Abstracts
PURPOSE: Evaluate the cardiovascular and hematological effects produced by chronic treatment with two dosis of etoricoxib in Wistar normotensive rats. METHODS: Thirty rats have been used and divided into one control group and two etoricoxib (10mg/kg and 30mg/kg) treatments groups for 60 days. The mean arterial pressure (MAP) was taken during the whole experimental period and at the end of this period, under anesthesia blood samples were taken, and further the withdrawn of the aorta, heart, brain, liver, and kidneys for the anatomopathologic study. RESULTS: The treatment with etoricoxib (30mg/Kg) produced a significant increase of the MAP from the 28th day of the experiment and from the platelets when compared to the control group and to the group treated with 10mg/Kg, besides producing a highly significant difference in hematocrit and in the red blood cells in relation to the control group. On the other hand the treatment with etoricoxib has not caused histopathological changes when compared to the control. CONCLUSION: These data show that the chronic treatment with etoricoxib leads to increase of the MAP, and to important hematological changes which seem to be associated to the hemoconcentration although not producing anatomopathological significant changes.
Anti-inflammatory Agents; Hematology; Cardiovascular Agents; Rats
OBJETIVO: Avaliar os efeitos cardiovasculares e hematológicos produzidos pelo tratamento crônico com duas doses de etoricoxib em ratos Wistar normotensos. MÉTODOS: Foram utilizados 30 ratos divididos em um grupo controle e dois grupos tratamentos (10mg/kg e 30mg/kg) de etoricoxib por 60 dias. A pressão arterial média (PAM) dos animais foi aferida durante todo o período experimental e, ao final deste, sob anestesia, foram coletadas amostras de sangue, além da retirada da aorta, coração, cérebro, fígado e rins para estudo anatomopatológico. RESULTADOS: O tratamento com etoricoxib (30mg/Kg) produziu aumento significativo da PAM a partir do 28° dia do experimento e das plaquetas quando comparado ao grupo controle e ao grupo tratado com etoricoxib 10 mg/Kg, além de produzir diferença altamente significativa no hematócrito e nas hemácias em relação ao grupo controle. Por outro lado, o tratamento com etoricoxib, não produziu alterações histopatológicas quando comparado ao controle. CONCLUSÃO: Estes dados indicam que o tratamento crônico com etoricoxib produz aumento da PAM, além de importantes alterações hematológicas que parecem estar associadas à hemoconcentração, porém sem produzir alterações anatomopatológicas significativas.
Antiinflamatórios; Hematologia; Agentes do Sistema Cardiovascular; Ratos
8 - ORIGINAL ARTICLE
EFFECTS OF DRUGS
Cardiovascular and hematologic effects produced by chronic treatment with etoricoxib in normotensive rats1 Correspondence: Nilo César do Vale Baracho Rua Marechal Juarez Távora, 180 37502-106 Itajubá - MG Brazil Phone: (55 35)3621-4610 nilocvbaracho@yahoo.com.br
Efeitos cardiovasculares e hematológicos produzidos pelo tratamento crônico com etoricoxib em ratos normotensos
Nilo César do Vale BarachoI; Guilherme Pedrosa GuizelliII; Beatriz Leone CarmelloII; Danielle de Souza SanchesII; Felipe Moraes Costa SilvaII; José Marcos dos ReisIII; Jarbas de BritoIV
IMaster, Associate Professor, Pharmacology and Biochemistry, FMIt, Minas Gerais, Brazil
IIGraduate student, FMIt, Minas Gerais, Brazil
IIIPhD, Associate Professor, Anatomy and Surgical Techniques, FMIt, Minas Gerais, Brazil
IVMaster, Full Professor of Pathology, FMIt, Minas Gerais, Brazil
Correspondence Correspondence: Nilo César do Vale Baracho Rua Marechal Juarez Távora, 180 37502-106 Itajubá - MG Brazil Phone: (55 35)3621-4610 nilocvbaracho@yahoo.com.br
ABSTRACT
PURPOSE: Evaluate the cardiovascular and hematological effects produced by chronic treatment with two dosis of etoricoxib in Wistar normotensive rats.
METHODS: Thirty rats have been used and divided into one control group and two etoricoxib (10mg/kg and 30mg/kg) treatments groups for 60 days. The mean arterial pressure (MAP) was taken during the whole experimental period and at the end of this period, under anesthesia blood samples were taken, and further the withdrawn of the aorta, heart, brain, liver, and kidneys for the anatomopathologic study.
