Abstract
PURPOSE: To evaluate if the ileum resection changes the functioning liver cell mass, the hepatic metabolism and the biodistribution of radiopharmaceutical in rats. METHODS: Twelve Wistar rats weighing 285g±34g were randomly divided into the ileum resection group (n = 6) and sham group rats (n = 6). After 30 days, they were anesthetized and 0.1mL of 99m-Tc-phytate (0.66MBq) was injected via femoral vein. After 30 minutes, blood samples were collected for red blood cells radioactive labeling and serum ALT, AST and gammaGT. Liver samples were used for 99m-Tc-phytate percentage of radioactivity/gram of tissue and histopathology. Student 's t test was used with significance 0.05. RESULTS: There was a higher uptake of 99m-Tc-phytate in the liver of sham rats, compared to the ileum resection group (p<0.05). GammaGT, ALT and AST were increased in ileum resection rats compared to sham (p<0.05). The he patocytes count was significantly lower in ileum resection group than in sham (p<0.05). Liver: body mass ratio was lower in experimental animals than in sham group (p<0.05). CONCLUSION: These data support that the ileum has important role in liver function and liver mass regulation, and they have potential clinical implications regarding the pathogenesis of liver injury following lower bowel resection.
Ileum; Liver; Radioisotopes; Rats
9 ORIGINAL ARTICLE
ALIMENTARY TRACT
The ileum as a determinant organ of the functional liver cell mass in rats1 1 Research performed at Department of Surgery, School of Medicine, Federal University of Rio Grande do Norte (UFRN), Natal, Brazil.
Aldo Cunha MedeirosI; Alexandra Régia Dantas BrígidoII; Suyana Meneses SilvaII; Vanessa Ramalho BritoII; Keyla Borges Ferreira RochaIII; Cláudia Nunes OliveiraIII
IChairman, Full Professor, Department of Surgery, UFRN, Natal, Brazil. Conception, design, intellectual and scientific content of the study, performed the majority of the experiment, analysis and interpretation of data, critical revision and provided financial support
IIGraduate student, Scientific Initiation Program, UFRN, Natal, Brazil. Acquisition, analysis and interpretation of data, manuscript writing
IIIAssociate Professor, Department of Pathology, UFRN, Natal, Brazil. Acquisition, analysis and interpretation of data
Correspondence Correspondence: Aldo Cunha Medeiros Avenida Nilo Peçanha, 620 59012-300 Natal RN Brasil aldo@ufrnet.br
ABSTRACT
PURPOSE: To evaluate if the ileum resection changes the functioning liver cell mass, the hepatic metabolism and the biodistribution of radiopharmaceutical in rats.
METHODS: Twelve Wistar rats weighing 285g±34g were randomly divided into the ileum resection group (n = 6) and sham group rats (n = 6). After 30 days, they were anesthetized and 0.1mL of 99m-Tc-phytate (0.66MBq) was injected via femoral vein. After 30 minutes, blood samples were collected for red blood cells radioactive labeling and serum ALT, AST and gammaGT. Liver samples were used for 99m-Tc-phytate percentage of radioactivity/gram of tissue and histopathology. Student 's t test was used with significance 0.05.
RESULTS: There was a higher uptake of 99m-Tc-phytate in the liver of sham rats, compared to the ileum resection group (p<0.05). GammaGT, ALT and AST were increased in ileum resection rats compared to sham (p<0.05). The he patocytes count was significantly lower in ileum resection group than in sham (p<0.05). Liver: body mass ratio was lower in experimental animals than in sham group (p<0.05).
CONCLUSION: These data support that the ileum has important role in liver function and liver mass regulation, and they have potential clinical implications regarding the pathogenesis of liver injury following lower bowel resection.
Key words: Ileum. Liver. Radioisotopes. Rats.
Introduction
The liver mass is regulated in a precise ratio with body mass in healthy individuals, and this ratio (liver/body weight) is specifically restored after liver injury or resection1. Extensive analyzes have been conducted to investigate the mechanisms that regulate liver mass and regeneration after resection, but the precise nature of the involved signals have not been fully elucidated. It has been hypothesized that some mechanisms from intestine and portal circulation contribute to the regulation of the relative liver mass/body mass1. It has been shown that 50% resection of the proximal intestine is associated with a significant reduction in hepatic total mass2. Furthermore, the role of the small intestine in regulating the recovery of liver mass after injury has been investigated, suggesting that intestine is the source of a humoral factor essential for liver regeneration after partial hepatectomy3. It has been shown that there is a factor which affects the absorption of bile acids, which may regulate the hepatic mass and its recovery after resection, supporting the role of the small intestine in regulating the hepatic mass4.
