Abstract
Nitroaromatic compounds have significant economic and industrial relevance in various inputs such as dyes, inks, agrochemicals, and explosives. In the manufacture of the explosive 2,4,6 trinitrotoluene (TNT), there is the formation of contaminated wastewater that is difficult to treat because it contains, among other compounds, high concentrations of 2,4 and 2,6-trinitrotoluene (DNT). In this work, the application of advanced oxidation processes of the O3/UV type was studied in sequential integration with catalytic material based on metallic iron slag (SZVI) and mineral matrix to treat an industrial effluent from the explosives industry contaminated with compounds nitroaromatics. Two types of mineral matrix composition were studied, kaolinite (Kau) and pumice powder (Pum), having been observed in the conditions of best efficiency (O3/UV-SZVI/Kau) 100% removal of both nitroaromatic compounds and residual ozone. On the other hand, photo-ozonation alone could only partially remove these components, with 38% DNT removal and 3.2 mg/L residual O3 observed, proving the importance of integration with the catalytic matrix for more effective treatment of the studied effluent.
Keywords:
DNT degradation; explosives industry effluent; Scrap zero-valent iron
Resumo
Os compostos nitroaromáticos possuem importante relevância econômica e industrial fazendo parte de diversos insumos como corantes, tintas, agroquímicos e explosivos. Na fabricação do explosivo 2,4,6 trinitrotoluneo (TNT), há a formação de águas residuárias contaminadas de difícil tratamento por conter, dentre outros compostos, elevadas concentrações de 2,4 e 2,6-trinitrotolueno (DNT). Neste trabalho foi estudada a aplicação de Processos oxidativos avançados do tipo O3/UV em integração sequencial com material catalítico a base de escória de ferro metálico (SZVI) e matriz mineral, para o tratamento de um efluente industrial oriundo da indústria de explosivos contaminado com compostos nitroaromáticos. Foram estudados dois tipos de composição de matriz mineral, caulinita (Kau) e pedra pomes em pó (Pum), tendo sido observado, nas condições de melhor eficiência (O3/UV-SZVI/Kau), 100% de remoção tanto dos compostos dinitroaomáticos quanto de ozônio residual. Por outro lado, a foto-ozonização isoladamente, foi capaz de remover apenas parcialmente estes componentes, tendo sido observados 38% de remoção de DNT e 3,2 mg/L de O3 residual, comprovando a importância da integração com a matriz catalítica para um tratamento mais efetivo do efluente em estudo.
Palavras-chave:
degradação de DNT; efluentes da indústria de explosivos; escória de ferro
1. INTRODUCTION
The contamination of water by emerging pollutants has been identified as one of today’s most relevant challenges, mainly due to its possible effects on the endocrine, hormonal and genetic systems. These contaminants can also be converted into chiral metabolites (chiral pollution), which can cause enantioselective carcinogenesis and diverse impacts on soil and water ecosystems (Basheer, 2018bBASHEER, Al A. New generation nano-adsorbents for the removal of emerging contaminants in water. Journal of Molecular Liquids, v. 261, p. 583-593, 2018b. https://doi.org/10.1016/j.molliq.2018.04.021
https://doi.org/10.1016/j.molliq.2018.04...
; Basheer and Ali, 2018BASHEER, Al A.; ALI, I. Stereoselective uptake and degradation of (±)‐o, p‐DDD pesticide stereomers in water‐sediment system. Chirality, v. 30, n. 9, p. 1088-1095, 2018. https://doi.org/10.1002/chir.22989
https://doi.org/10.1002/chir.22989...
; Khan et al., 2020KHAN, N. A. et al. Occurrence, sources and conventional treatment techniques for various antibiotics present in hospital wastewaters: a critical review. TrAC Trends in Analytical Chemistry, v. 129, p. 115921, 2020. https://doi.org/10.1016/j.trac.2020.115921
https://doi.org/10.1016/j.trac.2020.1159...