RESULTS: The treatment with etoricoxib (30mg/Kg) produced a significant increase of the MAP from the 28th day of the experiment and from the platelets when compared to the control group and to the group treated with 10mg/Kg, besides producing a highly significant difference in hematocrit and in the red blood cells in relation to the control group. On the other hand the treatment with etoricoxib has not caused histopathological changes when compared to the control.
CONCLUSION: These data show that the chronic treatment with etoricoxib leads to increase of the MAP, and to important hematological changes which seem to be associated to the hemoconcentration although not producing anatomopathological significant changes.
Key words: Anti-inflammatory Agents. Hematology. Cardiovascular Agents. Rats.
RESUMO
OBJETIVO: Avaliar os efeitos cardiovasculares e hematológicos produzidos pelo tratamento crônico com duas doses de etoricoxib em ratos Wistar normotensos.
MÉTODOS: Foram utilizados 30 ratos divididos em um grupo controle e dois grupos tratamentos (10mg/kg e 30mg/kg) de etoricoxib por 60 dias. A pressão arterial média (PAM) dos animais foi aferida durante todo o período experimental e, ao final deste, sob anestesia, foram coletadas amostras de sangue, além da retirada da aorta, coração, cérebro, fígado e rins para estudo anatomopatológico.
RESULTADOS: O tratamento com etoricoxib (30mg/Kg) produziu aumento significativo da PAM a partir do 28° dia do experimento e das plaquetas quando comparado ao grupo controle e ao grupo tratado com etoricoxib 10 mg/Kg, além de produzir diferença altamente significativa no hematócrito e nas hemácias em relação ao grupo controle. Por outro lado, o tratamento com etoricoxib, não produziu alterações histopatológicas quando comparado ao controle.
CONCLUSÃO: Estes dados indicam que o tratamento crônico com etoricoxib produz aumento da PAM, além de importantes alterações hematológicas que parecem estar associadas à hemoconcentração, porém sem produzir alterações anatomopatológicas significativas.
Descritores: Antiinflamatórios. Hematologia. Agentes do Sistema Cardiovascular. Ratos.
Introduction
Inflammation is a defense mechanism which has the purpose of eliminating the initial cause of the cellular lesion and its consequences by means of a vascular reaction, with the departure of plasmatic proteins and blood cells to the place where the action is happening, and a cell reaction which recruits and activates leukocytes against the aggressive agents, creating a storage of liquid and leukocytes in the extravascular mean1. The suppression of this process can be done with the use of anti-inflammatory drugs which can be the "hormonal" type (glucocorticoids) or the "non-hormonal" types (NSAID)2.
As anti-inflammatory drugs the glucocorticoids act inhibiting the production of substances by many cell types, which leads to the decrease of the vasoactive and chemoattractant factors , reduction of lipolytic and proteolytic enzymes, reduction of the fibrosis and to a smaller extravasation of leukocytes to the place of the lesion2.
The NSAID also act as analgesic and as antipyretic. The basic action of this medicament occurs with the inhibition of the cycloxigenase enzyme (COX) which acts as a catalyzer of the conversion reaction from arachidonic acid into prostaglandins (PG's) and thromboxanes (TX's)3.
Although they had an anti-inflammatory efficiency, the first NSAID developed showed an increase in gastrointestinal symptoms and were, therefore, not indicated for chronical use3.
In this way, some studies were initiated with the purpose of solving such problems and it was found out that the COX had two isoenzymes: cyclooxigenase-1 (COX-1), responsible for the physiological production of prostanoids, and was known as constitutive enzyme; and the cyclooygenase-2 (COX-2) which is associated to the pathological processes, and therefore, is called inducible enzyme. From this discovery, new research has been done, with the purpose of obtaining substances which are able to inhibit the specific action related to the COX-24.
Thus, two new molecules were developed: rofecoxib and celecoxib. These were able to inhibit selectively the COX-23. At the same time, it was found out that the COX-2 was the main source of prostaciclin (PGI2). Further research involving these medicaments showed a potential of these subclass in increasing the risk of cardiovascular problems, for as the COX-2 is responsible for the production of PGI2, and the COX-1 for the production of the TXA2 when inhibiting only the COX-2, there is a super production of TXA2, leading to the cardiovascular effects such as acute myocardial infarction (AMI), unstable angina, cardiac thrombus, stroke and transient ischemic attack (TIA)5. Such observation has been showed by the study VIGOR, which indicated bigger risk of cardiac affections in the treatment with rofecoxib, when compared to naproxen6.
