ABSTRACT
In recent years the immunomodulatory actions of vitamin D, a steroid hormone, have been extensively studied. In 2020, due to the COVID-19 pandemic, the question arose as to 25(OH)D status would be related to susceptibility to SARS-CoV-2 infection, since several studies pointed out a higher prevalence and severity of the disease in populations with low levels of 25(OH)D. Thus, we investigated the 25(OH)D levels in adults “Detected” positive for SARS CoV-2 by RT-PCR (reverse transcriptase polymerase chain reaction) test, and in negative controls, “not Detected”, using the Fleury Group's examination database, in Sao Paulo, Brazil. Of a total of 14.692 people with recent assessments of 25(OH)D and RT-PCR tests for COVID-19, 2.345 were positive and 11.585 were negative for the infection. The groups did not differ in the percentage of men and women, or in the age distribution. There were no differences in the distribution of 25(OH)D between the two groups (p = 0.08); mean 25(OH)D of 28.8 ± 21.4 ng/mL and 29.6 ± 18.1 ng/mL, respectively. In the specific population studied, clinical, environmental, socioeconomic and cultural factors should have greater relevance than 25(OH)D in determining the susceptibility to COVID-19.
Keywords
Vitamin D; coronavirus; COVID-19
INTRODUCTION
The musculoskeletal effects of vitamin D are widely studied, as well as its endocrine actions in regulating the homeostasis of calcium and phosphorus. Cholecalciferol or “vitamin D”, synthesized in the skin, is metabolized in the liver to 25-hydroxycholecalciferol (25(OH)D) and then in the kidney to its biologically active form, 1,25-dihydroxycholecalciferol (1,25(OH)2D). The metabolite 25(OH)D is the major circulating form of vitamin D in humans, and it is used to reflect person's vitamin D status.
Vitamin D deficiency causes secondary hyperparathyroidism, osteomalacia, osteopenia and an increased risk of fractures. In addition to its classic functions in osteomineral metabolism, the extensive distribution of vitamin D (VDR) receptors in human tissues and the action of the active hormone, 1,25(OH)2D (calcitriol), in regulating the transcription and expression of countless genes, indicate the importance of nonskeletal actions of this hormone. Experimental and clinical studies have revealed the intracrine action of vitamin D in the immune system, particularly in monocytes and macrophages, with a modulating role for both innate and adaptive immune responses against a number of microorganisms, including viruses (11. Charoenngam N, Holick M. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12:2097.,22. Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr Rev. 2019;40(4):1109-51.).
Autophagic encapsulation of viral particles is also a cellular process enhanced by both 25(OH)D and 1,25(OH)2D (33. Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, et al. Mechanisms in Endocrinology: Vitamin D and COVID-19. European J Endocrinol. 2020;183(5):R133-R147.), with a fundamental role in reducing viral infectivity, for example for human immunodeficiency virus type 1 (44. Campbell GR, Spector SA. Toll-like receptor 8 ligands activate a vitamin D mediated autophagic response that inhibits human immunodeficiency virus type 1. PLoS Pathog. 2012;8(11):e1003017.). In addition, other data revealed a role of vitamin D in pulmonary protection against acute respiratory infections, in particular its action on capillary permeability, which plays a fundamental role in the pathophysiology of many diseases with pulmonary involvement (55. Ginde AA, Mansbach JM, Camargo CA. Association between Serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169(4):384-90.,66. Santos RND, Maeda SS, Jardim JR, Lazaretti-Castro M. Reasons to avoid vitamin D deficiency during COVID-19 pandemic. Arch Endocrinol Metab. 2020 Aug 28:S2359-39972020005006214. doi: 10.20945/2359-3997000000291.
https://doi.org/10.20945/2359-3997000000...
). Respiratory epithelial cells constitutively express 1α-hydroxylase resulting in local activation of vitamin D. Vitamin D-dependent genes including cathelicidin and CD14 are upregulated after the exposure of airway epithelial cells to the inactive vitamin D precursor (77. Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181(10):7090-9.).
