, oxidative processes and expression of the tumor suppressor proteins p53, p16 and p21, as well as cyclins (D and E), were determined. We also evaluated the profile of the short-chain fatty acids (SCFA) acetate, propionate and butyrate in animal feces in the presence and absence of DMH.
Methods
Experiment approved by the Ethics Committee of the Department of Veterinary Medicine of the Federal University of Viçosa, processed as protocol number 61/2009/DVT-UFV, and the experiment was carried out according to the Ethical Principles in Animal Experimentation, adopted from the National Council for Control of Animal Experimentation (CONCEA).
Defatted flaxseed meal
The defatted flaxseed meal used in the experiment was acquired from the company Cisbra® Foods, and had the following chemical composition: 6.6% carbohydrates, 22.1% protein, 16% total fat (0.3% saturated fatty acids, 3.3% monounsaturated fatty acids (w-9) and 10% polyunsaturated fatty acids (8% alpha-linolenic acid and 2% alpha-linolenic acid)) and 40.26 % dietary fiber, giving a total caloric value of 1086.7 kj/100g meal.
Experimental design
A case-control study was performed that lasted 15 weeks. Thirty-six 12-week-old C57BL/6 mice, weighing approximately 25 grams, were used. The animals were obtained from the Animal Colony of the Biological Sciences Center at Federal University of Viçosa and maintained in collective boxes in a controlled environment with a temperature of 23 ± 2°C and a light/dark cycle of 12h. Animals were given water and a modified AIN-93M diet ad libitum16. The experimental groups were divided into mice with and without DMH administration, and with and without 10% of defatted flaxseed meal instead of cellulose added to their diet over 15 weeks. The animals were divided into three treatment groups (n=12 in each group): F - AIN93M diet with defatted flaxseed meal; FCA- AIN93M diet with defatted flaxseed meal and precancerous colon lesions; and CA- AIN93M diet and precancerous colon lesions.
Induction of ACF via the administration of DMH and collection of material
ACF was induced by giving an intraperitoneal injection of 20 mg of 1.2-dimethylhydrazine/kg of body weight (SIGMA Chemical Co., St. Louis, MO) once a week for eight weeks. DMH was prepared immediately before use, dissolved in 0.9% NaCl containing 1 mM EDTA and 10 mM sodium citrate as the vehicle, at a final concentration of 20 mg/kg, and the final pH was adjusted to pH 817. The animals in the control group (F) received the vehicle without DMH. Seven weeks after the last DMH application, the animals were euthanized by CO2 asphyxiation. Following euthanization, we weighed the liver of the mice on an analytic balance Shimadzu® with an accuracy of 0.01 mg. The colon and liver were stored at -80°C for future analysis.
The large intestine was removed from the cecum to the anus to assess the presence of ACF. Catalase activity was measured in the distal colon and liver of animals to evaluate the oxidative stress caused by DMH. mRNA levels were also quantified in the distal colon to evaluate the gene expression of cyclin-D1, cyclin-E, p16, p21 and p53. Feces were collected from the colon and cage to quantify SCFA (acetic, propionic and butyric). The liver, spleen, kidney and heart were weighed to evaluate the homeostasis of the animal.
Aberrant crypt foci counts
The large intestine was removed, washed in saline solution, and fixed in Carson's formalin18. After 48h of fixation, the intestine was divided into three fragments of equal length, i.e., proximal, middle, and distal regions. They were stained with 0.1% methylene blue for approximately 2 min and washed in phosphate buffer. Lesions were counted using optical microscopy with a ×40 magnification, according to the technique of Bird19. ACF was counted across the mucosal surface of the colon, from the cecum to the rectum, double-blindly, by two observers. Categorization of the ACF was based on the number of aberrant crypts per focus, or foci with three or fewer crypts and foci with more than three crypts, according to the method of Rosa et al.1.
Extraction and concentration of SCFA by HPLC in feces from the colon and cage
Feces analysis was performed in duplicate. Approximately 400 mg of feces were weighed in microtubes and 500 µL of m-phosphoric acid were added at 25%, the mixture then being agitated and left to stand for 30 min at room temperature. Following centrifugation at 13.200 g for 30 min, the supernatant was collected, centrifuged at 13.200 g for 20 min and frozen at - 20ºC.