After that, as the results of "Adenomatous Polyp Prevention on Vioxx®"(APPROVe) study, there was an immediate withdrawn of the product from the market, because during the study the group treated with rofecoxib showed an increase in the cardiovascular risks. Even though, it was considered not so sure that such effect was exclusively because of the rofecoxib or because of the class of specific inhibitors of the COX-27.
Recently, etoricoxib, a new selective COX-2 inhibitor was developed. It is a strong member of the COX-2 NSAID selective family, with a potential to reduce the gastrointestinal risks when compared to the others molecules of same pharmacological class8. Many studies proved its anti-inflammatory and analgesic efficiency in the acute and chronical pain, besides its higher security and tolerability4.
Although it shows less effects on the gastrointestinal treat, adverse reactions may appear, such as, e.g. cardiovascular problems. But the evaluation of such risks is still difficult, as well as the evaluation of its possible hematological effects, since there have been no proper studies or long duration studies in high and low risks patients3,4.
Thus, the objective of this study was to evaluate the cardiovascular and hematological effects produced by a chronical treatment with two different dosages of etoricoxib in Wistar normotensive rats.
Methods
The project had its beginning only after the approval from the Research Ethics Committee from FMIt under the number 05/2006 dated from 12/05/2006.
The study followed the federal law 6638 and the orientations from the Brazilian College of Animal Experimentation COBEA)
Thirty male wistar rats had been used with weight ranging from 220 to 300 g and age between 60 and 90 days. The animals were kept in plastic cages with water and "ad libitium" food and submitted to a light-dark cycle of 12 hours. The animals were randomized in 03 groups of 10 rats (n=10)10.
The rats received the following treatment in one single oral dosage (gavage) for a 60 day period.
-Group 01(Control-n=10): 1 mL of distilled water.
-Group 02 (n=10): 10 mg/kg of etoricoxib11.
-Group 03 (n=10): 30 mg/kg of etoricoxib11.
To measure the Mean arterial Pressure (MAP) it was used the Plethysmography of Tail. This method was applied in awake animals, in the indirect way for the system uses a tail cuff attached to a mercury column and a hermetic rubber chamber joined to a water column which promotes the compression of the tail of the animal and the emptiness of its vessels. After being heated for five minutes in a pre-heated container the animal was kept in a mechanical acrylic container and following this the pressure the pressure in the mercury column was measured after the oscillation in the water column. This procedure was performed twice a week, during the whole period of the study. Therefore, 18 values for the MAP from day zero (0) to day sixty (60) were obtained.
At the end of the experimental period, the animals were anesthetized with Ketamine (50mg/kg) Xylazine (25mg/kg and by intraperitonial means and submitted to intracardiac puncture. The blood was collected in test tubes containing the anticoagulant EDTA and processed so that the following blood exams could be made: hemoglobin, hematocrit, leukocytes global count, red blood cells count, platelets count, hematimetrics index, differential leukocytes count and hematoscopy (red blood cells, leukocytes and platelets).
After these procedures, a wide incision was done in the abdomen, thorax and at last in the cranium of the animal, so that it would be possible to withdraw the following organs: abdominal and thoracic aorta, heart, brain, liver and kidneys. From each withdrawn organ, segments of about 1 millimeter depth were taken and placed in plastic labeled pots for each animal, containing a 10% Buffered formol solution for 4 hours and sent to the Pathology Laboratory, so that they could be processed making it possible to perform the histological analysis.
The hematological dosages and the calculus were performed in a hematological automated analyzer K-800 from Sysmex® (Kobe, Japan). The total and differential count of leukocytes, red blood cells and platelets were performed by methodology of detection of the difference in the conductivity between these particles and the diluents in which they are in suspension. For the electronic detection of the hematocrit, it was taken the principle of the alteration of the voltage produced by the passage of the blood cells trough the transductor opening which is proportional to the volume of the cell. The cianometahemoglobin method was used to obtain the concentration of hemoglobin which is proportional to the absorbance.