In 2020, during the coronavirus disease (COVID-19) pandemic, several retrospective studies were published, showing an association between low 25(OH)D status and increased susceptibility to and severity of SARS-CoV-2 infection, suggesting a deleterious effect of hypovitaminosis D on the incidence and clinical evolution of COVID-19 (88. D'Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, Keller F, Cantù M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):1359.–1212. Faul JL, Kerley CP, Love B, O'Neill E, Cody C, Tormey W, Hutchinson K, Cormican LJ, Burke CM. Vitamin D Deficiency and ARDS after SARS-CoV-2 Infection. Ir Med J. 2020;113(5):84.). Experimental research has shown that 1,25(OH)2D modulates the expression of angiotensin-converting enzyme 2, which is the receptor for the entry of SARS CoV-2 into cells. VDR-null mice showed more severe acute lung injury in a sepsis model than their wild-type counterparts (1313. Kong J, Zhu X, Shi Y, Liu T, Chen Y, Bhan I, Zhao Q, Thadhani R, Li YC. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol Endocrinol. 2013;27(12):2116-25.). Thus, it became essential to study the relationship between 25(OH)D and SARS CoV-2 incidence, with the aim of identifying an easily modifiable factor that can play a preventive role in all populations susceptible to infection.
OBJECTIVE
To compare 25(OH)D levels between individuals infected with SARS-CoV-2, with diagnostic confirmation by RT-PCR (reverse-transcriptase polymerase chain reaction), and individuals negative for SARS-CoV-2, using the Fleury Group's database.
SUBJECTS AND METHODS
Data source
Fleury Group is a medical organization that provides supplemental health services in Brazil. Data were collected from the Fleury Group's Caché database, of 14692 individuals who underwent RT-PCR tests for the diagnosis of COVID-19, from March to July 2020, who also had 25(OH)D measured; participants were identified by a unique register number. The study protocol was approved by the research and ethics committee of Fleury Group (protocol number 4.409.445, CAAE 39961120.7.00005474). Informed consent was not required since the data were anonymized.
Study design
This was a retrospective study that collected records from individuals of both genders, between 18 and 90 years old, with RT-PCR results for SARS CoV-2 and who simultaneously had their 25(OH)D measured over a period of 30 days before or after the collection of the sample for COVID-19 RT-PCR test. In cases of patients with more than one vitamin D test, the most recent in relation to the RT-PCR date, was selected. Records with 25(OH)D above 100 ng/mL were excluded to avoid distortions in the analysis of vitamin D averages. After removing entries with missing data and inconclusive diagnostic tests for COVID-19, the new dataset (n = 13930) was divided into two groups: “Detected” or positive for COVID-19 (2345 patients) and “Not Detected” or negative for COVID-19 (11585 patients).
Biochemical analysis
25OH vitamin D – Liason, CLIA, DiaSorin, Saluggia, Italy, reference range: 20-60 ng/mL, intra and inter-assay coefficient of variation are 6.0% and 8.0%, respectively; RT-PCR – molecular test developed entirely in house according to the Charité protocol and a confirmatory test by the CDC protocol when necessary for confirmation, using clinical samples from the respiratory tract.
Statistical analysis
To assess the significant differences between the groups, the normality of the two groups was confirmed (Kolmogorov-Smirnov Normality Test) and then, the difference between the means of the groups was verified using the Welch T test (Software R, www.r-project.org). This is a parametric test, adapted from the Student's t-test, whose objective is to compare two independent groups, without the hypothesis of equal population variance. The test considers the difference between the number of patients in each group when calculating the real difference between the means (1414. Derrick B, Toher D, White P. Why Welchs test is Type I error robust. Tutor Quant Methods Psychol. 2016;12(1):30-8.). Statistical significance was defined as p < 0,05.
RESULTS
There was no difference between groups regarding the percentage of men and women, or regarding the age distribution. There was no significant difference for the mean 25(OH)D between men and women, or between adults and the elderly (over 60 years), (multiple T tests with Bonferroni correction). The Detected Group had a mean 25(OH)D of 28.8 ± 21.4 ng/mL, with a median of 26.0 ng/mL. The not Detected Group had a mean 25(OH)D of 29.6 ± 18.1 ng/mL, with a median of 27.0 ng/mL. Table 1 shows the percentage of individuals and the means of 25(OH)D, separated by the ranges of the values, < 12 ng/mL, 12-20 ng/mL, 20-30 ng/mL and > 30 ng/mL, in both Detected and not Detected groups (1515. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96(7):1911-30.,1616. Moreira CA, Ferreira CEDS, Madeira M, Silva BCC, Maeda SS, Batista MC, et al. Reference values of 25-hydroxyvitamin D revisited: a position statement from the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC). Arch Endocrinol Metab. 2020;64(4):462-78.).
