Abstract
Background
The Parkinson disease (PD) is frequently associated with autonomic dysfunctions. However, data regarding the influence of PD on the autonomic responses to postural changes is limited.
Objective
To analyze and compare the autonomic responses, evaluated through linear and non-linear methods of heart rate variability, and cardiorespiratory parameters in two groups: Parkinson disease (PDG) and control (CG), at rest and during the active tilt test.
Methods
A total of 48 participants were analyzed (PDG: n = 25;73.40 ± 7.01 years / CG: n = 23;70.17 ± 8.20 years). The autonomic modulation and cardiorespiratory parameters were evaluated at rest and during the active tilt test. To assess the autonomic modulation the linear indices, at the time (rMSSD, SDNN) and frequency (LF, HF, LF/HF) domains, and the non-linear indices, obtained through the Poincaré plot (SD1, SD2, SD1/SD2), were calculated. The cardiorespiratory parameters evaluated were heart rate (HR), systolic (SBP), and diastolic blood pressure (DBP), peripheral oxygen saturation (SpO2), and respiratory rate.
Results
At rest, the PDG presented significantly lower values of rMSSD, SDNN, LF, HF, SD1, SD2, and DBP, and higher values of SpO2. During test, in the PD group, modifications were observed in HR, and SBP, besides a reduced parasympathetic response, and an increased global modulation. The qualitative analysis of the Poincaré plot showed that the PDG has a lower dispersion of the RR intervals during rest and the active tilt test.
Conclusion
Individuals with PD present reduced global variability and parasympathetic modulation at rest, and reduced parasympathetic response and damage in HR regulation when performing the active tilt test, compared with controls.
Keywords:
Arterial Pressure; Autonomic Nervous System Diseases; Heart Rate; Parkinson Disease; Respiratory Rate
Resumo
Antecedentes
A doença de Parkinson (DP) está frequentemente associada a disfunções autonômicas. Porém, dados sobre a influência da DP nas respostas autonômicas às mudanças posturais são limitados.
Objetivos
Analisar e comparar as respostas autonômicas, avaliadas por métodos lineares e não lineares de variabilidade da frequência cardíaca e parâmetros cardiorrespiratórios em dois grupos: DP (GDP) e controle (CG), em repouso e durante o tilt test ativo.
Métodos
Foram analisados 48 participantes (GDP: n = 25;73,40 ± 7,01 anos/GC: n = 23; 70,17 ± 8,20 anos). A modulação autonômica e os parâmetros cardiorrespiratórios foram avaliados em repouso e durante o tilt test ativo. Para avaliar a modulação autonômica foram calculados os índices lineares, nos domínios do tempo (rMSSD, SDNN) e frequência (LF, HF, LF/HF), e os índices não lineares, obtidos através do plot de Poincaré (SD1, SD2, SD1/SD2). Os parâmetros cardiorrespiratórios avaliados foram frequência cardíaca (FC), pressão arterial sistólica (PAS), diastólica (PAD), saturação periférica de oxigênio (SpO2) e frequência respiratória.
Resultados
Em repouso, o GDP apresentou valores menores de rMSSD, SDNN, LF, HF, SD1, SD2 e PAD, e maiores valores de SpO2. No teste, foram observadas modificações na FC e na PAS, redução da resposta parassimpática e aumento da modulação global no GDP. A análise qualitativa do plot de Poincaré mostrou que o GDP apresentou menor dispersão dos intervalos RR no repouso e no tilt test ativo.
Conclusão
Indivíduos com DP apresentam redução da variabilidade global e modulação parassimpática em repouso, redução da resposta parassimpática e prejuízo na regulação da FC ao realizar o tilt test ativo, em comparação aos controles.
