ABSTRACT:
Weeds compete with agricultural crops for water, light, nutrients and space, besides having an extensive seed bank. However, another aspect to be considered relates to few studies pointing out weeds as hosts of phytopathogenic fungi. Many fungi, the main cause of diseases in plants, are known to use seeds as an efficient means of survival and dispersal. The objective of this work was to evaluate the health of weed seeds and the pathogenicity of fungi associated to plants of agricultural importance. The seeds were collected manually in Cerrado areas located in the municipality of Gurupi, Tocatins, Brazil. The blotter test method was used to evaluate seed health. The incidence of fungi was evaluated through an individual analysis of seeds using a stereoscopic and an optical microscope. The pathogenicity of fungi from weed seeds was evaluated by inoculation in plants of agronomic interest and, when pathogenic, we inoculated them in the host plant of the fungus. Weed seeds have been identified in fungi of the genus Alternaria, Aspergillus, Bipolaris, Cladosporium, Curvularia, Fusarium, Nigrospora, Papularia, Rhizopus and Pythium. The seeds of Acanthospermum australe, Bidens pilosa, Cenchrus echinatus, Digitaria horizontalis, Echinochloa crus-pavonis, Eleusine indica, Ipomoea sp., Pennisetum setosum, Sida rhombifolia, Spermacoce latifolia, Tridax procumbens and Vernonia polyanthes carry and disseminate fungi that, once inoculated, cause infection in plants of agricultural importance, such as Oryza sativa, Phaseolus vulgaris, Vigna unguiculata, Zea mays and Glycine max.
KEYWORDS:
diseases host; invasive plants; sanity; transport of fungi
RESUMO:
As plantas daninhas competem com culturas agrícolas por água, luz, nutrientes e espaço, além de possuírem um extenso banco de sementes. Entretanto, outra vertente a ser considerada é quanto aos poucos estudos relacionando plantas daninhas como hospedeiras de fungos fitopatogênicos. É sabido que muitos fungos, principais causadores de doenças em plantas, utilizam as sementes como meio eficiente de sobrevivência e de dispersão. Dessa forma, o trabalho objetivou avaliar a sanidade de sementes de plantas daninhas e a patogenicidade dos fungos associados às plantas de importância agrícola. As sementes foram coletadas manualmente em áreas de cerrado localizadas no município de Gurupi, Tocantins, utilizando o método blotter test para avaliação da sanidade. A incidência dos fungos foi avaliada com auxílio de microscópio estereoscópico e ótico. A patogenicidade dos fungos oriundos das sementes de plantas daninhas foi avaliada por meio da inoculação em plantas de interesse agronômico e, quando patogênico, a inoculação foi na própria planta daninha hospedeira do fungo. Foram identificados os fungos dos gêneros Alternaria, Aspergillus, Bipolaris, Cladosporium, Curvularia, Fusarium, Nigrospora, Papularia, Rhizopus e Pythium. As sementes de Acanthospermum australe, Bidens pilosa, Cenchrus echinatus, Digitaria horizontalis, Echinochloa crus-pavonis, Eleusine indica, Ipomoea sp., Pennisetum setosum, Sida rhombifolia, Spermacoce latifolia, Tridax procumbens e Vernonia polyanthes transportam e disseminam fungos que, uma vez inoculados, causam infecção em plantas de importância agrícola, como Oryza sativa, Phaseolus vulgaris, Vigna unguiculata, Zea mays e Glycine max.
PALAVRAS-CHAVE:
hospedeiro de doenças; plantas invasoras; sanidade; transporte de fungos
INTRODUCTION
Brazilian agriculture has presented a constant growth in production each year, expanding to new agricultural frontiers. In the 2016/17 harvest, the grain production estimate was of 213.1 million tons, with growth of 14.2% in relation to the previous harvest, and a total planted area reaching 59.2 million hectares (CONAB, 2017COMPANHIA NACIONAL DE ABASTECIMENTO - CONAB. Acompanhamento da safra brasileira de grãos safra 2016/17, v.4, p.1-152, 2016. Available from: <Available from: http://www.conab.gov.br/OlalaCMS/uploads/arquivos/16_12_08_12_27_47_boletim_graos_dezembro_2016.pdf
>. Access on: Sep. 22 2017.
http://www.conab.gov.br/OlalaCMS/uploads...
). However, even with this volume of production and expansion of areas, there are several factors that may influence these results. One of the major current concerns of agriculture is precisely related to the damages caused by weeds to crops (VASCONCELOS et al., 2012VASCONCELOS, M.C.C.; SILVA, A.F.A.; LIMA, R.S. Interferência de plantas daninhas sobre plantas cultivadas. Agropecuária Científica no Semi-Árido, Campina Grande, v.8, n.1, p.1-6, 2012.).
In addition to damage and reduced crop yields, weeds may act as an alternative host of phytopathogens, serving as sources of inoculum and still playing a significant role in disease epidemiology (SALES JÚNIOR et al., 2012SALES JÚNIOR, R.; OLIVEIRA, O.F.; MEDEIROS, E.V.; GUIMARÃES, I.M.; CORREIA, K.C.; MICHEREFF, S.J. Ervas daninhas como hospedeiras alternativas de patógenos causadores do colapso do meloeiro. Revista Ciência Agronômica, Fortaleza, v.43, n.1, p.195-198, 2012.). They can also produce allelopathic substances, be toxic to animals and humans, cause depreciation of the soil and hinder the management of cultivated plants (VASCONCELOS et al., 2012VASCONCELOS, M.C.C.; SILVA, A.F.A.; LIMA, R.S. Interferência de plantas daninhas sobre plantas cultivadas. Agropecuária Científica no Semi-Árido, Campina Grande, v.8, n.1, p.1-6, 2012.).
