Abstracts
The aim of the study was to detect neurological abnormalities in human immunodeficiency virus (HIV) infected children. This was achieved by a prospective evaluation, from November/1995 to April/2000, of 43 HIV infected children (group I) and 40 HIV seroreverters children (group II) through neurological exam and neurodevelopmental tests: Denver Developmental Screening Test (DDST) and Clinical Adaptive Test / Clinical Linguistic and Auditory Milestone Scale (CAT/CLAMS). A control group (III), of 67 children, were evaluated by CAT/CLAMS. Hyperactivity, irritability and hypotonia were the findings on neurological examination, without statistical differences between group I and II. On CAT/CLAMS, the group I developmental quotient (DQ) was significantly lower than the other groups. The same occurred in DDST, with group I presenting significantly more failures than group II. Nineteen HIV children of group I had brain computed tomographic scan, with abnormalities in three of them (basal ganglia calcification, white matter hypodensity and asymmetry of lateral ventricles). We conclude that in HIV infected children a neurodevelopment delay occur early in the disease, and it can be detected by screening tests.
human immunodeficiency virus (HIV); neurodevelopment delay; neurological screening test
Objetivou-se com o presente estudo detectar anormalidades neurológicas em crianças infectadas pelo vírus da imunodeficiência humana (HIV). Avaliou-se prospectivamente, de novembro de 1995 a abril de 2000, 43 crianças infectadas pelo HIV (grupo I) e 40 crianças sororevertidas (grupo II), por meio de exame neurológico, testes de screening de neurodesenvolvimento: Teste Denver de Triagem de Desenvolvimento (DDST) e Teste de Adaptação Clínica / Escala Linguística e Desenvolvimento Auditivo (CAT/CLAMS). Como grupo controle (III), foram avaliadas 67 crianças pelo CAT/CLAMS. Hiperatividade, irritabilidade e hipotonia foram as alterações encontradas ao exame neurológico, não ocorrendo diferenças estatísticas entre as crianças infectadas ou sororevertidas. No CAT/CLAMS o quociente de desenvolvimento (QD) do grupo I foi significativamente mais baixo que dos outros . O mesmo ocorreu no DDST, com o grupo I apresentando reprovações mais frequentes que o grupo II. Tomografia axial computadorizada de crânio foi realizada em 19 crianças do grupo I, sendo anormal em três delas (calcificação de gânglios da base, hipodensidade de substância branca e assimetria de ventrículos laterais). Conclui-se que, nas crianças infectadas, o atraso do neurodesenvolvimento ocorre precocemente e pode ser detectado por testes de screening.
vírus da imunodeficiência humana (HIV); atraso no neurodesenvolvimento; testes neurológicos de screening
DEVELOPMENTAL MILESTONES OF VERTICALLY HIV INFECTED AND SEROREVERTERS CHILDREN
Follow up of 83 children
Isac Bruck1 1 Pediatric Department, Clinical Hospital (HC) Federal University of Parana (UFPR), Curitiba PR, Brazil: Professor Assistente em Neurologia Pediátrica, Centro de Neurologia Pediátrica (CENEP), Departamento de Pediatria (DP), HC/UFPR; 2 Médico Residente em Infectologia Pediátrica, DP/HC/UFPR; 3 Professor Assistente em Infectologia Pediátrica, DP/HC/UFPR; 4 Médico(a) Residente em Neurologia Pediátrica, DP/HC/UFPR. , Tony Tannous Tahan2 1 Pediatric Department, Clinical Hospital (HC) Federal University of Parana (UFPR), Curitiba PR, Brazil: Professor Assistente em Neurologia Pediátrica, Centro de Neurologia Pediátrica (CENEP), Departamento de Pediatria (DP), HC/UFPR; 2 Médico Residente em Infectologia Pediátrica, DP/HC/UFPR; 3 Professor Assistente em Infectologia Pediátrica, DP/HC/UFPR; 4 Médico(a) Residente em Neurologia Pediátrica, DP/HC/UFPR. , Cristina Rodrigues da Cruz3 1 Pediatric Department, Clinical Hospital (HC) Federal