Since its introduction in Nuclear Medicine in 1973, 99mTc-phytate radiocolloid has been widely used to study the liver, especially for diagnosis and progression of liver disease, and has been used for biodistribution studies, which is useful for determining parameters of liver function5,6. Biodistribution of radiocolloids to the liver has shown good correlation with the severity of liver diseases such as cirrhosis, fibrosis and its prognosis7. Thus, the measurement of the uptake of 99mTc-phytate is an excellent index of liver function. The ease of quantifying the amount and structure of the liver and the concentration of the radioisotope using scintigraphy, greatly encourage the use of 99m-Tc-phytate scintigraphy as a quantitative test of liver function. The biodistribution of radiopharmaceuticals has been studied by us in massive bowel resection model, having been shown to be a good tool8. Based on these data, we developed the hypothesis that the regulation of liver function can be done separately by ileum, and that this intestinal segment may be effective in the process.
The aim of this study was to determine whether resection of the ileum produces changes in physical and functional liver mass in rats, by using analysis of biodistribution of radiocoloid in liver and red blood cells, histopathological examination and measurement of liver enzymes.
Methods
Twelve Wistar rats, weighing 285g±34g were used. They were supplied by the animal colony of the Nucleus for Experimental Surgery of the Federal University of the Rio Grande do Norte (UFRN), Brazil. All the animals were weighed and observed in individual cages with water and food (Labina® Purina) ad libitum and acclimated in the laboratory for seven days. They were maintained under temperature control (21ºC), air humidity (6070%) and lighting (12/12 hours light/dark cycle). All experiments and procedures were approved by the Institutional Animal Care and use Committee of UFRN and conformed to the Brazilian guidelines involving animals in research. They were randomly divided into two groups of six rats each: the experimental group was subjected to 50 cm resection of the distal small bowel; and the control group underwent sham operation. Twelve hours before surgery, rats were fasted and only access to water was permited. The animals were anesthetized with an intramuscular (i.m.) injection of 0.1 ml/100g body weight of a solution consisting of 1.0 ml of ketamine (50 mg/ml) and 1.0 ml xylazine (20 mg/ml); they were operated on under sterile conditions. Tenoxicam (3 mg/Kg i.m.) was used for postoperative pain control once a day, for three days.
The partial small bowel resection was performed 5 cm proximal to the cecum and 50 cm of ileum were removed. A surgical microscope (DF Vasconcelos, São Paulo, Brazil), was used to perform end-to-end bowel anastomosis with interrupted sutures of 6-0 polypropilene. The sham group was subjected to a medium laparotomy, ileum transection 5 cm above the cecum and subsequent anastomosis. The animals were allowed water immediately after surgery and solid food on the second postoperative day. The rats were weighed before the surgery and weekly with a digital scale until the sacrifice, on the 30th day. They were handled in the operating room of the Nucleus for Experimental Surgery, and during the observation period after surgery they were kept in its postoperative control room.
Biodistribution of 99mTc-phytate and measurement of biochemical parameters
After 30 days, all the animals were anaesthetized and injected with 0.lmL of 99mTc-phytate in the jugular vein, corresponding to 0.66MBq radioactivity. After 30 minutes, blood samples were collected by cardiac puncture to measurement of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma GT (GGT) and radiopharmaceutical labeling in red blood cells.
The liver was quickly removed, washed in 0.9% NaCl and weighed on a high-precision digital scale. The total liver weight was divided by the weight of each animal before the liver removal, generating the ratio liver mass: body mass. A sample of the hepatic left lobe was subjected to radioactivity uptake using an automatic gamma counter, Wizard Gama Counter Perkin-Elmer®. The percentage of radioactivity per gram (%ATI/g) of liver was calculated by dividing the activity/g of the tissue by the total radioactivity administered to each animal.
The radioactive experiments were performed in compliance with the standards of radiation protection recommended by the National Nuclear Energy Comission-Brazil.
Histopathology
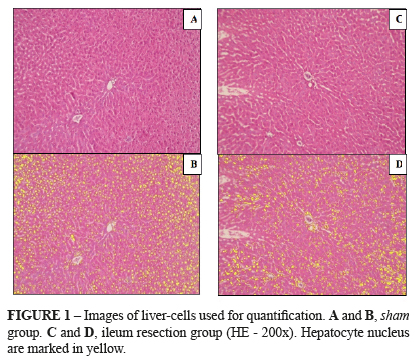
Euthanasia of the animals was done using lethal dose of anesthetic (thiopental 100 mg/Kg). If animals appeared ill (piloerection and lethargy) or had bowel obstruction (bowel dilatation proximal to the anastomosis) during observation, they were excluded. The right lobe of the liver was fixed in 10% neutral buffered formalin in order to analyze the liver histology and to quantify the number of hepatocytes. The formalin-fixed slices were embedded in paraffin, processed and stained with hematoxylin and eosin. A BX50 microscope (Olympus, Tokyo, Japan) was used to quantify the hepatocytes density by examining of six different images of each lamina. Quantification was obtained using a video-assisted computer program (Image-ProPlus 6.0 Media Cybernetics).