According to CAVERS; BENOIT (1989CAVERS, P.B.; BENOIT, D.L. Seed banks in amble land. In: CAVERS, P.B. Ecology of soil seed banks. New York: Academic Press, 1989. p.309-328.), weeds have an efficient seed bank. They constitute thus a major problem for the management of agricultural activity, since they guarantee infestations over a long period, even with the adoption of control measures. It occurs because these plants have mechanisms such as ease of dispersion, dormancy and allelopathic effects (CARMONA, 1992CARMONA, R. Problematic and management of weed seed banks in agricultural soils. Planta Daninha, Viçosa, v.10, n.1/2, p.5-16, 1992.). Seeds may shelter and transport microorganisms from all taxonomic groups, pathogenic or not. Therefore, the detection of such organisms becomes one of the most important tools for the sanitary management of diseases (BARROCAS; MACHADO, 2010BARROCAS, E.; MACHADO, J.D.C. Introdução a patologia de sementes e testes convencionais de sanidade de sementes para a detecção de fungos fitopatogênicos. Informativo ABRATES: Inovações tecnológicas em Patologia de Sementes, Londrina, v.20, n.3, p.74-75, 2010.).
For the dissemination and survival, pathogens depend on dissemination, which implies their movement, which is mostly provided by seeds (BARROCAS; MACHADO, 2010BARROCAS, E.; MACHADO, J.D.C. Introdução a patologia de sementes e testes convencionais de sanidade de sementes para a detecção de fungos fitopatogênicos. Informativo ABRATES: Inovações tecnológicas em Patologia de Sementes, Londrina, v.20, n.3, p.74-75, 2010.) and external agents such as water, air, humans and insects (AMORIM et al., 2011AMORIM, L.; REZENDE, J.A.M.; BERGAMIN FILHO, A. Manual de fitopatologia. Volume 1: Princípios e Conceitos. 4 ed. São Paulo: Agronômica Ceres LTDA, 2011. 920p.). Some authors describe the presence of phytopathogenic fungi associated with weeds causing diseases in crops, such as Paullinia cupana, Cucumis melo and Eucalyptus sp. (MILÉO et al., 2007MILÉO, L.J.; SILVA, J.F.; BENTES, J.L.S.; CHRISTOFFOLETI, P.J. Plantas daninhas hospedeiras alternativas de Colletotrichum guaranicola em cultivos de guaraná no Estado do Amazonas. Planta Daninha, Viçosa, v.25, n.4, p.771-782, 2007.; SALES JÚNIOR et al., 2012SALES JÚNIOR, R.; OLIVEIRA, O.F.; MEDEIROS, E.V.; GUIMARÃES, I.M.; CORREIA, K.C.; MICHEREFF, S.J. Ervas daninhas como hospedeiras alternativas de patógenos causadores do colapso do meloeiro. Revista Ciência Agronômica, Fortaleza, v.43, n.1, p.195-198, 2012.; VASCONCELOS et al., 2012VASCONCELOS, M.C.C.; SILVA, A.F.A.; LIMA, R.S. Interferência de plantas daninhas sobre plantas cultivadas. Agropecuária Científica no Semi-Árido, Campina Grande, v.8, n.1, p.1-6, 2012.).
Little is known about the importance of weeds and their seeds as an alternative host of disease-causing pathogens in cultivated plants. Thus, the objectives of this work were to evaluate the health of weed seeds and verify the pathogenicity of fungi on these seeds to plants of agronomic interest.
MATERIALS AND METHODS
Origin of seeds
Weed seeds were collected manually in Cerrado areas from September 2013 to August 2014 in the city of Gurupi, Tocatins, Brazil. Seeds collected and identified came from 12 weed species, including the families Asteraceae, Convolvulaceae, Malvaceae, Poaceae and Rubiaceae. The species collected were Acanthospermum australe, Bidens pilosa, Cenchrus echinatus, Digitaria horizontalis, Echinochloa crus-pavonis, Eleusine indica, Ipomoea sp., Pennisetum setosum, Sida rhombifolia, Spermacoce latifolia, Tridax procumbens and Vernonia polyanthes. After collection, the seeds were dried in an oven at 40 ºC for 45 minutes to remove excess of moisture and avoid external contaminations. Afterwards, they were packed in paper bags identified with the place and date of collection, and stored in a cold room (5 ± 2 ºC) until being used.
Sanity
For this experiment, the filter paper method (blotter test) was used according to the Manual of Sanitary Analysis of Seeds (BRASIL, 2009BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Manual de Análise Sanitária de Sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: Mapa/ACS, 2009. 200p.). The experimental design was completely randomized with 12 weed species and three replications.
Seed disinfestation was carried out by immersion for 30 seconds in alcohol (50%) and 40 seconds in sodium hypochlorite (1%), followed by three washes with distilled and sterilized water. The seeds were then distributed in sterile Petri dishes containing two layers of filter paper, sterilized and moistened with distilled and sterilized water. Ninety seeds were arranged for each treatment by placing 30 seeds per petri dish. The seeds were incubated in an incubation chamber with a 12-hour photoperiod at 25 ± 2 ºC for seven days until evaluation.