University of Parana (UFPR), Curitiba PR, Brazil: Professor Assistente em Neurologia Pediátrica, Centro de Neurologia Pediátrica (CENEP), Departamento de Pediatria (DP), HC/UFPR; 2 Médico Residente em Infectologia Pediátrica, DP/HC/UFPR; 3 Professor Assistente em Infectologia Pediátrica, DP/HC/UFPR; 4 Médico(a) Residente em Neurologia Pediátrica, DP/HC/UFPR. , Luzilma Terezinha Flenik Martins3 1 Pediatric Department, Clinical Hospital (HC) Federal University of Parana (UFPR), Curitiba PR, Brazil: Professor Assistente em Neurologia Pediátrica, Centro de Neurologia Pediátrica (CENEP), Departamento de Pediatria (DP), HC/UFPR; 2 Médico Residente em Infectologia Pediátrica, DP/HC/UFPR; 3 Professor Assistente em Infectologia Pediátrica, DP/HC/UFPR; 4 Médico(a) Residente em Neurologia Pediátrica, DP/HC/UFPR. , Sérgio Antonio Antoniuk1 1 Pediatric Department, Clinical Hospital (HC) Federal University of Parana (UFPR), Curitiba PR, Brazil: Professor Assistente em Neurologia Pediátrica, Centro de Neurologia Pediátrica (CENEP), Departamento de Pediatria (DP), HC/UFPR; 2 Médico Residente em Infectologia Pediátrica, DP/HC/UFPR; 3 Professor Assistente em Infectologia Pediátrica, DP/HC/UFPR; 4 Médico(a) Residente em Neurologia Pediátrica, DP/HC/UFPR. , Marcelo Rodrigues4 1 Pediatric Department, Clinical Hospital (HC) Federal University of Parana (UFPR), Curitiba PR, Brazil: Professor Assistente em Neurologia Pediátrica, Centro de Neurologia Pediátrica (CENEP), Departamento de Pediatria (DP), HC/UFPR; 2 Médico Residente em Infectologia Pediátrica, DP/HC/UFPR; 3 Professor Assistente em Infectologia Pediátrica, DP/HC/UFPR; 4 Médico(a) Residente em Neurologia Pediátrica, DP/HC/UFPR. , Sílvia Mara de Souza4 1 Pediatric Department, Clinical Hospital (HC) Federal University of Parana (UFPR), Curitiba PR, Brazil: Professor Assistente em Neurologia Pediátrica, Centro de Neurologia Pediátrica (CENEP), Departamento de Pediatria (DP), HC/UFPR; 2 Médico Residente em Infectologia Pediátrica, DP/HC/UFPR; 3 Professor Assistente em Infectologia Pediátrica, DP/HC/UFPR; 4 Médico(a) Residente em Neurologia Pediátrica, DP/HC/UFPR. , Laís Regina de Bruyn4 1 Pediatric Department, Clinical Hospital (HC) Federal University of Parana (UFPR), Curitiba PR, Brazil: Professor Assistente em Neurologia Pediátrica, Centro de Neurologia Pediátrica (CENEP), Departamento de Pediatria (DP), HC/UFPR; 2 Médico Residente em Infectologia Pediátrica, DP/HC/UFPR; 3 Professor Assistente em Infectologia Pediátrica, DP/HC/UFPR; 4 Médico(a) Residente em Neurologia Pediátrica, DP/HC/UFPR.
ABSTRACT - The aim of the study was to detect neurological abnormalities in human immunodeficiency virus (HIV) infected children. This was achieved by a prospective evaluation, from November/1995 to April/2000, of 43 HIV infected children (group I) and 40 HIV seroreverters children (group II) through neurological exam and neurodevelopmental tests: Denver Developmental Screening Test (DDST) and Clinical Adaptive Test / Clinical Linguistic and Auditory Milestone Scale (CAT/CLAMS). A control group (III), of 67 children, were evaluated by CAT/CLAMS. Hyperactivity, irritability and hypotonia were the findings on neurological examination, without statistical differences between group I and II. On CAT/CLAMS, the group I developmental quotient (DQ) was significantly lower than the other groups. The same occurred in DDST, with group I presenting significantly more failures than group II. Nineteen HIV children of group I had brain computed tomographic scan, with abnormalities in three of them (basal ganglia calcification, white matter hypodensity and asymmetry of lateral ventricles). We conclude that in HIV infected children a neurodevelopment delay occur early in the disease, and it can be detected by screening tests.