Number of patients, mean and percentage of patients by ranges of 25(OH)D, for the “Detected” and “not Detected” groups. Multiple T tests with Bonferroni correction were applied; t-test were performed using the log-transformed values of the means
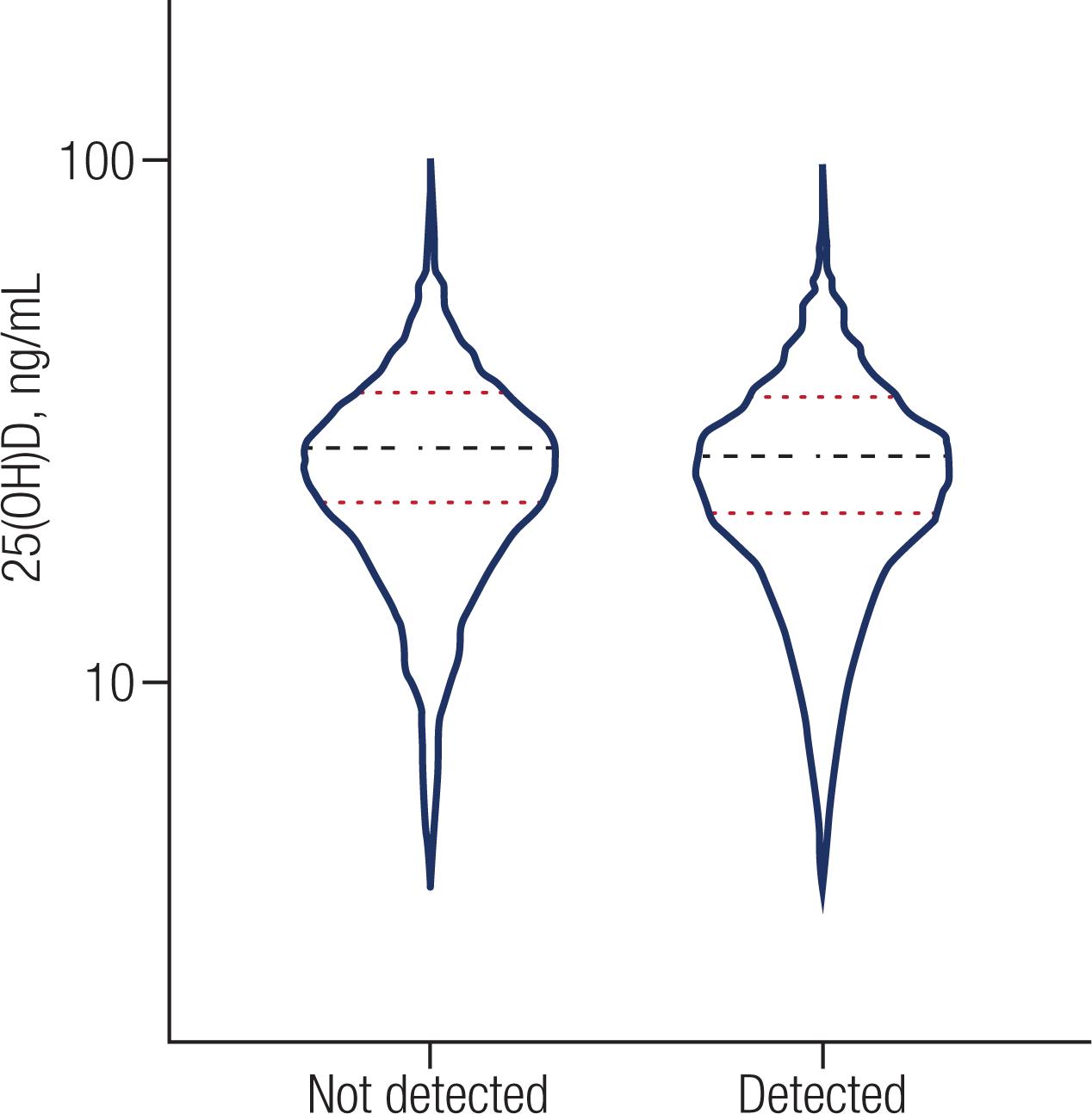
There was no difference in the distribution of 25(OH)D between the “Detected” and “not Detected” groups (p = 0.0811). Figure 1 shows the dispersion of the values of 25(OH)D between the Detected and not Detected groups.