To determine the concentration of SCFA, feces samples were centrifuged for 10 min at 13.200 g and applied to a SHIMADZU SPD-10A VP High Performance Liquid Chromatographer (HPLC) coupled to a UV detector using a 210-nm wavelength. To perform chromatographic separation, 20 µL of the sample were injected in a 101H SHIMADZU 30 cm x 7.9 mm SCR mobile column at a flow rate of 8.0 ml/min and 24 Kgf pressure using water in 1% metaphosphoric acid as the mobile phase, according to the method of Smirick-Tjardes et al.20.
Quantification of mRNA of cyclin-D1, cyclin E, p16, p21 and p53 by Real-Time PCR
RNA extraction
For RNA extraction, 100 mg of distal colon tissue (n=4/group) was collected and treated with proteinase K (Sigma Aldrich®, St. Louis, USA). Total mRNA was extracted by incubating the colon tissue with 500 µL Trizol (Invitrogen, CA, USA) in a 750-µL tube for 5 min at room temperature. Then, 300 µL of chloroform were added, and the mixture manually mixed for 15 min and left to stand at room temperature for 2 min before centrifugation (12,000 g for 15 min) at 4oC.
The aqueous phase was transferred to another 2-mL tube, into which 350 µL of isopropanol were added. The samples were incubated at room temperature for 10 min before being centrifuged (12,000 g for 10 min). The supernatant was carefully discarded and the precipitate was added to 750 µL of ethanol (75%) and kept overnight at -70 °C. The samples were then centrifuged at 7.500 g for 10 min, the supernatant discarded and the precipitate dried at 37°C. The samples were suspended in 20 µL of distilled water, autoclaved and incubated at 35°C for 10 min to solubilize the extracted RNA. Approximately 10 µg of each total RNA were used to synthesize the first cDNA strands, using primers for cyclin-D1, cyclin-E, p16, p21 and p53.
Synthesis of cDNA
After extracting total RNA from the samples, we synthesized the first cDNA strand of cyclin-D1, cyclin-E, p16, p21 and p53, using the Superscript™ kit (Invitrogen®, New York, USA). To do so, 1 µL of random primer (Amersham-Pharmacia, Buckinghamshire, England) was added to approximately 2 µg of RNA from the colon, then heated for 10 min at 70ºC. A mixture containing the reverse transcriptase buffer, DTT, reverse transcriptase and dNTPs was prepared, heated and added to the sample. The resulting solution was incubated for 1h at 42ºC and for 1 min at 70ºC.
Gene expression of cyclin-D1, cyclin-E, p16, p21 and p53 were evaluated in the distal colon. After 24 h, the colon was cleared by centrifugation at 10.000 g for 2 min and subjected to RNA extraction and synthesis of complementary DNA, as previously described. The evaluation of gene expression was performed by real-time PCR, where the quantification of these mRNAs was compared to the expression of mRNAs of a constitutively expressed housekeeping gene (b-actin). To this end, about 2 mg of cDNA from the colon were used for real-time PCR, in a 50ml reaction mixture containing 0.45 nmol of sense and antisense primers specific for each gene (Table 1), 20 U/ml of RNAse inhibitor and 12.5 ml of the SYBR Green PCR Master Mix. The cycling conditions were 95°C for 10 min followed by 40 cycles of 94°C for 1 min, 56°C for 1 min and 72°C for 2 min. Quantification of the transcriptional expression of each gene was performed using the Gene Amp® 5700 Sequence Detection System Version 1.3 software (Applied Biosystems)21,22.
Statistical analysis
The variables were tested for normality before the analysis of variance. When the assumptions of normality were not met, even after appropriate transformations, the data were submitted to a non-parametric test. Comparisons between three or more independent groups were performed by analysis of variance (ANOVA) for normally distributed data (Tukey's multiple comparison test) and the non-parametric Dunn's test. When the goal was to compare two treatments, the t-test was used.The value (p) was fixed up to 5% to obtain 95% of reliability in the comparisons for Tukey's test.