The survey of pathogens was carried out through an individual analysis of seeds using a stereoscopic and an optical microscope by visualizing the morphological characteristics of fungal structures. Using a sterile stylet, the fungal structures were transferred to Petri dishes containing potato-dextrose-agar (BDA) culture medium for growth and sporulation for further identification. It was performed based on specialized literature, such as WATANABE (2010WATANABE, T. Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species. 3 ed. New York: CRC Press, 2010. 426p.). The fungi incidence data were expressed as percentage.
Pathogenicity
Microorganisms considered potentially pathogenic - Alternaria sp., Bipolaris sp., Curvularia sp., Fusarium sp. (four isolates) and Pythium sp. (two isolates) - were isolated in the sanity test, grown in Petri dishes with BDA and incubated for seven days at 25 ± 2 ºC with a 12-hour photoperiod for inoculum production. The pathogenicity of fungi from weed seeds was evaluated by inoculations in plants of agronomic importance: Oryza sativa (rice), Phaseolus vulgaris (bean), Vigna unguiculata (cowpea), Zea mays (corn) and Glycine max (soybean). Only fungi that were pathogenic to plants of agronomic importance were inoculated in the same weed of origin in order to attest that it had a potential pathogenic effect to the host plant.
In order to prepare the weed and cultivated seedlings, sowing was initially performed in pots with capacity of 5 L containing a mixture of autoclaved sand and commercial substrate (Plantmax) at a 1:1 ratio. The material was kept with daily irrigation in a greenhouse until 20 days after sowing, and ten plants of each species were used for each inoculated microorganism.
For the genera Alternaria, Bipolaris and Curvularia, the inoculation was carried out using a hand spray, and suspensions with conidia of each genus were sprayed onto the leaves of the plants at the concentration of 1 x 106 conidia mL-1, adjusted with a Neubauer Chamber. For the genus Fusarium, we used sticks contaminated with fungal mycelium introduced directly in the tissue of the petiole, stem and culm of plants. The contamination of sterile wooden sticks was carried out by inserting them at the center of Petri dishes containing BDA along with a fungal mycelium disc. Then, the plates were incubated for ten days at 25 ± 2 ºC with a photoperiod of 12 hours for inoculum production. For the genus Pythium, disks with 5-mm-diameter mycelium were used. They were introduced and fixed to the stem and culm of the plants using a sterile pin. For each crop, control plants were only sprayed with water and kept under the same conditions.
After inoculation, the plants were kept in a humid chamber for 48 hours, and then in a greenhouse, where after ten days the pathogenicity evaluations were performed. When symptoms were observed in the inoculated tissue, the fungus was re-isolated and cultured in BDA medium (ALFENAS; MAFIA, 2007ALFENAS, A.C.; MAFIA, R.G. Métodos em fitopatologia. Viçosa: UFV, 2007. 382p.) with the purpose of confirming the causal agent, complying with the Koch Postulates.
Statistical procedure
After performing an analysis of variance, the means was compared by the Scott-Knott test at 5% probability using the software SISVAR.
RESULTS AND DISCUSSION
Sanity
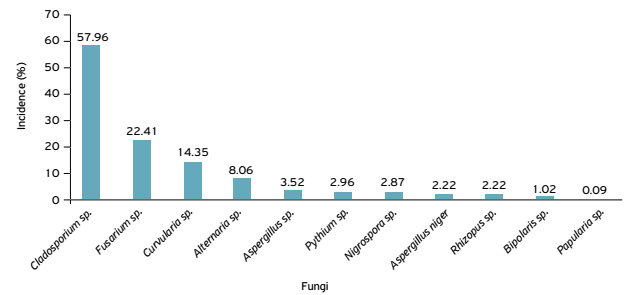
Eleven fungal genera associated with weed seeds were identified (Fig. 1). The genera Alternaria, Cladosporium, Curvularia and Fusarium had the highest incidence: 8.06, 57.96, 14.35 and 22.41%, respectively. The fungi identified with the lowest incidence were from the genera Aspergillus, Bipolaris, Nigrospora, Papularia and Rhizopus. They were also identified in 2.96% of the isolated seed samples of the genus Pythium. The genera Aspergillus, Cladosporium and Rhizopus are considered, in most cases, storage fungi and saprophytes, which may cause depreciation of grains and seeds (MARCHI et al., 2010MARCHI, C.E.; FERNANDES, C.D.; BUENO, M.L.; BATISTA, M.V.; FABRIS, L.R. Fungic microflora of Panicum maximum and Styosanthes spp. commercial seed. Semina: Ciências Agrárias, Londrina, v.31, n.3, p.575-584, 2010.).
Fungi considered potentially pathogenic have been identified as belonging to the genera Alternaria, Bipolaris, Curvularia and Fusarium, and also Pythium (Table 1). Among pathogenic microorganisms, fungi have a greater ability to penetrate directly into plant tissues and to lodge internally in the organs of plants, especially in the seed (MACHADO, 1988 MACHADO, J.C. Patologia de sementes - fundamentos e aplicações. Brasília: MEC/ESAL/FAEP, 1988. 107p.). The detection of these phytopathogenic fungi is easy, by the superficial disinfestation of the seeds, which makes it possible to reduce the incidence of saprophytic fungi located in the outer layers, usually with faster growth than pathogenic fungi.