KEY WORDS: human immunodeficiency virus (HIV), neurodevelopment delay, neurological screening test.
Desenvolvimento psicomotor de crianças verticalmente infectadas pelo HIV e sororevertidas: seguimento de 83 crianças
RESUMO ¾ Objetivou-se com o presente estudo detectar anormalidades neurológicas em crianças infectadas pelo vírus da imunodeficiência humana (HIV). Avaliou-se prospectivamente, de novembro de 1995 a abril de 2000, 43 crianças infectadas pelo HIV (grupo I) e 40 crianças sororevertidas (grupo II), por meio de exame neurológico, testes de screening de neurodesenvolvimento: Teste Denver de Triagem de Desenvolvimento (DDST) e Teste de Adaptação Clínica / Escala Linguística e Desenvolvimento Auditivo (CAT/CLAMS). Como grupo controle (III), foram avaliadas 67 crianças pelo CAT/CLAMS. Hiperatividade, irritabilidade e hipotonia foram as alterações encontradas ao exame neurológico, não ocorrendo diferenças estatísticas entre as crianças infectadas ou sororevertidas. No CAT/CLAMS o quociente de desenvolvimento (QD) do grupo I foi significativamente mais baixo que dos outros . O mesmo ocorreu no DDST, com o grupo I apresentando reprovações mais frequentes que o grupo II. Tomografia axial computadorizada de crânio foi realizada em 19 crianças do grupo I, sendo anormal em três delas (calcificação de gânglios da base, hipodensidade de substância branca e assimetria de ventrículos laterais). Conclui-se que, nas crianças infectadas, o atraso do neurodesenvolvimento ocorre precocemente e pode ser detectado por testes de screening.
PALAVRAS-CHAVE: vírus da imunodeficiência humana (HIV), atraso no neurodesenvolvimento, testes neurológicos de screening.
The Human Immunodeficiency Virus (HIV), the etiologic agent of the acquired immunodeficiency syndrome (AIDS), is a RNA retrovirus that belongs to lentiviridae subfamily, and it is a lymphotropic and neurotropic virus. Central nervous system (CNS) involvement is common and delay in neurodevelopmental milestones can be the first presenting symptom1-4. Other evidences that emphasizes the HIV infection in CNS are neuropathologic findings, showing cortical and subcortical atrophy, encephalitis, neuronal apoptosis, diffuse leucoencephalopathy and basal ganglia calcification2. At neuroimaging, brain computed tomographic (CT) scan can show brain atrophy and basal ganglia calcification1-2.
Some children with vertically transmitted HIV may show mild symptoms, such as muscle tone abnormality. Other children show progressive developmental growth deceleration characterized by slowing of developmental gain followed by loss of previously acquired milestones. A third group exhibits a static encephalopathy, functioning below the average range but continuing to progress along their own developmental growth curve without further decline3.
Some studies3-5 suggest that the neurodevelopment delay of pediatric HIV disease may be seen even before 6 months of age. The goals of this study were to compare mental and motor growth and to describe the spectrum of neurodevelopment outcome in a sample of infected and uninfected children born from HIV-infected mothers; the children were followed up prospectively from 6 to 72 months of age.
METHOD
Subjects were recruited between November 1995 to April 2000, from the outpatient clinic of Clinical Hospital (HC) of the Federal University of Paraná (UFPR), and followed by a prospective, longitudinal and transversal neurologic evaluation. We studied two groups of patients separated by HIV infection status and compared with a control group. Written consent to participate in the study was given by parent (s) or caregiver (s) and the study was approved by the ethical commission of the HC-UFPR.
The groups were: (I) HIV vertically infected patients (n=43); (II) HIV seroreverter patients (n=40); and (III) control group (n=67). Socioeconomic status was the same for the three groups. Maternal mean age was 25 years old (17 to 40 years old). Forty two percent were boys and 58% were girls.
Drug ingestion during pregnancy was not evaluated, because the information was not available in the medical record. It was also analyzed if the child lived with the parent(s), were adopted or institutionalized.
The HIV infected patients (Group I) were seen in Pediatric Infectious outpatient Clinic at HC-UFPR and they had a total of 61 assessments. Some of them were evaluated more than once. The mean age of this group was 50.7 months (11-72 months).