Results
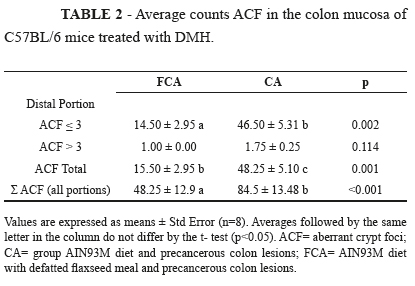
At the end of the experiment, no difference was found between the treatments with respect to weight gain and organ weights (the liver, heart, and kidney) (p>0.05). After 15 weeks of treatment with defatted flaxseed meal, there was no difference between treatments in the dosage of the enzyme catalase in liver tissue and gut (data not shown). The presence of ACF in the colon mucosa of mice treated with the carcinogen DMH demonstrated the efficiency of the induction of precancerous lesions. The total ACF count in the proximal and middle portions of the colon and the categorization of ACF incidence as ≤3 or >3 showed no differences between the treatments (p>0.05) (data not shown). In the distal portion, the animals with neoplastic lesions and pre-treated flaxseed meal defatted.
The defatted flaxseed meal elicited lesions with lower multiplicity (ACF distal ≤ 3) (p= 0.002). In the distal portion of the colon of FCA mice, there was a lower incidence of ACF (p= 0.001). When assessing the incidence of precancerous injury along the entire length of the colon, defatted flaxseed meal reduced the incidence of lesions (p<0.001), showing 43-% fewer ACF than the group CA mice (Table 2).
Values are expressed as means ± Std Error (n=8). Averages followed by the same letter in the column do not differ by the t- test (p<0.05). ACF= aberrant crypt foci; CA= group AIN93M diet and precancerous colon lesions; FCA= AIN93M diet with defatted flaxseed meal and precancerous colon lesions.
The SCFA profile of acetate, propionate and butyrate was evaluated in the feces collected from the colon and cage at the end of the experiment. No difference was observed (p>0.05) in the concentration of SCFA between treatments (Table 3).
Values are expressed as means ± Std Error (n=8). Averages followed by the same letter in the column do not differ by the Tukey test (p<0.05) F= group AIN93M diet; CA= group AIN93M diet and precancerous colon lesions; FCA= AIN93M diet with defatted flaxseed meal and precancerous colon lesions
Quantification of the mRNA levels of cyclin-D1, cyclin E, p16, p21 and p53 was performed in the colonic mucosa, using real-time PCR. The expression of these genes did not differ among the treatments (p>0.05), except for p16, which was expressed less in the CA group compared to the F group (p= 0.019) (Table 4).
Values are expressed as means ± Std Error (n=8). Averages followed by the same letter in the column do not differ by the Tukey test (p<0.05) F= group AIN93M diet; CA= group AIN93M diet and precancerous colon lesions; FCA= AIN93M diet with defatted flaxseed meal and precancerous colon lesions.
Discussion
The present study evaluated the preventive effects of defatted flaxseed meal on the incidence of ACF in the proximal, middle and distal colon of C57BL/6 mice administered DMH. For decades, experiments have been performed in rodents to induce colon cancer, using a variety of carcinogenic drugs such as the synthetic analogs DMH and azoxymethane (AOM). The disadvantage of these models is the lack of an invasive and metastatic phenotype; nevertheless, rodent models are widely used to elucidate the adenoma-carcinoma sequence, since they are similar to the events occurring in human CRC23.
Our results showed that defatted flaxseed meals could protect animals from precancerous colon lesions. The highest number of ACF induced by DMH and the protective effect of the meal was evident in the distal colon. The higher number of lesions in the distal portion of the colon in rodents treated with DMH has been reported in other studies1,24. DMH induces proliferation of colon tumors, especially in the rectum and sigmoid portion, which may also occur around the duodenum-jejunum junction. The mechanism of action of this drug probably involves the disruption of the DNA methylation mediated by cytochrome P45025.