). This class includes pesticides, phenols, polycyclic aromatic hydrocarbons and nitroaromatic compounds, such as dinitrotoluene (DNT), and conventional processes for the treatment of contaminated effluents (typically physicochemical and biological processes) have limited efficiency, mainly due to the persistent toxic effects that these compounds impart to the effluent (Bilal et al., 2021BILAL, M.; BAGHERI, A. M.; BHATT, P.; CHEN, S. Environmental occurrence, toxicity concerns, and remediation of recalcitrant nitroaromatic compounds. Journal of Environmental Management, v. 291, p. 112, 2021. https://doi.org/10.1016/j.jenvman.2021.112685 ; Barreto-Rodrigues et al., 2009BARRETO-RODRIGUES, M.; SILVA, F. T.; PAIVA, T. C. B. Characterization of wastewater from the Brazilian TNT industry, Journal of Hazardous Materials, v. 164, n. 1, p. 385-388, 2009. https://doi.org/10.1016/j.jhazmat.2008.07.152
https://doi.org/10.1016/j.jhazmat.2008.0...
). As an alternative, several processes have been studied to remove emerging pollutants, including UV photodegradation, reverse osmosis, removal by nano-adsorbents, and photocatalysis (Basheer, 2018aBASHEER, Al A. Chemical chiral pollution: impact on the society and science and need of the regulations in the 21st century. Chirality, v. 30, n. 4, p. 402-406, 2018a. https://doi.org/10.1002/chir.22808
https://doi.org/10.1002/chir.22808...
; Ali et al., 2018ALI, I. et al. Kinetics, thermodynamics, and modeling of amido black dye photodegradation in water using Co/TiO2 nanoparticles. Photochemistry and Photobiology, v. 94, n. 5, p. 935-941, 2018. https://doi.org/10.1111/php.12937
https://doi.org/10.1111/php.12937...
; Khan et al., 2020KHAN, N. A. et al. Occurrence, sources and conventional treatment techniques for various antibiotics present in hospital wastewaters: a critical review. TrAC Trends in Analytical Chemistry, v. 129, p. 115921, 2020. https://doi.org/10.1016/j.trac.2020.115921
https://doi.org/10.1016/j.trac.2020.1159...
).
In this context, advanced oxidative processes mediated by ozone and ultraviolet radiation have been presented as a promising alternative for the treatment of various contaminants, including nitroaromatic compounds, which can be used as a pre-treatment of biological processes due to their ability to increase biodegradability and dissolved oxygen concentration (Equation 1). The effect caused by the joint action of O3 and UV makes possible the simultaneous occurrence of degradation mechanisms of direct photolysis, direct ozonation, and oxidation by hydroxyl radicals. The process starts with the photolysis of ozone-producing hydrogen peroxide, followed by the ion reaction hydroperoxide (HO2 -) with O3 to produce O3 - and hydroxyl radicals, the latter of which can lead to effective oxidation and eventual mineralization of typically refractory organic compounds (Chen et al.,2007CHEN, W.; JUAN, C.; WEI, K. Decomposition of dinitrotoluene isomers and 2,4,6-trinitrotoluene in spent acid from toluene nitration process by ozonation and photo-ozonation. Journal of Hazardous Materials, v. 147, n. 1-2, p. 97-104, 2007. https://doi.org/10.1016/j.jhazmat.2006.12.052
https://doi.org/10.1016/j.jhazmat.2006.1...
). However, when applied to residues of greater complexity, such as industrial effluents with a high organic load and diversity of refractory compounds, such systems may have some limitations, mainly associated with the need to complement the treatments carried out to ensure the elimination of any intermediates (e.g., phenolic compounds) and remaining oxidants from the process, such as O3 and H2O2 (Bui and Minh, 2021BUI, D. N.; MINH, T. T. Investigation of TNT red wastewater treatment technology using the combination of advanced oxidation processes. Science of The Total Environment, v. 756, n. 143852, 2021. https://doi.org/10.1016/j.scitotenv.2020.143852.
https://doi.org/10.1016/j.scitotenv.2020...
).