Palavras-chave:
Pressão Arterial; Doenças do Sistema Nervoso Autônomo; Frequência Cardíaca; Doença de Parkinson; Taxa Respiratória
INTRODUCTION
The Parkinson disease (PD) is a neurodegenerative disorder, characterized by damage to the neurons from the substantia nigra pars compacta,11 Kalia LV, Lang AE. Parkinson’s disease. Lancet 2015;386 (9996):896–912 which affects approximately 1% of the population over 60-years-old.22 Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 2017;124(08):901–905
Besides motor symptoms, several non-motor dysfunctions have been reported in individuals with PD,33 Poewe W, Seppi K, TannerCM, etal. Parkinson disease. Nat Rev Dis Primers 2017;3:17013 including impairments related to the autonomic nervous system (ANS) which are associated with lower survival rate, worse disease progression,44 De Pablo-Fernandez E, Tur C, Revesz T, Lees AJ, Holton JL, Warner TT. Association of autonomic dysfunction with disease progression and survival in Parkinson disease. JAMA Neurol 2017;74(08): 970–976 and reduced quality of life.33 Poewe W, Seppi K, TannerCM, etal. Parkinson disease. Nat Rev Dis Primers 2017;3:17013
The heart rate variability (HRV) is a method that allows the investigation of ANS efficiency through the descriptionof the intervals between consecutive heartbeats.55 Vanderlei LCM, PastreCM, Hoshi RA, CarvalhoTD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc 2009;24(02):205–217 HRV analysis combined with autonomic tests,66 Giacon TR, Vanderlei FM, Christofaro DGD, Vanderlei LCM. Impact of diabetes type 1 in children on autonomic modulation at rest and in response to the active orthostatic test. PLoS One 2016;11 (10):e0164375 such as the active tilt test,77 Jones PK, Gibbons CH. Autonomic function testing: an important diagnostic test for patients with syncope. Pract Neurol 2015;15 (05):346–351 provides more complete ANS assessment.66 Giacon TR, Vanderlei FM, Christofaro DGD, Vanderlei LCM. Impact of diabetes type 1 in children on autonomic modulation at rest and in response to the active orthostatic test. PLoS One 2016;11 (10):e0164375
Reductions in HRV indices in the time and frequency domains have already been described in individuals with PD.88 Rocha RSB, De Oliveira Rocha LS, Pena ESM, Caldas LCP, Moreno MA. Analysis of autonomic modulation of heart rate in patients with Parkinson’s disease and elderly individuals submitted to game therapy training. Geriatr Gerontol Int 2018;18(01):20–25,99 Ke JQ, Shao SM, Zheng YY, Fu FW, Zheng GQ, Liu CF. Sympathetic skin response and heart rate variability in predicting autonomic disorders in patients with Parkinson disease. Medicine (Baltimore) 2017;96(18):e6523 However, studies evaluating HRV during autonomic tests have considered only the passive tilt test,1010 Bouhaddi M, Vuillier F, Fortrat JO, et al. Impaired cardiovascular autonomic control in newly and long-term-treated patients with Parkinson’s disease: involvement of L-dopa therapy. Auton Neurosci 2004;116(1-2):30–38,1111 Watano C, Shiota Y, Onoda K, et al. Evaluation of autonomic functions of patients with multiple system atrophy and Parkinson’s disease by head-up tilt test. J Neural Transm (Vienna) 2018; 125(02):153–162 which is not feasible in clinical settings. Thus, the active tilt test may represent a simple and economical alternative1212 Moura-Tonello SCG, Takahashi ACM, Francisco CO, et al. Influence of type 2 diabetes on symbolic analysis and complexity of heart rate variability in men. Diabetol Metab Syndr 2014;6(01):13 for the evaluation of individuals with PD. Furthermore, active tilt test seems to promote more evident changes in musculoskeletal and cardiovascular responses when compared with passive tilt test.77 Jones PK, Gibbons CH. Autonomic function testing: an important diagnostic test for patients with syncope. Pract Neurol 2015;15 (05):346–351 However, to date, no studies have evaluated the HRV of individuals with PD during the active tilt test. Moreover, studies that have evaluated cardiorespiratory system in PD are incipient and did not evaluate parameters such as respiratory rate (RR) and peripheral oxygen saturation (SpO2).
Understanding these aspects, it may provide a new perspective on the characteristics of the ANS of individuals with PD in the face of postural changes, which are frequently in the daily lives. Furthermore, this information may assist inthe consolidation of the literature regarding the most appropriate therapeuticinterventionsconcerning posturalchangesfor this population.
Therefore, the aim of this study was to analyze and compare the response of autonomic modulation, through linear and non-linear HRV methods, and cardiorespiratory parameters at rest and during the active tilt test in individuals with and without PD. We hypothesized that individuals with PD would present a reduced response of autonomic modulation and cardiorespiratory parameters at rest and in the active tilt test, when compared with individuals without the disease.
METHODS
Study design
Cross-sectional study developed in a city of west of São Paulo, Brazil, between August of 2017 and April of 2018, approved by the Institution’s Research Ethics Committee (CAEE:71395617.7.0000.5402). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed to report this study.