Statistics
Data were presented as mean±standard deviation and analyzed using SPSS software 17.0 (Chicago, IL, USA). The experiment was completely randomized, with statistical analysis performed using the Student t test. p<0.05 was considered statistically significant.
Results
Analysis of the liver mass: body mass ratio
After calculating the ratio of liver mass: body mass, we observed a significant decrease in this ratio in animals subjected to ileum resection compared to sham group (p=0.03), as shown in Table 1. We did not observe difference in body weight comparing the two groups during the experiment.
Biodistribution of Tc-99m-phytate
We observed a lower percentage of radioactivity per gram of tissue (%ATI/g) of the erythrocytes and the liver in the group undergoing ileal resection. However, the results were only significant in the statistical comparison of hepatic uptake (p=0.033). Thus, there was loss of functional liver cell mass as evidenced by lower uptake of radiocolloid by the liver of experimental group rats, compared to the sham group. The data of the radiopharmaceutical biodistribution in erythrocytes and liver are expressed using descriptive statistics on Table 2.
Biochemical results
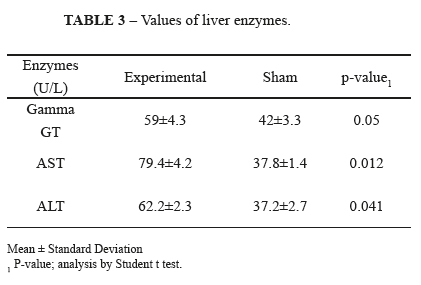
The ileum resection was associated to changes in parameters of liver enzymes. We observed increased levels of liver (ALT and AST) and canalicular (GGT) enzymes in the experimental group, compared to the sham group. Only the increase in AST and ALT was statistically significant (p=0.012 and p=0.041, respectively), showing a greater degree of parenchymal damage in the group undergoing ileal resection. These data are summarized in Table 3.
Histopathologic data
The liver cell mass also changed in the group undergoing ileum resection. Parallel to the reduction of functional liver mass and deterioration of biochemical parameters of liver function, the number of hepatocytes was higher in the sham group rats (Figure 1, A,B) compared to the experimental group (Figure 1, C,D). After marking the hepatocyte nucleus using yellow color, the mass difference became more perceived evidenced by the lowest number of hepatocytes per field, comparing in a same increase (p = 0.001) (Figures 1B and 2B). The data are summarized in Table 4.
Discussion
The adaptive capacity of the liver is known since ancient Greece by the legend of Prometheus9, and it begins with the process of compensatory hyperplasia in cases where there is a disproportion between the hepatic functional capacity and the organism's demand1. Although the most widely studied model of liver adaptation uses partial hepatectomy as the triggering stimulus of the regeneration process, other experimental models are also being used in the attempt of better understanding the precise signals involved in the regulation of liver mass. Combined models of partial hepatectomy and small bowel resection has successfully demonstrated that intestinal adaptation takes priority over hepatic adaptation and that intestinal resection reduces liver adaptive process. This issue has not yet been fully understood2,10. The interrelationship between intestine and liver has also been demonstrated in an experimental model in which a 70% hepatectomy was capable to induce trophic changes in the distal ileum that remained at short and long term, as well as causing atrophy of the ileum wall and reduction of the villi thickness11. In parallel, partial small bowel resections, particularly ileum resections, appear to change the hepatic fat composition, being observed a reduction in mitochondrial density and mild hepatic macrosteatosis in animals subjected to intestinal resection, confirming the existence of a close interaction between these organs12. In our study, this interaction was studied by functional tests, one of them examined by nuclear medicine. The loss of functional liver cell mass was evidenced by histology and by low liver uptake of radiocolloid by the experimental group rats.
Anatomically, through the portal circulation, liver and intestine also have a close metabolic relationship, which suggests the existence of a possible functional interrelation. Nelson et al.2 proposed that small bowel is a substantial source of hepatotrophic factors that are present in portal blood. Within this context, intestinal resections could reduce the ability to sustain the trophic effect in regenerating liver, resulting in decreased liver mass and function after resection.