The children of Group II who were born from HIV-infected women had a total of 111 assessments and were considered seroreverter after 2 negative tests by ELISA HIV antibodies, which were undertaken until 18 months of age. The mean age of this group was 39.6 months (20 ¾ 64 months).
The control group was selected from the same environment of the patients and matched by age6. The mean age of this group was 16.7 months (4 ¾ 35.2 months).
All children underwent at least one clinical assessment between 6 and 72 months of age. Five children with congenital infection or neonatal meningitis were excluded from the study. We detected 2 patients with congenital toxoplasmosis in the seroreverter group and one in HIV-infected group, and one child in each group with neonatal meningitis.
The evaluation consisted by neurological examination and neurodevelopmental screening tests - Denver Developmental Screen Test (DDST)7 and Clinical Adaptive Test/Clinical Linguistic and Auditory Milestone Scale (CAT/CLAMS)8-12. These were applied to group I and II, while group III only performed CAT/CLAMS. Nineteen children of group I underwent CT scan, as part of the neurologic assessment.
CAT/CLAMS is a 100-item' scale administered in a standardized manner in two parts and is obtained through observation (CAT) and parental report (CLAMS). This test is used in children between 1 and 36 months old. CAT consists of visual-motor problem-solving items, which are performed directly with the child. CLAMS items consist of language acquisition. A score is derived for CAT and CLAMS separately. CAT/CLAMS score is the numerical average of the two, converted to a developmental quotient (DQ) by dividing total score by the chronological age in months and multiplying by 1008-12. The DQ of 85 to 120 is normal; 70 to 85 is borderline; under 70 is delayed.
The DDST was chosen because it could be applied in children between 1 and 72 months of age. This test evaluates four developmental areas: language, personal-social, fine motor-adaptive and gross motor. The child can pass or fail on each of the items, in which 90% of children pass at this age7.
These tests (DDST and CAT/CLAMS) were applied at 6 months intervals by a pediatric neurologist (IB), or residents (neuropediatric and infectopediatric), under direct supervision by the former. In case of a premature child, defined as under 37 weeks of gestational age (7 patients), both tests were corrected for the degree of prematurity until they achieved 24 months old.
Assessment was established by age, as follows: 1) children under 13 months old had 9 assessments in group I, 40 in group II and 33 in group III. The small number of HIV infected children is due to the fact that most of them are asymptomatic at this age. 2) between 14 to 24 months of age we performed 11 assessments in group I, 37 in group II and 16 in group III. 3) between 25 and 36 months of age we had 16 assessments in group I, 21 in group II and 18 in group III. 4) finally in children between 36 and 72 months we applied only DDST with 25 assessments in group I and 13 in group II.
Statistical Methods
Data were analyzed using the SAS statistical package. The variables were created when data existed for the 1-13, 14-24, 25-36, 37-72 months, for CAT/CLAMS test and DDST. The t Student test was used to identify relationships between outcome measures. Fisher test was used to percentile correlation. It was considered significant when p < .05.
RESULTS
We found no significant statistical differences in the 3 groups when comparing parameters of sex, prenatal intercurrences, gestational age, birth weight, obstetrical characteristics and mother's mean age.
When analyzing family structure, 29% of children in group I and none in group II lived in a institution. Considering the children who live with one or both parent(s) our study showed: group I: 32% and 18%; and group II: 50% and 42%, respectively. Considering the adopted children the results were group I: 21% and group II: 8%. All these results showed no significant statistical differences between them.
Head circumference was stable during evolution of both groups (I and II) with the mean in 50 percentile13. Mild neurological abnormalities were found in group I (55% of assessments) and group II (40% of assessments) such as hyperactivity, low grades of hypotonia and irritability but without significant differences between them. Two children in group I (1 boy and 1 girl) had spastic paraparesis.
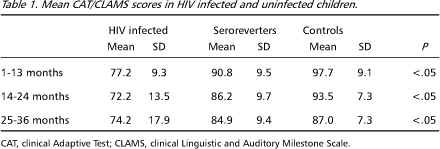
Mean values of CAT/CLAMS scores under 13, 14-24, and 25-36 months of age are shown in Table 1. Highly significant differences were noted among the HIV infected children when comparing with the other two groups at all ages. No significant differences were found between seroreverter and control group.