The experimental procedure was conducted in two evaluation sessions with a minimal interval of 48hours between them. Both sessions were performed in a climatized room, with temperature and humidity controlled and maintained between 21° and 23°C, and 40 and 60%,1313 de Rezende Barbosa MPC, Júnior JN, Cassemiro BM, et al. Effects of functional training on geometric indices of heart rate variability. J Sport Health Sci 2016;5(02):183–189 respectively, between 8:00 and 12:00 AM to minimize the circadian variation.1414 Catai AM, Pastre CM, Godoy MF, Silva ED, Takahashi ACM, Vanderlei LCM. Heart rate variability: are you using it properly? Standardisation checklist of procedures. Braz J Phys Ther 2020;24 (02):91–102 In the first evaluation session, the population profile regarding the participants’ physical, cognitive, and clinical characteristics were assessed. The second session was composed of the active tilt test and outcomes assessment.
Population
A total of 48 participants were evaluated and divided into two groups: PD (PDG) and control (CG). The PDG (n = 25) was composed of individuals with a medical diagnosis of PD, classified between stages 1 and 3 of the Hoehn and Yahr (HY) disability scale,1515 Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17(05):427–442 and who did not present cognitive deficits according to the Mini-Mental State Examination (MMSE).1616 Bertolucci PHF, Brucki SMD, Campacci SR, Juliano Y. O Mini-Exame do Estado Mental em uma população geral. Impacto da escolaridade. Arq Neuropsiquiatr 1994;52(01):1–7 The CP (n = 23) was composed of individuals without cognitive deficits and neurological diseases, paired by sex and age with the participants from the PDG.
All participants of the PDG were recruited at the neurology sector of the Center for Physical Therapy and Rehabilitation Studies and Treatmentof the São Paulo State University’s (UNESP) Faculty of Sciences and Technology, Presidente Prudente, Brazil, and the corresponding controls were recruited from health centers in the same city.
Current smokers, current heavydrinkers, individuals with active infections, as well as cardiovascular and respiratory diseases that could interfere with cardiac autonomic control were not included. Participants with >5% error in their HRV record were excluded.
The sample size was defined considering the rMSSD index. The magnitude of the significant difference assumed was 14 milliseconds1717 Brisinda D, Sorbo AR, Di Giacopo R, Venuti A, Bentivoglio AR, Fenici R. Cardiovascular autonomic nervous system evaluation in Parkinson disease and multiplesystem atrophy. JNeurol Sci 2014; 336(1-2):197–202 and a standard deviationof 12milliseconds, with an α risk of 5% and a β risk of 80% was considered, which resulted in 21 participants.
Participants were previously informed about the objectives and procedures of the study and provided written informed consent.
Experimental procedure
First evaluation
The anthropometric measures, such as weight, height, body mass index (BMI),1818 Associação Brasileira para o Estudo da Obesidade e da Síndrome Metabólica. Diretrizes brasileiras de obesidade.4°ed. São Paulo, SPABESO2016 ndwaist-hip ratio(WHR),1919 Ferreira MG, Valente JG, Gonçalves-Silva RMV, Sichieri R. [Accuracyof waist circumference and waist-to-hipratioaspredictors of dyslipidemia in a cross-sectional study among blood donors in Cuiabá, Mato Grosso State, Brazil]. Cad Saude Publica 2006;22 (02):307–314 aswellasthe cognitive deficits, according to the MMSE,1616 Bertolucci PHF, Brucki SMD, Campacci SR, Juliano Y. O Mini-Exame do Estado Mental em uma população geral. Impacto da escolaridade. Arq Neuropsiquiatr 1994;52(01):1–7 were assessed in all participants. For the participants with PD, the HY disability scale,1515 Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17(05):427–442 and the revised version of the Unified Parkinson Disease Rating Scale (MDS-UPDRS)2020 Goetz CG, Tilley BC, Shaftman SR, et al; Movement Disorder Society UPDRS Revision Task Force. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23(15):2129–2170 were applied.