The image shows the dispersion of 25(OH)D, in patients Detected and Not Detected for COVID-19; means are indicated by the black line and two standard deviations (SD) by the red line.
DISCUSSION AND CONCLUSIONS
Studies prior to the COVID-19 pandemic period have demonstrated the role of vitamin D in innate and adaptive immunity, particularly in protection against viral and bacterial infections. Martineau et al demonstrated, in a large meta-analysis of randomized controlled trials, that vitamin D supplementation reduced the risk of experiencing at least one acute respiratory tract infection (1717. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.). The initial spread of COVID-19 occurred in countries that were going through the winter, had a high prevalence of hypovitaminosis D. Together, these data raised the question of the role of vitamin D to susceptibility and the severity of the disease.
Numerous studies initially linked 25(OH)D status to susceptibility and mortality from SARS-CoV-2, although causality cannot be demonstrated (88. D'Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, Keller F, Cantù M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):1359.–1212. Faul JL, Kerley CP, Love B, O'Neill E, Cody C, Tormey W, Hutchinson K, Cormican LJ, Burke CM. Vitamin D Deficiency and ARDS after SARS-CoV-2 Infection. Ir Med J. 2020;113(5):84.). The clinical evolution and severity of COVID-19 respiratory disease has enormous complexity and competition from numerous other confounding factors, such as obesity, hypertension, socioeconomic level, quality of medical care, comorbidities and probably, the degree of exposure and genetic susceptibility. However, individuals with inadequate 25(OH)D could have an additional risk of contracting a viral infection such as COVID-19, and possibly a greater risk of an unfavorable clinical course (33. Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, et al. Mechanisms in Endocrinology: Vitamin D and COVID-19. European J Endocrinol. 2020;183(5):R133-R147.,66. Santos RND, Maeda SS, Jardim JR, Lazaretti-Castro M. Reasons to avoid vitamin D deficiency during COVID-19 pandemic. Arch Endocrinol Metab. 2020 Aug 28:S2359-39972020005006214. doi: 10.20945/2359-3997000000291.
https://doi.org/10.20945/2359-3997000000...
,1818. Biesalski HK. Vitamin D deficiency and co-morbidities in COVID-19 patients – A fatal relationship? NFS Journal. 2020;20:10-21.). Additionally the social isolation, imposed to control the pandemic, could be a predisposing factor to less sun exposure.
However, our study showed no difference in 25(OH)D status in a large group of Brazilian infected individuals with SARS CoV-2 and non infected controls. The same conclusion was reached by Hastie and cols. (1919. Hastie CE, Mackay DF, Ho F, Celis-Morales CA, Katikireddi SV, Niedzwiedz CL, et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr. 2020;14(4):561-5.) and Raisi-Estabragh and cols. (2020. Raisi-Estabragh Z, McCracken C, Bethell MS, Cooper J, Cooper C, Caulfield MJ, et al. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK Biobank. J Public Health (Oxf). 2020;42(3):451-60.), using UK Biobank data. Neither study supports the hypothesis of a link between vitamin D levels and the risk of SARS CoV-2 infection, nor does 25(OH)D explain the ethnic differences in COVID-19 prevalence.
The population sample evaluated in this study has a high socioeconomic level, has access to private medical services, and is predominantly of Caucasian origin; therefore, we were unable to assess socioeconomic or ethnic-racial factors that could affect infectivity. Another aspect to be considered is that the pandemic spread in Brazil during late summer and early fall, periods characterized by higher levels of solar irradiation; therefore, low 25(OH)D is less prevalent. Unfortunately, we were also unable to control for other clinical risk parameters for COVID-19, such as weight, diabetes and other comorbidities.