The genus Alternaria was found in seeds of eight weed species, with significance for T. procumbens, which presented incidence of 45.55%. It was followed by the species Bidens pilosa, Digitaria horizontalis and Echinochloa crus-pavonis, with incidence of 12.20, 13.33 and 12.22%, respectively. The other weed species presented incidence below 4.44%, and others had no incidence. TÖFOLI; DOMINGUES (2004TÖFOLI, J.G.; DOMINGUES, R.J. Alternarioses em hortaliças: sintomas, etiologia e manejo integrado. Biológico, São Paulo, v.66, n.1/2, p.23-33, 2004.) point out that species of the genus Alternaria are among the main causes of fungal diseases in vegetables. There are still few reports of the incidence of fungi of the genus Alternaria on weed seeds.
The incidence of Bipolaris sp. was associated with the seeds of two weed species, Echinochloa crus-pavonis (4.44%) and Pennisetum setosum (7.77%), both belonging to the family Poaceae. For species of Bipolaris, there are reports of necrotic leaf lesions, causing the death of the tissues in several cultures. As an example, MANAMGODA et al. (2014MANAMGODA, D.S.; ROSSMAN, A.Y.; CASTLEBURY, L.A.; CROUS, P.W.; MADRID, H.; CHUKEATIROTE, E.; HYDE, K.D. The genus Bipolaris. Studies in Mycology, Utrecht, v.79, p.221-288, 2014.) reported the occurrence of Bipolaris sp. in plants of the family Poaceae, such as Pennisetum clandestinum, Zea mays, Chrysopogon aciculatus, Brachiaria ruziziensis, Oryza sativa, Panicum maximum, Setaria sp., Sorghum sp. and Triticum aestivum, distributed in Australia, India, Africa, Brazil, Canada, New Zealand, Nigeria, Zimbabwe, Denmark, among others.
The genus Curvularia presented incidence in seeds of seven weed species, with emphasis again on T. procumbens, which obtained the highest incidence: 47.78%. According to SHAN et al. (2008SHAN, F.Y.; SHI, W.; LIU, Y.; ZHANG, J.; DONG, J. Parasitic fitness and RAPD analysis of Curvularia species on corn. Journal of Agricultural University of Hebei, Baoding, v.31, p.37-41, 2008.), several species of Curvularia are associated with diseases in plants, causing necrotic spots on leaves and other parts of the plant, death of seedlings etc. There are reports of Curvularia sp. causing diseases in grasses, such as rice, sorghum and millet crops (LIMA; FURTADO, 2007LIMA, A.; FURTADO, M. Curvulariaspecies (anamorphicfungi: Hyphomycetes) from Santiago Island, Cape Vert. Portugaliae Acta Biologica, Lisboa, v.22, p.145-156, 2007.; YAGO et al., 2011YAGO, J.I.; ROH, J.H.; BAE, S.D.; YOON, Y.N.; KIM, H.J.; NAM, M.H. The effect of seed-borne mycoflora from sorghum and foxtail millet seeds on germination and disease transmission. Mycobiology, Seul, v.39, n.3, p.206-218, 2011).
The second genus with the highest incidence in seeds was Fusarium, which is a widely distributed fungus. Most of the species act on the decomposition of substrates derived from cellulose, or has saprophytic habits. In some cases, plants are pathogenic and produce toxins (LESLIE; SUMMERELL, 2006LESLIE, J.F.; SUMMERELL, B.A. The Fusarium laboratory manual. Ames: Blackwell Publishing Professional, 2006. 388p.). S. rhombifolia presented the highest incidence of this fungus (82.22%), followed by T. procumbens (70.00%). The other weed species had incidence lower than 26.67%.
In seeds of T. procumbens (0.779%), C. echinatus (16.44%), and S. latifolia (0.33%), fungi were found with structures similar to Pythium, such as a white and cotton hyaline mycelium, the presence of hyphae and the formation of sporangia containing zoospores (AGRIOS, 2005AGRIOS, G.N. Plant pathology. 5ed. San Diego: Academic Press, 2005. 952p.). This microorganism can cause damping-off, seed rot, seedling rot and root rot (AMORIM et al., 2011AMORIM, L.; REZENDE, J.A.M.; BERGAMIN FILHO, A. Manual de fitopatologia. Volume 1: Princípios e Conceitos. 4 ed. São Paulo: Agronômica Ceres LTDA, 2011. 920p.).
Table 2 shows fungal genera incident in weed seeds related to saprophytic conditions and grain and seed contaminants, such as Aspergillus, Cladosporium, Nigrospora, Papularia and Rhizopus.
The most recurrent genus in the analyzed seeds was Cladosporium, being verified in all samples examined. This genus is normally considered a contaminant of grains and seeds poorly stored. However, there are species pathogenic to passion fruit (NEGREIROS et al., 2004NEGREIROS, J.R.S.; BRUCKNER, C.H.; CRUZ, C.D.; SIQUEIRA, D.L.; PIMENTEL, L.D. Seleção de progênies de maracujazeiro amarelo vigorosas e resistentes à verrugose (Cladosporium cladosporioides). Revista Brasileira Fruticultura, Jaboticabal, v.26, n.2, p.272-275, 2004.) and peach (MARTINS et al., 2006MARTINS, M.C.; LOURENÇO, S.A.; GUTIERREZ, A.S.D.; JACOMINO, A.P.; AMORIM, L. Quantificação de danos pós-colheita em pêssegos no mercado atacadista de São Paulo. Fitopatologia Brasileira, Brasília, v.31, n.1, p.5-10, 2006.). Among the other fungal genera found in Table 2, the main cause of occurrence is the poor quality or conservation of seeds and stored grains. Fungi are commonly found by sanitary seed analyses.