Defatted flaxseed meal has a preventive effect against precancerous lesions in the distal colon of rodents. Jenab et al.26 studied rats with colon cancer induced by AOM and fed a control high-fat diet or treatment diets containing: 2.5% flaxseed, 5% flaxseed; 2.5% defatted flaxseed, 5% flaxseed and defatted control diet with high-fat daily gavage with 5 mg of SDG (a lignan present in flaxseed). The authors noted that with the exception of the control group (diet with a high fat content), the number of ACF was significantly reduced in the distal colon of rats in all the treatment groups. Four micro adenomas and two polyps were found only in the control group. These results reinforce our findings that flaxseed has a protective effect on the formation of ACF.
In another study, the authors compared the effect of flaxseed meal and corn in rats that were induced colon cancer with AOM. The number, location and size of the tumors were significantly lower in those receiving flaxseed meal, as was the protein expression of COX-1 and COX-2, which are involved in the inflammation process27. Williams et al.11 compared the protective effect of flaxseed oil and flaxseed meal in rats treated with AOM. They found that flaxseed oil and meal reduced the incidence of ACF in the rat intestine after induction by AOM, therefore acting as CRC preventive agents. The protective effect of oils (corn and flaxseed) and meal (corn and flaxseed) were investigated in APC mutant mice. The multiplicity and size of tumors in the small intestine and colon of rats were significantly lower in the groups receiving the meal and flaxseed oil, further confirming the chemopreventive effect of flaxseed28.
Given the results, we need to clarify how the flaxseed meal could exert protective effects. One hypothesis is that it does so via antioxidant activity. Recent studies indicate that a high level of catalase expression could be associated with less genetic damage, thereby reducing the risk of CRC29 and the oxidative stress generated by DMH. However, we observed no difference in the expression of catalase in the liver and intestine following the treatments.
In the present study, we attempted to relate the defatted flaxseed meal effects with the increased expression of the tumor suppressor proteins p53, p21 and p16 and a decreased expression of proteins involved in cell cycle control, cyclin E and cyclin D1. Defatted flaxseed meal in DMH-treated animals did not affect the expression of these proteins.
The consumption of defatted flax flour did not increase the concentration of fecal SCFA. We had expected defatted flaxseed meal to increase the production of SCFA due to the presence of soluble fiber in the food. Our results are not consistent with those in the literature since it was not possible to determine whether the SCFAs protected the colon from an increased incidence of ACF.
Flaxseed contains 58% of α-linolenic acid (C18: 3 w-3)30, but this decreases to only 2% following food processing. Some studies show the potential of α-linolenic acid in the prevention of colon cancer11,31.
Our results demonstrated that defatted flaxseed bran protected the distal colon of mice from ACF. However, the mechanisms underlying this protection are not clear. The animals fed defatted flaxseed meal showed no change in the expression of tumor suppressor genes and those involved in cell cycle control. The protective effect could be related to lignan, the main source of fiber in flaxseed meal. According to Qu et al.32, lignan metabolites act as antioxidants and free radical scavengers, which may decrease the risk of cancer development. They have already been shown to reduce epithelial cell proliferation and the number of aberrant crypt foci in animal models.
Taken together, our findings suggest that more studies need to be performed to try to elucidate the mechanisms, focusing on the relationship between fiber intake and the modulation of gut microbiota. According to Davis and Milner33, the colonic microbiota has a critical role in setting the tone for a healthy bowel, including the risk for developing colorectal cancer. Key physiological functions that might be related to cancer risk include the control of epithelial cell proliferation and differentiation, production of essential nutrients and/or bioactive food components, prevention of overgrowth of pathogenic organisms, and stimulation of intestinal immunity. Thus, the clinical application of defatted flaxseed meal could be justified.
Conclusion
In this study, none of the tests showed a potential beneficial effect against pre-cancerous lesions. Nevertheless, the defatted flaxseed was able to protect the development of lesion in the distal colon of mice.
Received: April 15, 2013
Review: June 17, 2013
Accepted: July 18, 2013
Conflict of interest: none
Financial sources: Minas Gerais Research Foundation (FAFEMIG) and National Council of Technological and Scientific Development (CNPq)
- 1. Rosa DD, Lourenço FC, da Fonseca ACM, de Sales RL, Ribeiro SMR, Neves CA, Peluzio MCG. Fish oil improves the lipid profile and reduces inflammatory cytokines in wistar rats with precancerous colon lesions. Nutr Cancer. 2012;64(4):569-79.