Although all mean values are within the normal range for the test, 33.3% of HIV infected children had values below 70 at age under 13 months, 45.4% between 14 and 24 months and 37.5% between 25 and 36 months (Table 2).
DDST, showed more failures (50-80%) in HIV infected group at all ages when comparing with seroreverter group (20-40%) and the results were statistically significant at ages under 13 and between 25 and 36 months (Table 3).
Nineteen HIV infected children had brain CT scans: 16 were normal and 3 abnormal - white matter hypodensity, asymmetry of lateral ventricles and basal ganglia calcification (Fig 1).
DISCUSSION
HIV infected children with neurologic and neurodevelopmental manifestations of central nervous system infection have been termed neuro-AIDS1,4,14. Although the prevalence of neurodevelopmental manifestations of HIV infection has been reported in several series to vary between 8% and 62%1, few studies have been done prospectively.
Similar results were reported by Wachtel et al.4, who demonstrated worse performance in CAT/CLAMS of HIV infected children when comparing with seroreverter and control groups. While no significant differences could be detected at 6 months of age, highly significant differences were noted over this age among the three groups. Actually CAT/CLAMS is more sensible in detecting abnormalities at ages over 12 months, as reported by Kube et al12.
Recently, it has been mentioned that a highly proportion of HIV infected infants show early marked cognitive and motor delays or declines, that may be an important early indication of HIV disease progression. These abnormalities are independent of other risk factors for developmental delay15,16 and were also seen in the present study. The aim of the study was to examine the frequency and the age when occurred during the first 6 years of life, abnormalities in cognitive and motor development in infants born from HIV-infected women. The CAT/CLAMS seems to fit well for the first 36 months of life. Based on studies comparing CAT/CLAMS with Bayley Scales of Infant Development (BSID), both have good correlation, as mentioned in the literature4, 12,15.
The DDST has low sensitivity17-21 but in this study it achieved a good correlation with CAT/CLAMS; probably it was more accurate in our cases because it was assessed by the same Pediatric Neurologist. Our results are in agreement with Greer S et al.22 who showed that a child with an abnormal DDST is likely to have a poor school outcome.
When comparing with other Brazilian Centers, as in the study of Tellechea et al.14, there was also a trend of developmental delay in HIV infected children in the first few months of life, until 5 years, an indication of early encephalopathy.
De Carli et al.23, analyzed 100 consecutive brain computed tomographic (CT) scans in symptomatic acquired immunodeficiency syndrome children. They found that cerebral calcification on CT scan suggested vertically acquired HIV infection and the presence of encephalopathy, as in our case (Fig 1).
A great number of HIV infected children lived in institutions or were adopted. This fact could be a cause of developmental delay, but there was not statistical significant differences when comparing with the other two groups.
We conclude that HIV contributes to the damage of developing brain and can be detected by the presence of alterations in the neurological assessment, including neurological examination and neurodevelopmental tests.
The low CAT/CLAMS scores and the prevalence of failures on DDST demonstrate that some degree of encephalopathy occurs in HIV infected children group since early ages.
Finally it must be emphasized that pediatric AIDS is considered a chronic and systemic disease and for this reason the follow up must be multidisciplinary, including a sequential neurological evaluation.
Acknowledgements ¾ We are grateful for Dra Monica Cat for the statistical analysis and Marlene Terezinha Gomes da Silva, social worker, who helped us on contacting parents and caregivers.
Received 13 February 2001, received in final form 9 May 2001. Accepted 18 May 2001.
Dr. Isac Bruck - CENEP, Centro de Neurologia Pediátrica - R. Floriano Essenfelder 81 ¾80060-270 Curitiba PR - Brasil. FAX: 41 2649101 / 41 3629385
- 1. Belman AL. Acquired immunodeficiency syndrome and the child's central nervous system. Pediatr Clin North Am 1992;39:691-714.
- 2. Tardieu M. HIV-1 and the developing central nervous system. Dev Med Child Neurol 1998;40:843-846.
- 3. Chase C, Vibbert M, Pelton SI, Coulter DL, Cabral H. Early neurodevelopmental growth in children with vertically transmitted human immunodeficiency virus infection. Arch Pediatr Adolesc Med 1995;149: 850-855.