Considering the sanitary analysis of seeds, the weed species P. setosum, S. rhombifolia and T. procumbens were the ones that presented the highest level of contamination of seeds by potentially phytopathogenic fungi.
Pathogenicity
The pathogenicity test may confirm or exclude the hypothesis that fungi associated with weed seeds are potential carriers of microorganisms pathogenic to crops of agricultural importance. According to the obtained results, the isolates of Curvularia sp., Fusarium sp. and Pythium sp. were able to cause disease-specific symptoms of each pathogen in rice, beans, cowpea, corn and soybean (Table 3).
The isolate of Curvularia sp. provoked oval necrotic spots with reddish edges on the clean leaf of corn plants, causing the loss of photosynthetic area. Several species of this genus may cause leaf spots, mainly in grasses (LIMA; FURTADO, 2007LIMA, A.; FURTADO, M. Curvulariaspecies (anamorphicfungi: Hyphomycetes) from Santiago Island, Cape Vert. Portugaliae Acta Biologica, Lisboa, v.22, p.145-156, 2007.; YAGO et al., 2011YAGO, J.I.; ROH, J.H.; BAE, S.D.; YOON, Y.N.; KIM, H.J.; NAM, M.H. The effect of seed-borne mycoflora from sorghum and foxtail millet seeds on germination and disease transmission. Mycobiology, Seul, v.39, n.3, p.206-218, 2011). It may also be transmitted by seed-plant (SILVA et al., 2014SILVA, M.S.B.S.; RODRIGUES, A.A. C. ; OLIVEIRA, L.J.M.G.; SILVA, E.K.C.; PEREIRA, T.S. Sanidade de sementes de arroz, biocontrole, caracterização e transmissão de Curvularia lunata em semente-plântula de arroz. Revista Ceres, Viçosa, v.61, n.4, p.511-517, 2014.).
The four isolates of Fusarium sp. obtained from weed seeds presented pathogenicity to all agricultural crops tested in the experiment. As pathogens of this group, there are species of the complex Fusarium fujikuroi, in which the species Fusarium verticillioides, Fusarium proliferatum and Fusarium subglutinans (LESLIE; SUMMERELL, 2006LESLIE, J.F.; SUMMERELL, B.A. The Fusarium laboratory manual. Ames: Blackwell Publishing Professional, 2006. 388p.) stand out. They are responsible for root rot, radicle rot and grain rot, and seedling tipping (SOUZA et al., 2007SOUZA, A.E.; ARAÚJO, E.; NASCIMENTO, L.C. Atividade antifúngica de extratos de alho e capim-santo sobre o desenvolvimento de Fusarium proliferatum isolado de grãos de milho. Fitopatologia Brasileira, Brasília, v.32, n.6, p.465-471, 2007.).
We observed that two isolates of Fusarium spp. caused lesions on the culm of corn plants after 15 days of inoculation. These lesions evolved to rot, causing the death of all plants. This result demonstrates the pathogenicity of this fungus, which, if introduced in an area, may seriously compromise the stand and plant growth in commercial crops. SCOTT; EVANS (1984SCOTT, S.W.; EVANS, D.R. Sclerotia of Sclerotinia trifoliorum in red clover seed. Transaction of the British Mycological Society, v.82, p.567-569, 1984.) described that the initial infection of seeds by pathogens occurs by spores, survival structures, or residues of infected plants close to the seeds. In general, species of Fusarium can infect a plant through different pathways, and the penetration of the pathogen commonly occurs close to root apexes, as well as natural wounds and root openings (BIANCHINI et al., 1997BIANCHINI, A.; MARINGONI, A.C.; CARNEIRO, S.M.T.P.G. Doenças do feijoeiro. In: KIMATI, H.; AMORIM, L. ; BERGAMIN FILHO, A .; CAMARGO, L.E.A.; REZENDE, J.A.M. (Eds.) Manual de fitopatologia: doenças das plantas cultivadas. 3 ed. São Paulo: Agronômica Ceres, 1997. v.2, p.376-399.). The dissemination of the inoculum in the cultivation area is favored by irrigation water or rains, winds, agricultural implements, etc. POLETTO et al. (2009POLETTO, I.; MUNIZ, M.F.B.; CECONI, D.E.; ARAUJO, M.M.; RODRIGUES, J.; MEZZOMO, R. Inoculação de Fusarium exysporume Fusarium solani e níveis de sombreamento na erva-mate: influência na severidade da podridão-de-raízes. Ciência Florestal, Santa Maria, v.19, n.3, p.267-278, 2009.) reported that fungi of this genus are also classified as soil-dwelling pathogens. In the absence of the host plant, they have a saprophytic habit, forming resistance structures (chlamydospores) that can make them survive for a long period. Thus, control measures are even more difficult to be adopted, making them ineffective and costly. Thus, preventive diagnosis, before sowing, as well as a chemical treatment of seeds, is a measure that helps against diseases caused by species of Fusarium sp. (COSTA et al., 2003COSTA, M.L.N.; MACHADO, J.C.; GUIMARÃES, R.M.; POZZA, E.A.; ORIDE, D. Inoculação de Fusarium oxysporum f. sp. phaseoli em sementes de feijoeiro através de restrição hídrica. Ciência e Agrotecnologia, Lavras, v.27, n.5, p.1023-1030, 2003.).