The hematimetric index were obtained by the instrument trough calculus using the hematocrit values, red blood cells count and hemoglobin concentration in the sample. The hematimetric indexes were obtained: Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), and Mean Corpuscular Hemoglobin Concentration (MCHC).
The hematoscopy was made by setting a blood drop on a glass blade, followed by an extension of this drop, forming a thin layer of blood, which after drying was submitted to the coloration by the Panotico method. Afterwards, the blades were observed in optical microscopes of 400x and 1000x where the morphology of the leukocytes, the red blood cells and the platelets were analyzed.
The materials were processed by the paraffin inclusion technique and colored by the Hematoxilin-Eosin (HE) technique. Further the blades were analyzed in optical microscopes of 40 fold, 100 fold, 400 fold, and 1000 fold. From the microcopy fields, images were collected going to the digitalization phase for comparative study and quantitative evaluation.
For the statistics analysis, the program Statistica® version 6.1. was used. The obtained data were submitted to the ANOVA test for the checking of the discordance among the k groups, and to the post-hoc tests of Tukey and Scheffé for the checking of the discordance in the pairs, besides the calculus of the statistic p and Box Plots for the construction of the graphics. In the cases where p was smaller than 0.05, the difference was considered significant and highly significant if p was smaller than 0.0112.
Results
Effect in the mean arterial pressure (MAP)
The treatment with etoricoxib (10 or 30 mg/Kg/day) produced a statistically significant increase in mean arterial pressure (MAP) when compared to the control group (Figure 1). At the beginning of the experimental period, statistical significant difference in the MAP of the animals of the three studied groups was not observed (day 0 - p=0,2411).
The chronic etoricoxib administration (10 or 30 mg/Kg/day) increased significantly the MAP from the day 28 compared to the control group (etoricoxib 10mg/Kg/day - p=0,0102 and etoricoxib 30mg/Kg/day - p=0,0036) . The increase in MAP produced by both doses of etoricoxib was observed until the end of experimental period (day 60) when compared to the control group (etoricoxib 10mg/Kg/day - p=0,0001 and etoricoxib 30mg/Kg/day - p=0,0001).
Hematological effects
The chronic etoricoxib administration (10 or 30 mg/Kg/day) produced a important and statistically significant increase in the levels of red blood cells (Table 1), hematocrit (Table 1) and platelets when compared to the control group (Table1). Etoricoxib administration (10 or 30mg/Kg/day) did not produce significant alterations in the other studied hematologic parameters when compared to the control group (p>0,05).
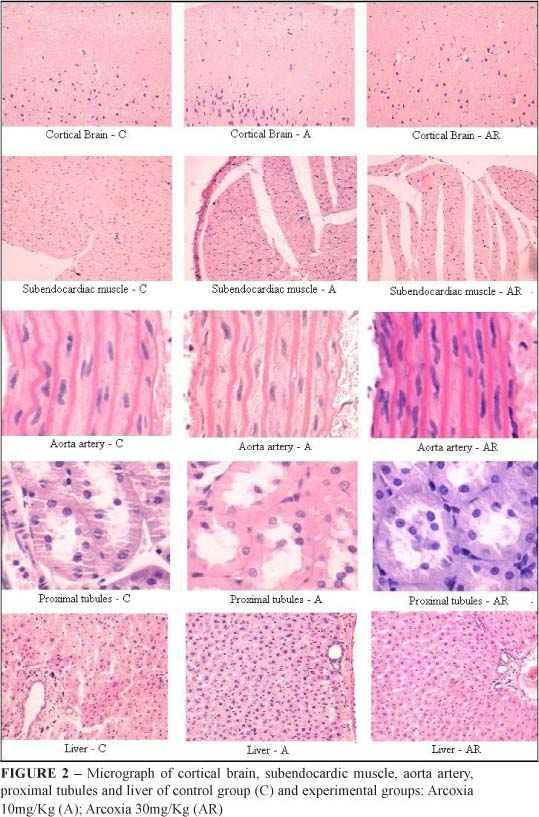
Histological analysis
The histological analysis of the cerebral cortex, subendocardic muscles, aorta, proximal tubules and hepatic lobules, showed normal appearance elements in the three groups studied. Thus, the etoricoxib administration (10 or 30mg/Kg/day) did not produce significant changes in the target organs when compared to the control group (Figure 2).