To overcome these limitations, alternatives such as the sequential association with ferrous catalysts can eliminate the remaining oxidants by converting them into additional doses of HO• through Fenton-type reactions or catalytic ozonation emerge (Bhanot et al., 2020BHANOT, P.; CELIN, S. M.; SREEKRISHNAN, T.R.; KALSI, A.; SAHAI, S.K.; SHARMA, P. Application of integrated treatment strategies for explosive industry wastewater-A critical review. Journal of Water Process Engineering, v. 35, n. 101232, 2020. https://doi.org/10.1016/j.jwpe.2020.101232.
https://doi.org/10.1016/j.jwpe.2020.1012...
). It is essential to point out that various forms of chemical synthesis can obtain ferrous catalytic materials, but also by using metallurgical/steel industrial waste, which can be used in combination with inert matrices such as calcium alginate, chitosan, bentonite, cellulose, zeolite, stone-pumice, carboxymethylcellulose, kaolinite, among others, to control the hydraulic conductivity and reduce the self-aggregation of particles which reduces the reactivity (Limper et al., 2018LIMPER, D.; FELLINGER, G. P.; EKOLU, S. O. Evaluation and microanalytical study of ZVI/scoria zeolite mixtures for treating acid mine drainage using reactive barriers - Removal mechanisms. Journal of Environmental Chemical Engineering, v. 6, n. 5, p. 6184-6193, 2018. https://doi.org/10.1016/j.jece.2018.08.064
https://doi.org/10.1016/j.jece.2018.08.0...
; Abukhadra et al., 2021ABUKHADRA, M. R.; ALHAMMADI, A.; EL-SHERBEENY, A. M.; SALAM, M. A.; EL-MELIGY, M. A.; AWWAD, E. M.; LUQMAN, M. Enhancing the removal of organic and inorganic selenium ions using an exfoliated kaolinite/cellulose fibers nanocomposite. Carbohydrate Polymers, v. 252, n. 117163, 2021. https://doi.org/10.1016/j.carbpol.2020.117163.
https://doi.org/10.1016/j.carbpol.2020.1...
; Sewwandi and Nitisoravut, 2020SEWWANDI, K. A. H. S.; NITISORAVUT, R. Nano zero-valent iron embedded on chitosan for enhancement of biohydrogen production in dark fermentation. Energy Reports, v. 6, p. 392-396, 2020. https://doi.org/10.1016/j.egyr.2020.11.225
https://doi.org/10.1016/j.egyr.2020.11.2...
; Pereira et al., 2021PEREIRA, C. A. A.; NAVA, M. R.; WALTER, J. B.; SCHERER, C. E.; DALFOVO, A. D. K.; BARRETO-RODRIGUES, M. Application of zero valent iron (ZVI) immobilized in Ca-Alginate beads for C.I. Reactive Red 195 catalytic degradation in an air lift reactor operated with ozone. Journal of Hazardous Materials, v. 401, 2021. https://doi.org/10.1016/j.jhazmat.2020.123275
https://doi.org/10.1016/j.jhazmat.2020.1...
; Ali et al., 2018ALI, I. et al. Kinetics, thermodynamics, and modeling of amido black dye photodegradation in water using Co/TiO2 nanoparticles. Photochemistry and Photobiology, v. 94, n. 5, p. 935-941, 2018. https://doi.org/10.1111/php.12937
https://doi.org/10.1111/php.12937...
). Among the matrices reported in the literature, kaolinite is a widely applied clay mineral used in several processes, mainly in the paper industry, with a load function and surface material. In this same sense, pumice has also stood out, as it is a porous material with a large surface area; it can be an efficient and low-cost adsorbent (Calabrò et al., 2012CALABRÒ, P. S.; MORACI, N.; SURACI, P. Estimate of the optimum weight ratio in zero-valent Iron/Pumice granular mixtures used in permeable reactive barriers for the remediation of nickel contaminated groundwater. Journal of Hazardous Materials, v. 207-208, p. 111-116, 2012. https://doi.org/10.1016/j.jhazmat.2011.06.094.
https://doi.org/10.1016/j.jhazmat.2011.0...