Two other isolates of Fusarium spp. caused lesions to beans, cowpea and soybeans, with symptoms of wilting and stem rot and petioles. The complex Fusarium solani is an important cause of lesions in legumes, provoking rot in roots, leaves and stems, especially the species Fusarium solani f. sp. glycines, which causes red root rot in soybean, and Fusarium solani f. sp. phaseoli, which causes root rot in beans (BALARDIN et al., 2005BALARDIN, C.R.; CELMER, A.F.; COSTA, E.C.; MENEGHETTI, R.C.; BALARDIN, R.S. Possible transmission of Fusarium solani f. sp. glycines, causal agent of Sudden Death Syndrome, through soybean seed. Fitopatologia Brasileira, Brasília, v.30, n.6, p.574-581, 2005.; TOLÊDO-SOUZA et al., 2009TOLÊDO-SOUZA, E.D.; LOBO JÚNIOR, M.; SILVEIRA, P.M.; CAFÉ FILHO, A.C. Interações entre Fusarium solani f. sp. phaseoli e Rhizoctonia solani na severidade da podridão radicular do feijoeiro. Pesquisa Agropecuária Tropical, Goiânia, v.39, n.1, p.13-17, 2009.).
The isolates of Pythium sp. presented pathogenicity to plants of different families, provoking symptoms of rot in the stem and culm, leading to the death of inoculated plants. The first isolate was pathogenic to rice and corn, while the second isolate presented pathogenicity to bean and cowpea plants.
The fungal isolates pathogenic to the cultivated plants were also inoculated in the plant of origin of the seed. Only the four isolates of Fusarium spp. caused wilting symptoms when inoculated in T. procumbens. It is the same isolate that caused wilt and rot in soybean plants.
The fact that the four isolates of Fusarium spp. was pathogenic to the weed from which it was isolated and also to the soybean crop demonstrates that, besides the weed being an alternative host of this fungus, it can also transmit this microorganism through the seeds to growing areas free from the pathogen, causing damage to the crop. Once established at the site, the pathogen spreads to short and long distances between crops by the movement of infested soil, cultural remains, irrigation water, contaminated equipment and seeds (ARAÚJO, 2008ARAÚJO, D.V. Caracterização molecular, patogenicidade e transmissão pela semente de Fusarium oxysporumf. sp. vasinfectum em algodoeiro. 2008. 102p. Thesis (Doutorado - Agronomia: Fitopatologia) - Universidade Federal de Lavras, Lavras, 2008. Available from: <Available from: http://prpg.ufla.br/_ppg/fitopatologia/
>. Access on: Aug. 10 2018.
http://prpg.ufla.br/_ppg/fitopatologia/...
). As for the other isolates, they did not present pathogenicity to weeds, which shows that the fungi can remain endophytic to them, that is, they can remain inside the tissues of the plants without causing damage or forming visible external structures (AZEVEDO et al., 2000AZEVEDO, J.L.; MACCHERONI JÚNIOR, W.; PEREIRA, J.O.; ARAÚJO, W.L. Endophytic microorganisms: a review on insect control and recent advances on tropical plants. Electronic Journal of Biotechnology, Valparaíso, v.3, n.1, p.15-16, 2000.), and may colonize leaves, branches, roots (PEIXOTO NETO et al., 2002PEIXOTO NETO, P.A.S.; AZEVEDO, J.L. ; ARAÚJO, W.L . Microrganismos endofíticos: interação com plantas e potencial biotecnológico. Revista Biotecnologia Ciência e Desenvolvimento, Brasília, v.29, p.62-76, 2002.) or seeds.
It is worth noting that fungi, even without developing symptoms of diseases in weeds, may remain as sources of inoculum for cultivated plants. RODRIGUES; MENEZES (2002RODRIGUES, A.A.; MENEZES, M. Detecção de fungos endofíticos em sementes de caupi provenientes de Serra Talhada e de Caruaru, Estado de Pernambuco. Fitopatologia Brasileira, Brasília, v.27, n.5, p.532-537, 2002.), working with endophytic fungi found in cowpea seeds, discovered species of F. solani, F. oxysporum and F. moniliforme, species already known as plant pathogens (LESLIE; SUMMERELL, 2006LESLIE, J.F.; SUMMERELL, B.A. The Fusarium laboratory manual. Ames: Blackwell Publishing Professional, 2006. 388p.).
The importance of interference of weed with cultivated plants, as a result of competition for light, nutrients and water, as well as being pest and disease hosts, is also highlighted. According to FONTES et al. (2003FONTES, J.R.A.; SHIRATSUCHI, L.S.; NEVES, J.L.; JÚLIO, L.; SODRÉ FILHO, J. Manejo integrado de plantas daninhas. Planaltina, DF: Embrapa Cerrados, 2003. 48p. Embrapa Cerrados. Documentos, 103.), the intensity of this interference depends on the characteristics of weeds and the crop, such as growth speed, size, plant architecture, growth stage, duration of coexistence period and the environment, making management fundamental to guarantee the productivity of crops.