- 4. Wachtel RC, Tepper VJ, Houck D, McGrath CJ, Thompson C. Neurodevelopment in pediatric HIV infection: the use of CAT/CLAMS. Clin Pediatr 1994;33:416-420.
- 5. Chase C, Coulter D, Vibbert M, Woodward P, Harris M, Mitrano J. Mental and motor development in HIV-infected and seroreverter infants. In Programs and abstracts of neuroscience of HIV infection: basic clinical frontiers. Eighth International Conference on AIDS/STD World Congress. Amsterdam 1992:Abstract 34.
- 6. França SN. Análise clínico laboratorial dos pacientes com hipotiroidismo congęnito diagnosticado pelo programa de rastreamento neonatal no Estado do Paraná: pacientes do grupo controle avaliados no período de junho a agosto de 1995. Dissertaçăo de Mestrado, Universidade Federal do Paraná, Curitiba, 1997.
- 7. Frankenburg WK, Dodds JB. The Denver Developmental Screening Test. Pediatrics 1967;71:181-191.
- 8. Wachtel RC, Shapiro BK, Palmer BF, Allen MC, Capute AJ. CAT/CLAMS: a tool for the Pediatric Evaluation of infants and young children with developmental delay. Clin Pediatr 1994,33:410-415.
- 9. Capute AJ, Shapiro BK, Wachtel RC, Gunter VA, Palmer FB. The Clinical Linguistic and Auditory Milestone Scale (CLAMS). Identification of cognitive defects in motor-delayed children. AJDC 1986;140:694-698.
- 10. Capute AJ, Accardo PJ. The infant neurodevelopmental assessment: a clinical interpretive manual for CAT-CLAMS in the first two years of life. Part 1. Curr Probl Pediatr 1996;26:238-256.
- 11. Rossman MJ, Hyman SL, Rorabaugh ML, Berlin LE, Allen MC, Modlin JF. The CAT / CLAMS assessment for early intervention services. Clin Pediatr 1994;33:404-409.
- 12. Kube DA, Wilson WM, Petersen MC, Palmer FB. CAT / CLAMS: its use in detecting early childhood cognitive impairment. Pediatr Neurol 2000;23:208-215.
- 13. Nellhaus G. Head circumference from birth to eighteen years. Pediatrics 1968;41:106.
- 14. Tellechea N, Silva CLO, Colvero M, Schirmer M, Afonso-Galvăo N. Neurosida. Rev Neurol (Barc) 1997;25:903-905.
- 15. Chase C, Ware J, Hittelman J, et al. Early cognitive and motor development among infants born to women infected with human immunodeficiency virus (Abstract). Pediatrics 2000;106:334.
- 16. Smith R, Malee K, Charutat M, et al. Timing of perinatal human immunodeficiency virus type 1 infection and rate of neurodevelopment. Pediatr Infect Dis J 2000;19: 862-871.
- 17. Borowitz KC, Glascoe FP. Sensitivity of the Denver Developmental Screening Test in speech and language screening. Pediatrics 1986; 78:1075-1078.
- 18. Meisels SJ, Margolis LH. Is the early and periodic screening, diagnosis, and treatment program effective with developmentally disabled children? Pediatrics 1988;81:262-271.
- 19. Meisels SJ. Can developmental screening tests identify children who are developmentally at risk? Pediatrics 1989;83:578-585.
- 20. Applebaum A. Validity of the revised Denver Developmental Screening Test for referred and non-referred samples. Psychol Rep 1978;43:227-233.
- 21. Sciarrilo WG, Brown MM, Robinson NM, Bennett FC, Sells CJ. Effectiveness of the Denver Developmental Screening Test with biologically vulnerable infants. J Dev Behav Pediatr 1986;7:77-83.
- 22. Greer S, Bauchner H, Zuckerman B. The Denver developmental screening test: How good is its predictive validity? Dev Med Child Neurol 1989;31:774-781.
- 23. De Carli C, Civitello LA Brouwers P, Pizzo PA. The prevalence of computed tomographic abnormalities of the cerebrum in 100 consecutive children symptomatic with the human immune deficiency virus. Ann Neurol 1993;34:198-205.
Publication Dates
-
Publication in this collection
05 Oct 2001 -
Date of issue
Sept 2001
History
-
Accepted
18 May 2001 -
Reviewed
09 May 2001 -
Received
13 Feb 2001