). It is interesting to note that the application of materials of this nature, whether in the form of filters or reactive barriers, tends to improve the efficiency and cost of the process (Ali et al., 2018ALI, I. et al. Kinetics, thermodynamics, and modeling of amido black dye photodegradation in water using Co/TiO2 nanoparticles. Photochemistry and Photobiology, v. 94, n. 5, p. 935-941, 2018. https://doi.org/10.1111/php.12937
https://doi.org/10.1111/php.12937...
).
In this context, this work describes a study of the potential of catalytic ozonation mediated by mixtures of ZVSI and clay mineral materials (kaolinite and pumice) in an effluent composed of 2,4 and 2,6-Dinitrotoluene. The main innovative element of the research is the operation of two advanced oxidative processes (O3/UV + Fe/O3) in a single reaction system.
2. MATERIAL AND METHODS
2.1. Chemicals and supplies
All chemicals used were of analytical grade and were obtained from Merck, Reagen, or Sigma. The effluent samples were collected from an explosives-manufacturing industry in the State of São Paulo, Brazil. The wastewater resulted from the purification stage of the trinitrotoluene manufacturing process. After being collected at room temperature, it was stored under refrigeration (4⁰C), and the concentration of nitroaromatic compounds present were estimated in the form of 2,4 and 2,6-dinitrotoluene 316 ± 10 mg.L-1. Kaolinite and pumice were dried in an oven at 110°C for approximately three h, then ground and processed through a 0.135 mm sieve. The scrap zero-valent iron (SZVI) used was donated by a company in the Grinding area, located in Pato Branco and Francisco Beltrão, Paraná. After collection, the material was sampled using the splitting method, processed in a 0.150mm sieve. The splitting of the samples was performed according to the technical standard (ABNT, 2001ABNT. NBR NM 27, A. Agregados - Redução da amostra de campo para ensaios de laboratório Rio de Janeiro, 2001.).
2.2. Analytical control
The following parameters were used to determine the pollution potential of the effluent and ensure analytic control of the system:
UV-visible spectroscopy (UV-Vis): Spectroscopic analyses were performed on a spectrophotometer Thermal Scientific Evolution 60S Model-UV-visible, using quartz cuvettes with an optical path of 1 cm. Absorbance measurements at ʎmax = 275nm were performed to quantify the degradation rate of nitroaromatic compounds in the effluent.
Gas chromatography/mass spectrometer analysis: One hundred millimeters (100 mL) of effluent were dried under reduced pressure, and the residue was solubilized with methanol chromatographic grade. After treatment with anhydrous sodium sulfate for moisture extraction and filtering on 0.45µm cellulose membrane, it was transferred to a sample vial. The proportional sample amount (0.4µL) was injected into a gas chromatograph/mass spectrometer (Varian 431-GC/210MS) equipped with a capillary column (DB5, 30 m×0.25mm, film thickness 0.25µm), operated from 313 to 573K at a programming rate of 20 K.min−1. Compared to authentic standard compounds, the obtained mass spectra were used to identify 2,4 and 2,6-dinitrotoluene in the wastewater.
Red Water Contaminant Toxicity: The level of oral toxicity was determined based on the chemical structure of the substance through the classification method, which uses a decision tree model, developed by Cramer in 1978, using the ToXtree software for data analysis.
For the characterization of the catalytic material and mineral matrices, the following analytical techniques were used:
X-Ray Diffractometry: The diffractograms were obtained in a Rigaku diffractometer (model Mini Flex 600), with copper radiation (CuKα λ=1.5418 Å), power of 40 kV, and current of 15 mA. The acquisition will occur in Bragg angle interval from 3 to 90º, step scan mode, 0.02º step, and 2 seconds per step.
Energy Dispersive Spectroscopy (EDS): Elementary chemical analyses (mapping) were performed by EDS (“Energy Dispersive Spectroscopy”) using Oxford equipment, with the voltage of 15 kV and electron backscattered detector.
2.3. Effluent treatment
Experimental Design: To select and evaluate the effect of the nature of the mineral clay matrix as well as the SZVI concentration on the removal efficiency and permeability coefficient, a complete 22 factorial design with three genuine replicas was carried out preliminarily in the absence of ozone and ultraviolet radiation. This study evaluated the effects of X1: matrix type (pumice and kaolinite) and X2: % SZVI (10 and 50% w/w) on the removal of nitroaromatic compounds measured by the percentage reduction in absorbance at ʎmax = 275 nm. For statistical calculations, the values of the independent variables were coded into two levels (- and +), as shown in Table 1.