Discussion
In the present study there has been noticed polycythemia and hemoconcentration in the animals treated with etoricoxib in relation to the animals from the control group, which can be due to a possible dehydration of those animals, influenced by the medication, which would be no dosage-dependent, since the statistical meaning of the experiment was observed only related to the control, in the research of red blood cells (RBC). Similar result has been observed in an experiment in rats presenting hypothyroidism induced by the propiltiouracil which decreased the bloody delta-aminolevulinate dehydratase and, when treated withT3, there was an increase of 10% in the levels of hemoglobin and red blood cells13.
The hemoglobin level of the treated rats increased, and seems to be dosage dependent, for there has been statistical meaning in the higher dosage(30mg/kg), in the same way that the selective inhibitors of COX-2, including the etoricoxib, cause renal toxicity when used in high dosages which may limit the use of this class of medicaments14.
Clinical pharmacological results of COX inhibitors support the concept that the COX-1 of platelets may be translated into an increased incidence of gastrointestinal bleeding as well as an increase in the cardiovascular risk, associated to a deep inhibition of the prostaglandin dependent from COX-2 (PGI2)13.
In the experiment, it may also observed an increase in the levels of platelet levels (PLT) in the treatment with 30 mg, in relation to the control treatment , probably due to the increase in the prostaciclin, which increases the gastrointestinal and cardiovascular risks of this medicament , which may contra indicate the chronical use of high doses. The medicament may increase the risk of thromboembolic phenomenon even when associated with platelets aggregation inhibitors, such as low dosages of acetylsalicylic acid (aspirin) and clopidrogel, due to a reduction in the levels of its markers15.
The data obtained, showed that the chronical treatment with etoricoxib, 10 or 30mg/kg/day produced a discrete but meaningful increase of the MAP when compared to the control group, and this effect has not been dosage dependent. The adverse cardiovascular effects are present in different degrees of importance in patients treated with NSAID these being conventional or selective, as showed in many studies made in many countries. However, such risks may suffer changes in its importance according to the substance which is involved16.
In a study which analyzed the gastrointestinal tolerability, security and efficiency of the treatment with etoricoxib and sodium diclofenac in patients with osteoarthritis it was observed similar rates of thromboembotic events in both groups and a more meaningful increase in the blood pressure of the group treated with 90mg etoricoxib when compared to the 150mg diclofenac, leading to the withdrawn from the studies of the patients presenting such effects. Furthermore there was a higher incidence of myocardial events in the group treated with etoricoxib and a higher prevalence of ischemic stroke in the group treated with diclofenac17.
Related to the target organs which were analyzed, there has been no changes due to hypertension, taking into consideration that the increase that occurred in the pressure was not enough to be characterized as such nor due to the chronical treatment with etoricoxib, for there were no adverse events such as strokes or myocardial infarction and others showed in the literature3. A possible explanation to what has happened is that the doses used in the study were inferior to the ones necessary to cause lesions in the target organs and that the experiment time of 60 days may have been insufficient to lead to such lesions.
The results obtained in this study show that the chronic treatment with etoricoxib increase the blood pressure in normotensive rats, suggesting that chronical treatment of hypertensive rats with this drug may produce important increases in the MAP and may even represent a decision taking factor to produce important lesions in the target organs such as big vessels, brain, kidneys and heart.
Conclusion
The results have showed that the chronical treatment with etoricoxib, in the dosage used, produces an increase of the MAP without produce significant changes in morphology of the target organs when compared to the control group. Besides that, the treatment with etoricoxib produces important hematological changes in normotensive rats which seem to be associated to the hemoconcentration. Further studies are necessary for elucidate the mechanisms involved in these cardiovascular and hematological effects.
Acknowledgements
We thank Dr. Rosimeire da Silveira and biologist Wanderley Nogueira Vilas Boas for their precious technical contribution in the realization of this study.
Received: December 22, 2008
Review: February 16, 2009
Accepted: March 18, 2009
Conflict of interest: none
Financial source: PDIC-FMIt and FAPEMIG
How to cite this article
Baracho NCV, Guizelli GP, Carmello BL, Sanches DS, Silva FMC, Reis JM, Brito J. Cardiovascular and hematologic effects produced by chronic treatment with etoricoxib in normotensive rats. Acta Cir Bras. [serial on the Internet] 2009 May-Jun;24(3). Available from URL: http://www.scielo.br/acb
* Color figures available from www.scielo.br/acb
1 Research performed at Pharmacology, Biochemistry and Pathology laboratories, Itajuba School of Medicine (FMIt), Minas Gerais, Brazil.