On the other hand, bile acids, important hepatic products whose levels are strictly controlled, are also related to the liver regeneration capacity, as shown by Huang et al.13. They have identified a nuclear bile-acid-dependent receptor that works signaling to normal hepatic regeneration. Low levels of circulating bile acids, such as in the absence of the nuclear primary bile acid receptor, would inhibit this process. Thus, reducing the absorption of vitamin B12 and bile salts, distal bowel resection could culminate in reduced levels of circulating bile acids and in consequences on the liver adaptation process.
However, although the process of compensatory liver hypertrophy and hyperplasia, there are other mechanisms to regulate liver mass. It is possible that the intestinal resection is responsible for the decrease in function a liver mass, in order to reestablish optimal ratio. Our results clearly demonstrated that ileal resection interfered significantly in the liver cells hyperplasia and in the functional liver mass.
The mechanism involved in regulating the reduction of liver mass, however, remains without a complete clarification. Although the causes of cell death are various, liver cells typically dye by one of two modes: oncotic necrosis or apoptosis14. Evidences support apoptosis as a major possibility, having been observed an increase in the ratio between pro-apoptotic and anti-apoptotic proteins after partial small bowel resection15.
The liver mass reduction observed in our study as a consequence of ileum resection had already been successfully shown after proximal intestine resection and suggested to distal intestine by Qiu et al.15. Using an experimental model, they evaluated liver parameters at various time intervals after proximal bowel resection, and characterized temporal changes in liver mass after surgery, that returned to normal ranges in seven days. In the present study the hepatic evaluation was performed on the 30th day after ileum resection in order to observe if this procedure causes a persistent liver mass alteration in the late postoperative period. The literature provides evidence that function as well as histological liver data are affected in animals subjected to intestinal resection, and the intestine adaptation makes the liver function return to normal levels within the low postoperative period2. This did not happen in our study.
To evaluate functional liver mass, we used pertechnetate labeled phytate, a radiocolloid which biodistribution has already been studied and validated in the assessment of liver function in postoperative period of intestinal resection in an animal model8. In the present study we observed a significant reduction of functional liver capacity in rats subjected to ileum resection, examined by measuring aspartate aminotransferase (AST) and alanine aminotransferase (ALT), intracellular enzymes released in the interstitium and circulation after hepatocytes injury16.
Thus, in our study, ileum resection alone was shown to negatively influence the process of liver adaptation, since the reduction of physical and functional liver mass was persistent in late postoperative period of the animals submitted to surgery. In addition to the significant increase of aminotransferases in the experimental group rats compared to sham group, we also observed a significant reduction on the hepatocytes number in the histological analysis, a method used to examine liver damage17.
Another parameter that we studied was the ratio between liver mass and body mass. In all cases of liver adaptation, it has been observed that the examination of liver function must be related to the hepatic mass and lean body mass ratio, and not to liver mass per se2,15. In our study, we observed a reduction of this ratio in the animals subjected to ileum resection. This may have been caused by reduced nutrients absorption, associated to the reduction of liver mass, possibly in a higher proportion, in an attempt to restore the optimal liver mass/body mass ratio. Qiu et al.15 also observed the reduction of liver mass in rats following intestinal resection and identified autophagy as the mediator of this effect.
The existence of a gut-liver axis, as well as the liver failure that occurs in patients suffering from short bowel syndrome, which is characterized by inadequate digestion and absorption of nutrients after extensive intestinal resection, is already consensus in literature12,13,18. However, our study, by showing liver injury identified by increased aminotransferases and through weight and functional hepatic losses, confirmed significant changes in liver. In fact, there is evidence that ileum resection represents an independent risk factor for cholelithiasis19, and that ileum resection is an important component in hepatic disorder observed in short bowel syndrome20,21.
Three days after partial liver resection, the original organ mass is almost restored. However, at this stage of liver regeneration, hepatic histology differs substantially from normal. After this time point, hepatocyte proliferation decreases and stellate cells migrate into the clusters. At the same time, new vascular branches are formed. Finally, normal liver histology and function is re-established five to seven days after surgery22. In our study we performed the liver biopsis at 30th postoperative day.
Therefore, further studies are needed in order to evaluate the clinical consequences related to the deleterious effects of ileum resection in liver structure and function, as demonstrated in our study using an animal model.
Conclusion
The data of the present study support that the ileum has important role in liver function and liver mass regulation, and they have potential clinical implications regarding the pathogenesis of liver disfunction following lower bowel resection.