CONCLUSIONS
Weeds are a dissemination vehicle and an alternative host of phytopathogenic fungi of the genera Curvularia sp., Fusarium sp. and Pythium sp., which cause diseases in cultivated plant species such as Oryza sativa, Phaseolus vulgaris, Vigna unguiculata, Zea mays and Glycine max. The occurrence and incidence of the identified fungal genera vary according to the weed species, and their control is an important measure to allow a better development of crops
REFERENCES
- AGRIOS, G.N. Plant pathology 5ed. San Diego: Academic Press, 2005. 952p.
- ALFENAS, A.C.; MAFIA, R.G. Métodos em fitopatologia Viçosa: UFV, 2007. 382p.
- AMORIM, L.; REZENDE, J.A.M.; BERGAMIN FILHO, A. Manual de fitopatologia Volume 1: Princípios e Conceitos. 4 ed. São Paulo: Agronômica Ceres LTDA, 2011. 920p.
- ARAÚJO, D.V. Caracterização molecular, patogenicidade e transmissão pela semente de Fusarium oxysporumf. sp. vasinfectum em algodoeiro. 2008. 102p. Thesis (Doutorado - Agronomia: Fitopatologia) - Universidade Federal de Lavras, Lavras, 2008. Available from: <Available from: http://prpg.ufla.br/_ppg/fitopatologia/ >. Access on: Aug. 10 2018.
» http://prpg.ufla.br/_ppg/fitopatologia/ - AZEVEDO, J.L.; MACCHERONI JÚNIOR, W.; PEREIRA, J.O.; ARAÚJO, W.L. Endophytic microorganisms: a review on insect control and recent advances on tropical plants. Electronic Journal of Biotechnology, Valparaíso, v.3, n.1, p.15-16, 2000.
- BALARDIN, C.R.; CELMER, A.F.; COSTA, E.C.; MENEGHETTI, R.C.; BALARDIN, R.S. Possible transmission of Fusarium solani f. sp. glycines, causal agent of Sudden Death Syndrome, through soybean seed. Fitopatologia Brasileira, Brasília, v.30, n.6, p.574-581, 2005.
- BARROCAS, E.; MACHADO, J.D.C. Introdução a patologia de sementes e testes convencionais de sanidade de sementes para a detecção de fungos fitopatogênicos. Informativo ABRATES: Inovações tecnológicas em Patologia de Sementes, Londrina, v.20, n.3, p.74-75, 2010.
- BIANCHINI, A.; MARINGONI, A.C.; CARNEIRO, S.M.T.P.G. Doenças do feijoeiro. In: KIMATI, H.; AMORIM, L. ; BERGAMIN FILHO, A .; CAMARGO, L.E.A.; REZENDE, J.A.M. (Eds.) Manual de fitopatologia: doenças das plantas cultivadas. 3 ed. São Paulo: Agronômica Ceres, 1997. v.2, p.376-399.
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Manual de Análise Sanitária de Sementes Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: Mapa/ACS, 2009. 200p.
- CARMONA, R. Problematic and management of weed seed banks in agricultural soils. Planta Daninha, Viçosa, v.10, n.1/2, p.5-16, 1992.
- CAVERS, P.B.; BENOIT, D.L. Seed banks in amble land. In: CAVERS, P.B. Ecology of soil seed banks New York: Academic Press, 1989. p.309-328.
- COMPANHIA NACIONAL DE ABASTECIMENTO - CONAB. Acompanhamento da safra brasileira de grãos safra 2016/17, v.4, p.1-152, 2016. Available from: <Available from: http://www.conab.gov.br/OlalaCMS/uploads/arquivos/16_12_08_12_27_47_boletim_graos_dezembro_2016.pdf >. Access on: Sep. 22 2017.
» http://www.conab.gov.br/OlalaCMS/uploads/arquivos/16_12_08_12_27_47_boletim_graos_dezembro_2016.pdf - COSTA, M.L.N.; MACHADO, J.C.; GUIMARÃES, R.M.; POZZA, E.A.; ORIDE, D. Inoculação de Fusarium oxysporum f. sp. phaseoli em sementes de feijoeiro através de restrição hídrica. Ciência e Agrotecnologia, Lavras, v.27, n.5, p.1023-1030, 2003.
- FONTES, J.R.A.; SHIRATSUCHI, L.S.; NEVES, J.L.; JÚLIO, L.; SODRÉ FILHO, J. Manejo integrado de plantas daninhas Planaltina, DF: Embrapa Cerrados, 2003. 48p. Embrapa Cerrados. Documentos, 103.
- LESLIE, J.F.; SUMMERELL, B.A. The Fusarium laboratory manual Ames: Blackwell Publishing Professional, 2006. 388p.
- LIMA, A.; FURTADO, M. Curvulariaspecies (anamorphicfungi: Hyphomycetes) from Santiago Island, Cape Vert. Portugaliae Acta Biologica, Lisboa, v.22, p.145-156, 2007.
- MACHADO, J.C. Patologia de sementes - fundamentos e aplicações Brasília: MEC/ESAL/FAEP, 1988. 107p.
- MANAMGODA, D.S.; ROSSMAN, A.Y.; CASTLEBURY, L.A.; CROUS, P.W.; MADRID, H.; CHUKEATIROTE, E.; HYDE, K.D. The genus Bipolaris. Studies in Mycology, Utrecht, v.79, p.221-288, 2014.