- 2. Jullumstrø E, Lydersen S, Møller B, Dahl O, Edna TH. Duration of symptoms, stage at diagnosis and relative survival in colon and rectal cancer. Eur J Can. 2009;45(13):2383-90.
- 3. Aune D, Chan DSM, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617.
- 4. Wallace H, Caslake R. Polyamines and colon cancer. Eur J Gastroenterol Hepatol. 2001;13:1033-9.
- 5. Marmot M, Atinmo T, Byers T, Chen J, Hirohata T, Jackson A, James W, Kolonel L, Kumanyika S, Leitzmann C, Mann J, Powers H, Reddy K, Riboli E, Rivera J, Schatzkin A, Seidell J, Shuker D, Uauy R, Willett W, Zeisel S. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: WCRF/AICR Expert Report; 2007.
- 6. Labianca R, Beretta GD, Kildani B, Milesi L, Merlin F, Mosconi S, Pessi MA, Prochilo T, Quadri A, Gatta G, de Braud F, Wils J. Colon cancer. Crit Rev Oncol Hematol. 2010;74(2):106-33.
- 7. Chan D, Lau R, Aune D, Vieira R, Greenwood D, Kampman E, Norat T. P1-109 The WCRF/AICR continuous update project: dietary fibre intake and colorectal cancer incidence. J Epidemiol Commun H. 2011;65(Suppl 1):A97.
- 8. Lipkin M, Reddy B, Newmark H, Lamprecht SA. Dietary factors in human colorectal cancer. Ann Rev Nutr. 1999;19(1):545-86.
- 9. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133(7):2485S-93S.
- 10. Gupta N, Martin PM, Prasad PD, Ganapathy V. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci. 2006;78(21):2419-25.
- 11. Williams D, Verghese M, Walker LT, Boateng J, Shackelford L, Chawan CB. Flax seed oil and flax seed meal reduce the formation of aberrant crypt foci (ACF) in azoxymethane-induced colon cancer in Fisher 344 male rats. Food Chem Toxicol. 2007;45(1):153-9.
- 12. Thompson LU, Robb P, Serraino M, Cheung F. Mammalian lignan production from various foods. Nutr Cancer. 1991;16:43-52.
- 13. Harris R, Haggerty W. Assays for potentially anticarcinogenic phytochemicals in flaxseed. Cereal Food World. 1993;38:147-51.
- 14. Sauer J, Richter KK, Pool-Zobel BL. Physiological concentrations of butyrate favorably modulate genes of oxidative and metabolic stress in primary human colon cells. J Nutr Biochem. 2007;18(11):736-45.
- 15. Felin IPD, Grivicich I, Felin CR, Regner A, Rocha ABd. Expressão de p53, p16 E COX-2 em carcinoma escamoso de esôfago e associação histopatológica. Arq Gastroenterol. 2008;45:308-12.
- 16. Reeves P, Nielsen F, Fahey GJ. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc 73 writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939-51.
- 17. Newell L, Heddle J. The potent colon carcinogen, 1,2 dimethylhydrazine induced mutations primarily in the colon. Mutat Res. 2004;564:1-7.
- 18. Carson F, Martin J, Lynn J. Formalin fixation for electron microscopy: a re-evaluation. Am J Clin Pathol. 1973;59:365-73.
- 19. Bird R. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37:147-51.
- 20. Smirick-Tjardes M, Grieshop C, Flickinger E, Bauer L, Fahey Jr. G. Dietary galactooligosaccharides affect ileal and total-tract nutrient digestibility, ileal and fecal bacterial concentrations, and ileal fermentive characteristics of growing pigs. Am Soc Anim Sc. 2003;81:2535-45.
- 21. Hailfinger S, Jaworski M, Marx-Stoelting P, Wanke I, Schwarz M. Regulation of P53 stability in p53 mutated human and mouse hepatoma cells. Int J Cancer. 2007;120(7):1459-64.