Received: October 22, 2012
Review: December 20, 2012
Accepted: January 24, 2013
Conflict of interest: none
Financial source: CNPq (National Council of Technological and Scientific Development
- 1. Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286300.
- 2. Nelson LA, O'Brien DP, Kemp CJ, Williams JL, Dunke-Jacobs E, Erwin CR, Warner BW. Intestinal and hepatic response to combined partial hepatectomy and small bowel resection in mice. Am J Surg. 2002;183:43540.
- 3. Iyer KR, Horslen S, Torres C, Vanderhoof JA, Langnas AN. Functional liver recovery parallels autologous gut salvage in short bowel syndrome. J Pediatr Surg. 2004;39:340-4.
- 4. Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312(5771):2336.
- 5. Huet P-M, Chartrand R, Marleau D. Extrahepatic uptake of Tc-99m-phytate: its mechanism and significance in chronic liver disease. Gastroenterology. 1980;78:7680.
- 6. Jago JR, Gibson CJ, Diffey BL. Evaluation of subjective assessment of liver function from radionuclide investigations. Br J Radiol. 1987;60:12732.
- 7. Hoefs JC, Wang F, Kanel G, Braunstein P. The liver-spleen scan as a quantitative liver function test: correlation with liver severity at peritoneoscopy. Hepatology. 1995;22:111321.
- 8. Chacon DA, Araújo-Filho I, Villarim-Neto A, Rêgo AC, Azevedo IM, Bernardo-Filho M, Brandão-Neto J, Medeiros AC. Biodistribution of the radiophamarceutical sodium pertechnetate (Na99mTcO4) after massive small bowel resection in rats. Acta Cir Bras. 2007;22:430-5.
- 9. Rosenthal N. Prometheus's vulture and the stem-cell promise. N Engl J Med. 2003;349:26774.
- 10. Miyazaki M, Kohda S, Itoh H, Kaiho T, Kimura F, Ambiru S, Hayashi S, Gohchi E, Takanishi K, Nagai M. Inhibition of hepatic regeneration after 70% partial hepatectomy by simultaneous resection of the bowel in rats. Eur Surg Res. 1995;27:396405.
- 11. Sanz MBR, García JA, Tomás FJR, Puerta CV. Effects of partial hepatectomy on the distal ileum in rats. Rev Esp Enferm Dig. 2004;96:185-90.
- 12. Correa Antúnez MI, MoránPenco JM, Amaya Lozano JL, Leal Macho A, MaciáBotejara E, Saenz Santamaría J. Changes in the fat composition and histomorphology of the liver after partial intestinal resections. Nutr Hosp. 2010;25:999-1005.
- 13. Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312(5771):2336.
- 14. Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: A tale of two deaths? Hepatology. 2006;43(2 suppl 1):S31-43.
- 15. Qiu Z, Longshore SW, Warner BW, Rudnick DA. Murine Functional Liver Mass is Reduced Following Partial Small Bowel Resection. J Gastrointest Surg. 2009;13:217682.
- 16. Mangus RS, O'Connor MG, Tector AJ, Lim JD, Vianna RM. Use of the aspartate aminotransferase to platelet ratio index to follow liver fibrosis progression in infants with short gut. J Pediatr Surg. 2010;45:126673.
- 17. Otsubo T. Control of the inflow and outflow system during liver resection. J Hepatobiliary Pancreat Sci. 2012;19:15-8.
- 18. Seetharam P, Rodrigues G. Short bowel syndrome: a review of management options. Saudi J Gastroenterol. 2011;17:229-35.
- 19. Thompson JS. The role of prophylactic cholecystectomy in the short-bowel syndrome. Arch Surg. 1996;131:556-9.
- 20. Aprahamian CJ, Chen M, Yang Y, Lorenz RG, Harmon CM. Two-hit rat model of short bowel syndrome and sepsis: independent of total parenteral nutrition, short bowel syndrome is proinflammatory and injurious to the liver. J Pediatr Surg. 2007;42:992-7.
- 21. Ljungmann K, Grofte T, Kissmeyer-Nielsen P, Flyvbjerg A, Vilstrup H, Tygstrup N, Laurberg S. GH decreases hepatic amino acid degradation after small bowel resection in rats without enhancing bowel adaptation. Am J Physiol Gastrointest Liver Physiol. 2000;279:G700-6.
- 22. Kountouras J, Boura P, Lygidakis NJ. Liver regeneration after hepatectomy. Hepatogastroenterology. 2001;48:556-62.
Publication Dates
-
Publication in this collection
06 Mar 2013 -
Date of issue
Mar 2013
History
-
Received
22 Oct 2012 -
Accepted
24 Jan 2013 -
Reviewed
20 Dec 2012