Red water effluent treatment: A modified Hellma model HP UV 50F W 500V photochemical reactor was used, with 1000 mL capacity, water cooling, homogenization promoted by a magnetic stirrer, air/O3 feed through the base with a flow of 1LMP (liter per minute) and passage inflow by catalytic mixture based on SZVI (preliminarily selected) supported on a sidearm, as illustrated in Figure 1. For characterization of the proposed treatment system (O3/UV-SZVI/Kau/Pum), tests were also conducted without the assistance of UV radiation (O3/SZVI/Kau/Pum) and the use of catalytic matrix (O3/UV).
The photochemical reactor used in the research; (1): advanced oxidation zone UV/O3 and (2): secondary oxidation zone and depletion of O3/H2O2.
3. RESULTS AND DISCUSSION
3.1. Characterization of catalytic materials, mineral matrices, and effluent
The X-ray diffractograms of the SZVI showed peaks at 27, 45, 55, 65, and 83° of 2ϴ, while those at 27 and 45° were narrow and intense, evidencing the crystalline character of the material. According to the Crystallographic Identification Chart 31829 of the Inorganic Crystal Structure Database (ICSD) database, the peaks at 27 and 55° are characteristic of carbon graphite, while the peaks located at 45, 65 and 83° refer to metallic iron (iron zero), based on ICSD identification letter 64999.
The results of the elemental quantitative chemical analysis of kaolinite, based on the intense peaks obtained from detected atoms, confirm the presence of oxygen (58.91%), silicon (14.14%), aluminum (13.49%), carbon (11.96%), and iron (1.50%). Regarding pumice, the presence of oxygen (58.60%), silicon (31.24%), carbon (8.15%), iron (1.61%), and aluminum (0.40%), was confirmed on the analyzed surface. Finally, in the SZVI samples, elements such as Iron (Fe), Carbon (C), Oxygen (O), Silicon (Si), Chromium (Cr), Manganese (Mn), and Calcium (Ca) were identified, with Carbon and Iron have the highest percentages, which were 48.5 and 43.6%, respectively.
The toxicity of 2,4,6-trinitrotoluene (TNT) and its respective degradation products was investigated using the Toxtree program. According to Cramer's rules, it was concluded that both TNT and its two degradation products identified in the studied effluent (2,4 and 2,6-DNT) have high toxicity (Class III) and biodegradability classified as persistent.
3.2. Multivariate study for the composition of the catalytic matrix
Table 1 shows the codes, real tests, and the results obtained in each experiment carried out with the same experimental errors. The data (responses) received based upon the statistical design was assessed by analysis of variance (ANOVA). Levene's test checked the homogeneity of variance, and the normal distribution of results was studied using the Shapiro-Wilk test with a 5% significance level. In general, degradation rates of nitroaromatic compounds ranged from 12.6 to 36.7%, obtained in Tests 4 and 7, which used kaolinite with 10% and 50% of SZVI, respectively. The permeability coefficients obtained indicated that the hydraulic flow through the catalytic material is favored with the increase in the concentration of SZVI, with the lowest and highest permeability being observed for the matrices of kaolinite with 10% of SZVI and of pumice with 50% of SZVI.
It is also interesting to note that the hydraulic permeability of the matrix with the best efficiency (Test 4) was five times lower than the matrix composed only of SZVI, suggesting that a longer hydraulic retention time increases the effectiveness of the interactions that lead to DNT degradation in the matrix.
For better interpretation, a statistical analysis carried out with Statgraphics Plus 5.1 software estimated the effects of the variables of interest on the removal rate of nitroaromatic compounds; the results are shown in Table 2, in which it's possible to observe estimated effect values, regression coefficients, interactions with significant and non-significant parameters, in addition to associated errors and level of significance attributed to each parameter. Factors in bold and marked with an asterisk were considered adequate for the 95% confidence interval (X2: SZVI and interactions X1.X2).