- MARCHI, C.E.; FERNANDES, C.D.; BUENO, M.L.; BATISTA, M.V.; FABRIS, L.R. Fungic microflora of Panicum maximum and Styosanthes spp. commercial seed. Semina: Ciências Agrárias, Londrina, v.31, n.3, p.575-584, 2010.
- MARTINS, M.C.; LOURENÇO, S.A.; GUTIERREZ, A.S.D.; JACOMINO, A.P.; AMORIM, L. Quantificação de danos pós-colheita em pêssegos no mercado atacadista de São Paulo. Fitopatologia Brasileira, Brasília, v.31, n.1, p.5-10, 2006.
- MILÉO, L.J.; SILVA, J.F.; BENTES, J.L.S.; CHRISTOFFOLETI, P.J. Plantas daninhas hospedeiras alternativas de Colletotrichum guaranicola em cultivos de guaraná no Estado do Amazonas. Planta Daninha, Viçosa, v.25, n.4, p.771-782, 2007.
- NEGREIROS, J.R.S.; BRUCKNER, C.H.; CRUZ, C.D.; SIQUEIRA, D.L.; PIMENTEL, L.D. Seleção de progênies de maracujazeiro amarelo vigorosas e resistentes à verrugose (Cladosporium cladosporioides). Revista Brasileira Fruticultura, Jaboticabal, v.26, n.2, p.272-275, 2004.
- PEIXOTO NETO, P.A.S.; AZEVEDO, J.L. ; ARAÚJO, W.L . Microrganismos endofíticos: interação com plantas e potencial biotecnológico. Revista Biotecnologia Ciência e Desenvolvimento, Brasília, v.29, p.62-76, 2002.
- POLETTO, I.; MUNIZ, M.F.B.; CECONI, D.E.; ARAUJO, M.M.; RODRIGUES, J.; MEZZOMO, R. Inoculação de Fusarium exysporume Fusarium solani e níveis de sombreamento na erva-mate: influência na severidade da podridão-de-raízes. Ciência Florestal, Santa Maria, v.19, n.3, p.267-278, 2009.
- RODRIGUES, A.A.; MENEZES, M. Detecção de fungos endofíticos em sementes de caupi provenientes de Serra Talhada e de Caruaru, Estado de Pernambuco. Fitopatologia Brasileira, Brasília, v.27, n.5, p.532-537, 2002.
- SALES JÚNIOR, R.; OLIVEIRA, O.F.; MEDEIROS, E.V.; GUIMARÃES, I.M.; CORREIA, K.C.; MICHEREFF, S.J. Ervas daninhas como hospedeiras alternativas de patógenos causadores do colapso do meloeiro. Revista Ciência Agronômica, Fortaleza, v.43, n.1, p.195-198, 2012.
- SCOTT, S.W.; EVANS, D.R. Sclerotia of Sclerotinia trifoliorum in red clover seed. Transaction of the British Mycological Society, v.82, p.567-569, 1984.
- SHAN, F.Y.; SHI, W.; LIU, Y.; ZHANG, J.; DONG, J. Parasitic fitness and RAPD analysis of Curvularia species on corn. Journal of Agricultural University of Hebei, Baoding, v.31, p.37-41, 2008.
- SILVA, M.S.B.S.; RODRIGUES, A.A. C. ; OLIVEIRA, L.J.M.G.; SILVA, E.K.C.; PEREIRA, T.S. Sanidade de sementes de arroz, biocontrole, caracterização e transmissão de Curvularia lunata em semente-plântula de arroz. Revista Ceres, Viçosa, v.61, n.4, p.511-517, 2014.
- SOUZA, A.E.; ARAÚJO, E.; NASCIMENTO, L.C. Atividade antifúngica de extratos de alho e capim-santo sobre o desenvolvimento de Fusarium proliferatum isolado de grãos de milho. Fitopatologia Brasileira, Brasília, v.32, n.6, p.465-471, 2007.
- TÖFOLI, J.G.; DOMINGUES, R.J. Alternarioses em hortaliças: sintomas, etiologia e manejo integrado. Biológico, São Paulo, v.66, n.1/2, p.23-33, 2004.
- TOLÊDO-SOUZA, E.D.; LOBO JÚNIOR, M.; SILVEIRA, P.M.; CAFÉ FILHO, A.C. Interações entre Fusarium solani f. sp. phaseoli e Rhizoctonia solani na severidade da podridão radicular do feijoeiro. Pesquisa Agropecuária Tropical, Goiânia, v.39, n.1, p.13-17, 2009.
- VASCONCELOS, M.C.C.; SILVA, A.F.A.; LIMA, R.S. Interferência de plantas daninhas sobre plantas cultivadas. Agropecuária Científica no Semi-Árido, Campina Grande, v.8, n.1, p.1-6, 2012.
- WATANABE, T. Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species 3 ed. New York: CRC Press, 2010. 426p.
- YAGO, J.I.; ROH, J.H.; BAE, S.D.; YOON, Y.N.; KIM, H.J.; NAM, M.H. The effect of seed-borne mycoflora from sorghum and foxtail millet seeds on germination and disease transmission. Mycobiology, Seul, v.39, n.3, p.206-218, 2011
Publication Dates
-
Publication in this collection
14 Nov 2018 -
Date of issue
2018
History
-
Received
13 Nov 2017 -
Accepted
10 Sept 2018