- 1. Lentsch AB, Ward PA. Regulation of inflammatory vascular damage. J Pathol. 2000;190:343-8.
- 2. Elenkov IJ, Chrousos GP. Stress hormones, Th1/ Th2 patterns, pro/antiinflammatory cytokines and susceptibility to disease. Trends Endocrinol Metabol. 1999;10:359-68.
- 3. Araujo LF, Soeiro AN, Fernandes JL, Serrano Junior CV. Eventos cardiovasculares: um efeito de classe dos inibidores de COX-2. Arq Bras Cardiol. 2005;85(3):222-9.
- 4. Rodrigues AD, Halpin RA, Leslie AG, Cui D, Woolf EJ, Matthews CZ, Gottesdiener KM, Larson PJ, Lasseter KC, Agrawal NGB. Absorption, metabolism, and excretion of etoricoxib, a potent and selective cyclooxygenase-2 inhibitor, in healthy male volunteers. Drug Metab Dispos. 2003;31(2):224-32.
- 5. Bannwarth B. Tienen futuro los inhibidores de la ciclooxigenasa-2? Drug Safety. 2005;28(3):183-9.
- 6. Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343(21):1520-8.
- 7. Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B , Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352(11):1092-102.
- 8. Perez RR, Silva MAML, Varzim FLSB, Oliveira SB, Hucke EETS. A ação do decanoato de nandrolona (Deca-durabolin®) sobre parâmetros hematológicos e proteína total plasmática de ratos (Rattus rattus) com depressão medular induzida após administração de sulfato de vincristina (Oncovin®). Cienc Rural. 2005;35(3):589-95.
- 9. Topol EJ. Failing the public health-rofecoxib, Merk and the FDA. N Engl J Med. 2004;351:1707-9.
- 10. Watanabe ALC, Watanabe LM. Efeitos do Tenoxicam sobre a cicatrização da parede abdominal: estudo experimental em ratos. Acta Cir Bras. 2005;20(2):140-3.
- 11. Singh VP, Patil CS, Kulkarni SK. Analysis of interaction between etoricoxib and tramadol against mechanical hyperalgesia of spinal cord injury in rats. Life Sci. 2006;78(11):1168-74.
- 12. Arango HG. Bioestatística: teórica e computacional. Testes paramétricos. 2ed. Rio de Janeiro: Guanabara Koogan; 2005.
- 13. Gravina FS, da Silveira CK, de Assis AM, Rieger DK, Guerini C, Müller AP, Farina M, Rotta LN, Perry ML. Experimental hypothyroidism inhibits {delta}-aminolevu-linate dehydratase activity in neonatal rat blood and liver. Exp Biol Med. 2007;232(8):1021-6.
- 14. Tacconelli S, Capone ML, Patrignani P. Clinical pharmacology of novel selective COX-2 inhibitors. Curr Pharm Des. 2004;10(6):589-601.
- 15. Steinhubl SR, Badimon JJ, Bhatt DL, Herbert JM, Lüscher TF. Clinical evidence for anti-inflammatory effects of antiplatelet therapy in patients with atherothrombotic disease. Vasc Med. 2007;12(2):113-22.
- 16. Helin-Salmivaara A, Virtanen A, Vesalainen R, Grönroos JM, Klaukka T, Idänpään-Heikkilä JE, Huupponen R. NSAID use and the risk of hospitalization for first myocardial infarction in the general population: a nationwide case-control study from Finland. Eur Heart J. 2006;27(14):1657-63.
- 17. Baraf HS, Fuentealba C, Greenwald M; Brzezicki J, O'brien K, Soffer B, Polis A, Bird S, Kaur A, Curtis SP, EDGE Study Group. Gastrointestinal side effects of etoricoxib in patients with osteoarthritis: results of the etoricoxib versus diclofenac sodium gastrointestinal tolerability and effectiveness (EDGE) trial. J Rheumatol. 2007;34:408-20.
Publication Dates
-
Publication in this collection
03 June 2009 -
Date of issue
June 2009
History
-
Accepted
18 Mar 2009 -
Received
22 Dec 2008