Equation 2 was generated considering only the significant coefficients listed in Table 2, and it explains mathematically how each variable affects the nitroaromatic compound’s removal during the treatment of red water effluent.
As it would be interesting to use Equation 1 as a model for predictive and interpretive purposes, the fit of the equation was evaluated with analysis of variance (ANOVA) (Barros Neto et al., 2002BARROS NETO, B.; SCARMÍNIO, S.; BRUNS, R. E. Como fazer experimentos: pesquisa e desenvolvimento na ciência e na indústria. 2. ed. Campinas: Editora UNICAMP, 2002. 401 p.). The results are shown in Table 3 and show that the model explains 97% of the variability in the DNT removal rate. Additionally, the Fcalc ratio value was higher than the Ftab value, suggesting that a regression involving the study variables can be considered significant and suitable to be used for predictive purposes (Box et al., 1978BOX, G. E. P.; HUNTER, W. G.; HUNTER, J. S. Statistics for experimenters. An introduction to design, data analysis and model building. Nova York: Wiley, 1978.). Thus, Equation 2 was used to construct response surfaces “Matrix versus SZVI” (X1.X2) illustrated in Figure 2. It is used to interpret the interaction of significant effects on the removal rate of dinitrotoluenes from the red water effluent.
The main effects and the response surface profile in Figure 2 show that an increase in the concentration of SZVI in the catalytic matrix promotes an increase in the DNT removal rate. This effect is more pronounced when the base of the catalytic matrix is based on kaolinite. The selected proportion (50:50) corroborates the studies by Moraci and Calabrò (2010)MORACI, N.; CALABRÒ, P. S. Heavy metals removal and hydraulic performance in zero-valent iron/pumice permeable reactive barriers. Journal of Environmental Management, v. 91, p. 2336-2341, 2010. https://doi.org/10.1016/j.jenvman.2010.06.019
https://doi.org/10.1016/j.jenvman.2010.0...
. They evaluated the potential of a mixture between ZVI and pumice for the removal of heavy metals in reactive permeable barriers.
3.3. Red Water treatment through the O3/UV-SZVI/Kao process
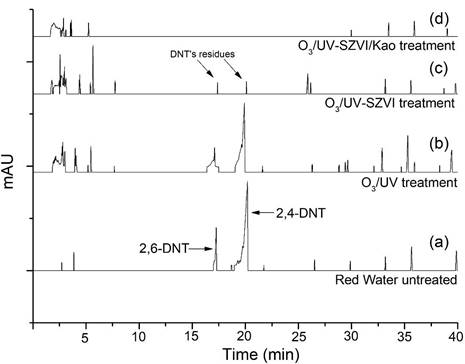
The composition of the catalytic matrix identified through factorial design (SZVI / kaolinite 50:50) was reproduced in a photochemical reactor (Figure 1), generating the results illustrated in Figure 3.
The chromatograms obtained for the samples submitted to the O3/UV-SZVI/Kau treatment (Figure 3d) indicated, about the untreated effluent (Figure 3a), total degradation of the nitroaromatic compounds in the red water effluent, and it was not possible to detect 2,4 and 2,6-DNTs after 120 minutes of treatment. On the other hand, for this same reaction time, treatments that were not integrated with the catalytic matrix (b) or that used a matrix composed only of SZVI (c) showed lower efficiency, being possible in these cases, the detection of both 2,4 and 2,6-DNT after both types of treatment. These results are also illustrated in Table 4.
Chromatograms of samples of untreated and subjected to different treatments: (a) Untreated red water; (b) Red water treated by O3/SZVI/Kao; (c) Red water treated by O3/UV e (c) Red water treated by O3/UV-SZVI/Kao. Experimental conditions: initial pH of 2.0; 10g/L of SZVI/Kao, and 120 minutes of treatment.