- 22. Rajkumar L, Kittrell F, Guzman R, Brown P, Nandi S, Medina D. Hormone-induced protection of mammary tumorigenesis in genetically engineered mouse models. Breast Cancer Res. 2007;9(1):R12.
- 23. Rosenberg DW, Giardina C, Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 2009;30(2):183-96.
- 24. Silva FGD, Penido LCP, Valente FX, Mendes MCS, Rosa DD, Glória MBA, Peluzio MdCG. Sodium butyrate does not decrease the evolution of precancerous lesions in rats. Acta Cir Bras. 2010;25:507-12.
- 25. Larangeira LLS, Taha MO, Ferme A, Lemos R, Plapler H. Localização de lesões tumorais induzidas pela 1,2-dimetilhidrazina e seu grau de atipia no colon de ratos. Acta Cir Bras. 1998;13(3).
- 26. Jenab M, Thompson LU. The influence of flaxseed and lignans on colon carcinogenesis and β-glucuronidase activity. Carcinogenesis. 1996;17(6):1343-8.
- 27. Bommareddy A, Arasada BL, Mathees DP, Dwivedi C. Chemopreventive effects of dietary flaxseed on colon tumor development. Nutr Cancer. 2006;54(2):216-22.
- 28. Bommareddy A, Zhang X, Schrader D, Kaushik RS, Zeman D, Matthees DP, Dwivedi C. Effects of dietary flaxseed on intestinal tumorigenesis in Apc (Min) mouse. Nutr Cancer. 2009;61(2):276-83.
- 29. Skrzydlewska E, Sulkowski S, Koda M, Zalewski B, Kanczuga-Koda L, Sulkowska M. Lipid peroxidation and antioxidant status in colorectal cancer. World J Gastroenterol. 2005;11:403-6.
- 30. Rosa DD, Sales RLd, Moraes LFdS, Lourenço FC, Neves CA, Sabarense CM, Ribeiro SMR, Peluzio MdCG. Flaxseed, olive and fish oil influence plasmatic lipids, lymphocyte migration and morphometry of the intestinal of Wistar rats. Acta Cir Bras. 2010;25:275-80.
- 31. Degen C, Habermann N, Piegholdt S, Glei M, Jahreis G. Human colon cell culture models of different transformation stages to assess conjugated linoleic acid and conjugated linolenic acid metabolism: Challenges and chances. Toxicol in Vitro. 2012;26(6):985-92.
- 32. Qu H, Madl RL, Takemoto DJ, Baybutt RC, Wang W. Lignans are involved in the antitumor activity of wheat bran in colon cancer SW480 cells. J Nutr. 2005;135(3):598-602.
- 33. Davis CD, Milner JA. Gastrointestinal microflora, food components and colon cancer prevention. J Nutr Biochem. 2009;20(10):743-52.
- 34. Rajkumar L, Kittrell F, Guzman R, Brown P, Nandi S, Medina D. Hormone-induced protection of mammary tumorigenesis in genetically engineered mouse models. Breast Cancer Res. 2007;9(1):R12.
- 35. Nakade K, Pan J, Yamasaki T, Murata T, Wasylyk B, Yokoyama KK. JDP2 (Jun Dimerization Protein 2)-deficient mouse embryonic fibroblasts are resistant to replicative senescence. J Biol Chemy. 2009;284(16):10808-17.
- 36. Mudhasani R, Zhu Z, Hutvagner G, Eischen C, Lyle S, Hall L. Loss of miRNA biogenesis induces p19 Arf-p53 signaling and senescence in primary cells. J Cell Biol. 2008;181:1055-63.
- 37. Hailfinger S, Jaworski M, Marx-Stoelting P, Wanke I, Schwarz M. Regulation of P53 stability in p53 mutated human and mouse hepatoma cells. Int J Cancer. 2007;120(7):1459-64.
Publication Dates
-
Publication in this collection
26 July 2013 -
Date of issue
Aug 2013
History
-
Received
15 Apr 2013 -
Accepted
18 July 2013 -
Reviewed
17 June 2013