Despite all of the evidence described in the literature on the immunological action of vitamin D, we did not observe differences between 25(OH)D status and COVID-19 susceptibility in a large Brazilian population sample. The strength of this study is the number of participants, mostly Sao Paulo residents, the largest city in Brazil located in the southeastern region of the country. The study population, both with and without SARS CoV-2 infection, has a lower prevalence of hypovitaminosis D, compared to that described in the European or American populations, or even within specific population subgroups living in Sao Paulo, such as the elderly over 80, institutionalized or chronically ill patients (1616. Moreira CA, Ferreira CEDS, Madeira M, Silva BCC, Maeda SS, Batista MC, et al. Reference values of 25-hydroxyvitamin D revisited: a position statement from the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC). Arch Endocrinol Metab. 2020;64(4):462-78.).
In conclusion, clinical, environmental, socio-economic and cultural factors have greater relevance than vitamin D status in determining the susceptibility to SARS-CoV-2 infections in the population studied.
-
Financial support: Fleury Group Research and Development Center funded study statistical analysis.
REFERENCES
-
1Charoenngam N, Holick M. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12:2097.
-
2Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr Rev. 2019;40(4):1109-51.
-
3Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, et al. Mechanisms in Endocrinology: Vitamin D and COVID-19. European J Endocrinol. 2020;183(5):R133-R147.
-
4Campbell GR, Spector SA. Toll-like receptor 8 ligands activate a vitamin D mediated autophagic response that inhibits human immunodeficiency virus type 1. PLoS Pathog. 2012;8(11):e1003017.
-
5Ginde AA, Mansbach JM, Camargo CA. Association between Serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169(4):384-90.
-
6Santos RND, Maeda SS, Jardim JR, Lazaretti-Castro M. Reasons to avoid vitamin D deficiency during COVID-19 pandemic. Arch Endocrinol Metab. 2020 Aug 28:S2359-39972020005006214. doi: 10.20945/2359-3997000000291.
» https://doi.org/10.20945/2359-3997000000291 -
7Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181(10):7090-9.
-
8D'Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, Keller F, Cantù M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):1359.
-
9Merzon E, Tworowski D, Gorohovski A, Vinker S, Golan Cohen A, Green I, Frenkel-Morgenstern M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287(17):3693-3702. doi: 10.1111/febs.15495.
» https://doi.org/10.1111/febs.15495 -
10Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020 Jul;32(7):1195-8.
-
11Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw Open. 2020;3(9):e2019722.
-
12Faul JL, Kerley CP, Love B, O'Neill E, Cody C, Tormey W, Hutchinson K, Cormican LJ, Burke CM. Vitamin D Deficiency and ARDS after SARS-CoV-2 Infection. Ir Med J. 2020;113(5):84.
-
13Kong J, Zhu X, Shi Y, Liu T, Chen Y, Bhan I, Zhao Q, Thadhani R, Li YC. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol Endocrinol. 2013;27(12):2116-25.
-
14Derrick B, Toher D, White P. Why Welchs test is Type I error robust. Tutor Quant Methods Psychol. 2016;12(1):30-8.
-
15Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96(7):1911-30.
-
16Moreira CA, Ferreira CEDS, Madeira M, Silva BCC, Maeda SS, Batista MC, et al. Reference values of 25-hydroxyvitamin D revisited: a position statement from the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC). Arch Endocrinol Metab. 2020;64(4):462-78.
-
17Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
-
18Biesalski HK. Vitamin D deficiency and co-morbidities in COVID-19 patients – A fatal relationship? NFS Journal. 2020;20:10-21.
-
19Hastie CE, Mackay DF, Ho F, Celis-Morales CA, Katikireddi SV, Niedzwiedz CL, et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr. 2020;14(4):561-5.
-
20Raisi-Estabragh Z, McCracken C, Bethell MS, Cooper J, Cooper C, Caulfield MJ, et al. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK Biobank. J Public Health (Oxf). 2020;42(3):451-60.
Publication Dates
-
Publication in this collection
29 Mar 2021 -
Date of issue
May-Jun 2021
History
-
Received
06 June 2020 -
Accepted
16 Feb 2021