The results also reveal that, compared to other processes, the treatment via photo-ozonation (O3/UV) presented a higher residual ozone concentration, revealing the importance of the integrated use of the catalytic matrix. Additionally, a comparison between the results obtained by the O3/UV-SZVI and O3/UV-SZVI/Kau processes about the degradation of DNTs reveals the effect of the mineral matrix, suggesting that the addition of kaolinite in the matrix could be promoting a reduction in the hydraulic permeability and self-aggregation of SZVI particles, increasing the effectiveness of advanced oxidation reactions on the matrix surface.
4. CONCLUSIONS
The results obtained in this work indicated that although advanced oxidative processes such as O3/UV (photo-ozonation) and O3/UV-SZVI (catalytic photo-ozonation) have shown significant efficiency for the degradation of nitroaromatic compounds present in red water, the addition of kaolinite to the catalytic matrix promotes an effect capable not only of fully degrading the 2,4 and 2,6-dinitrotoluene compounds but also removing residual concentrations of dissolved ozone, suggesting that the photo-ozonation process operating in coupling with a unit composed of a catalytic matrix based on SZVI and kaolinite, has significant potential for the treatment of effluents contaminated with nitroaromatic compounds. However, it is recommended to carry out additional tests to optimize the process and evaluate the effect of the treatment on other parameters of environmental relevance characteristic of the effluent.
5. ACKNOWLEDGMENTS
To CAPES, Araucária Fundation, UTFPR and to the Multiuser Central Analysis Laboratory.
6. REFERENCES
- ABNT. NBR NM 27, A. Agregados - Redução da amostra de campo para ensaios de laboratório Rio de Janeiro, 2001.
- ABUKHADRA, M. R.; ALHAMMADI, A.; EL-SHERBEENY, A. M.; SALAM, M. A.; EL-MELIGY, M. A.; AWWAD, E. M.; LUQMAN, M. Enhancing the removal of organic and inorganic selenium ions using an exfoliated kaolinite/cellulose fibers nanocomposite. Carbohydrate Polymers, v. 252, n. 117163, 2021. https://doi.org/10.1016/j.carbpol.2020.117163
» https://doi.org/10.1016/j.carbpol.2020.117163 - ALI, I. et al Kinetics, thermodynamics, and modeling of amido black dye photodegradation in water using Co/TiO2 nanoparticles. Photochemistry and Photobiology, v. 94, n. 5, p. 935-941, 2018. https://doi.org/10.1111/php.12937
» https://doi.org/10.1111/php.12937 - BARRETO-RODRIGUES, M.; SILVA, F. T.; PAIVA, T. C. B. Characterization of wastewater from the Brazilian TNT industry, Journal of Hazardous Materials, v. 164, n. 1, p. 385-388, 2009. https://doi.org/10.1016/j.jhazmat.2008.07.152
» https://doi.org/10.1016/j.jhazmat.2008.07.152 - BARROS NETO, B.; SCARMÍNIO, S.; BRUNS, R. E. Como fazer experimentos: pesquisa e desenvolvimento na ciência e na indústria. 2. ed. Campinas: Editora UNICAMP, 2002. 401 p.
- BASHEER, Al A. Chemical chiral pollution: impact on the society and science and need of the regulations in the 21st century. Chirality, v. 30, n. 4, p. 402-406, 2018a. https://doi.org/10.1002/chir.22808
» https://doi.org/10.1002/chir.22808 - BASHEER, Al A. New generation nano-adsorbents for the removal of emerging contaminants in water. Journal of Molecular Liquids, v. 261, p. 583-593, 2018b. https://doi.org/10.1016/j.molliq.2018.04.021
» https://doi.org/10.1016/j.molliq.2018.04.021 - BASHEER, Al A.; ALI, I. Stereoselective uptake and degradation of (±)‐o, p‐DDD pesticide stereomers in water‐sediment system. Chirality, v. 30, n. 9, p. 1088-1095, 2018. https://doi.org/10.1002/chir.22989
» https://doi.org/10.1002/chir.22989 - BILAL, M.; BAGHERI, A. M.; BHATT, P.; CHEN, S. Environmental occurrence, toxicity concerns, and remediation of recalcitrant nitroaromatic compounds. Journal of Environmental Management, v. 291, p. 112, 2021. https://doi.org/10.1016/j.jenvman.2021.112685
- BHANOT, P.; CELIN, S. M.; SREEKRISHNAN, T.R.; KALSI, A.; SAHAI, S.K.; SHARMA, P. Application of integrated treatment strategies for explosive industry wastewater-A critical review. Journal of Water Process Engineering, v. 35, n. 101232, 2020. https://doi.org/10.1016/j.jwpe.2020.101232
» https://doi.org/10.1016/j.jwpe.2020.101232 - BOX, G. E. P.; HUNTER, W. G.; HUNTER, J. S. Statistics for experimenters. An introduction to design, data analysis and model building. Nova York: Wiley, 1978.
- BUI, D. N.; MINH, T. T. Investigation of TNT red wastewater treatment technology using the combination of advanced oxidation processes. Science of The Total Environment, v. 756, n. 143852, 2021. https://doi.org/10.1016/j.scitotenv.2020.143852
» https://doi.org/10.1016/j.scitotenv.2020.143852 - CALABRÒ, P. S.; MORACI, N.; SURACI, P. Estimate of the optimum weight ratio in zero-valent Iron/Pumice granular mixtures used in permeable reactive barriers for the remediation of nickel contaminated groundwater. Journal of Hazardous Materials, v. 207-208, p. 111-116, 2012. https://doi.org/10.1016/j.jhazmat.2011.06.094
» https://doi.org/10.1016/j.jhazmat.2011.06.094 - CHEN, W.; JUAN, C.; WEI, K. Decomposition of dinitrotoluene isomers and 2,4,6-trinitrotoluene in spent acid from toluene nitration process by ozonation and photo-ozonation. Journal of Hazardous Materials, v. 147, n. 1-2, p. 97-104, 2007. https://doi.org/10.1016/j.jhazmat.2006.12.052
» https://doi.org/10.1016/j.jhazmat.2006.12.052 - KHAN, N. A. et al Occurrence, sources and conventional treatment techniques for various antibiotics present in hospital wastewaters: a critical review. TrAC Trends in Analytical Chemistry, v. 129, p. 115921, 2020. https://doi.org/10.1016/j.trac.2020.115921
» https://doi.org/10.1016/j.trac.2020.115921 - LIMPER, D.; FELLINGER, G. P.; EKOLU, S. O. Evaluation and microanalytical study of ZVI/scoria zeolite mixtures for treating acid mine drainage using reactive barriers - Removal mechanisms. Journal of Environmental Chemical Engineering, v. 6, n. 5, p. 6184-6193, 2018. https://doi.org/10.1016/j.jece.2018.08.064
» https://doi.org/10.1016/j.jece.2018.08.064 - MORACI, N.; CALABRÒ, P. S. Heavy metals removal and hydraulic performance in zero-valent iron/pumice permeable reactive barriers. Journal of Environmental Management, v. 91, p. 2336-2341, 2010. https://doi.org/10.1016/j.jenvman.2010.06.019
» https://doi.org/10.1016/j.jenvman.2010.06.019 - PEREIRA, C. A. A.; NAVA, M. R.; WALTER, J. B.; SCHERER, C. E.; DALFOVO, A. D. K.; BARRETO-RODRIGUES, M. Application of zero valent iron (ZVI) immobilized in Ca-Alginate beads for C.I. Reactive Red 195 catalytic degradation in an air lift reactor operated with ozone. Journal of Hazardous Materials, v. 401, 2021. https://doi.org/10.1016/j.jhazmat.2020.123275
» https://doi.org/10.1016/j.jhazmat.2020.123275 - SEWWANDI, K. A. H. S.; NITISORAVUT, R. Nano zero-valent iron embedded on chitosan for enhancement of biohydrogen production in dark fermentation. Energy Reports, v. 6, p. 392-396, 2020. https://doi.org/10.1016/j.egyr.2020.11.225
» https://doi.org/10.1016/j.egyr.2020.11.225
Publication Dates
-
Publication in this collection
17 June 2022 -
Date of issue
2022
History
-
Received
09 Nov 2021 -
Accepted
18 Apr 2022