Second evaluation
In this session, the active tilt test was performed. Participants were instructed not to consume stimulating substances such as coffee, tea, and chocolate at least 12 hours before this session. For participants with PD, this evaluation was performed during the “on” period of their medication (approximately one hour after ingestion).2121 Carpenter MG, Allum JHJ, Honegger F, Adkin AL, Bloem BR. Postural abnormalities to multidirectional stance perturbations in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2004;75 (09):1245–1254
To record the heart rate (HR) beat-to-beat, and to generate the RR intervals series used for the autonomic modulation evaluation, the Polar RS800CX (Polar Electro Oy, Kempele, Finland)66 Giacon TR, Vanderlei FM, Christofaro DGD, Vanderlei LCM. Impact of diabetes type 1 in children on autonomic modulation at rest and in response to the active orthostatic test. PLoS One 2016;11 (10):e0164375 was utilized.
Each participant was instructed to remain lying at rest, without talking, and in spontaneous breathing for 30 minutes. Then, the participant was instructed to stand up, and to remain in a standing position for 10 minutes. Cardiorespiratory parameters were assessed during rest (CR1), immediately after the participant stood up (CR2), and after 10 minutes in a standing position (CR3).
Outcomes
Cardiorespiratory parameters
Blood pressure (BP) was verified indirectly using a Tycos aneroid sphygmomanometer (WelchAllyn, Skaneateles Falls, NY, USA) and a Classic III Littman stethoscope (3M Company, Maplewood, MN, USA) on the participant’s left arm.2222 Malachias M, Plavnik FL, Machado CA, Malta D, Scala LCN, Fuchs S. 7th Brazilian Guideline of Arterial Hypertension: Chapter 1 -Concept, Epidemiology and Primary Prevention. Arq Bras Cardiol 2016;107(3, Suppl 3)1–6 HR was assessed by the Polar RS800CX heart monitor (Polar Electro Oy, Kempele, Finland) and SpO2 was assessed through a digital pulse oximeter (ChoiceMMed finger oximeter, MD300C29, China). RR was assessed by counting the number of respiratory incursions performed by the participant during 60 seconds.2323 Humberstone N, Tecklin JS. Avaliação Respiratória. Irwin S, Tecklin JS, organizadores. Fisioterapia cardiopulmonar. 3.ed. São Paulo: Manole; 2003. p. 334–55
Autonomic modulation
Autonomic modulation was evaluated by the HRV linear methods (time and frequency domains), and by the quantitative and qualitative analyzes of the Poincaré plot. HRV was assessed from the RR intervals obtained by a Polar RS800CX heart rate monitor (Polar Electro Oy, Kempele, Finland) with a sampling rate of 1000 Hz. Data on the RR intervals were sent to a microcomputer, by the pulse receptor’s data transmission port to the Polar Precision Performance software (Polar Electro Oy, Kempele, Finland), version 4.01.029, using an infrared signal interface. Initially, the RR intervals series were digitally filtered by Polar Precision Performance software (Polar Electro Oy, Kempele, Finland),2424 Kiviniemi AM, Hautala AJ, Kinnunen H, Tulppo MP. Endurance training guided individually by daily heart rate variability measurements. Eur J Appl Physiol 2007;101(06):743–751 using a moderate filter, and then, a manual filtering was performed using Microsoft Excel (Microsoft Corp. Redmond, WA, USA) software,55 Vanderlei LCM, PastreCM, Hoshi RA, CarvalhoTD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc 2009;24(02):205–217,1414 Catai AM, Pastre CM, Godoy MF, Silva ED, Takahashi ACM, Vanderlei LCM. Heart rate variability: are you using it properly? Standardisation checklist of procedures. Braz J Phys Ther 2020;24 (02):91–102 to eliminate the remaining ectopic beats and artifacts. Finally, a visual inspection of temporal series of the RR interval was performed in the computer monitor, which showed absence of artifacts that could interfere with the HRV analysis.
Only series with > 95% of sinus beats were analyzed,2525 Barreto GSC, Vanderlei FM, Vanderlei LCM, Leite AJM. Impact of malnutrition on cardiac autonomic modulation in children. J Pediatr (Rio J) 2016;92(06):638–644 this care was taken so that only traces of good quality were used in the analyzes. The RR intervals series were analyzed at rest period (TT1) and the active tilt test period (TT2). Two five-minute sections with at least 256 consecutive RR intervals were extracted from the period of greatest stability of the signal of these two periods (TT1 and TT2).
The HRV indices were calculated using the Kubios HRV (Kubios, Kuopio, Finland) software, version 54100230.2626 Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV–heart rate variability analysis software. Comput Methods Programs Biomed 2014;113(01):210–220 For the time domain were considered the indices: rMSSD (root mean square of the successive differences between adjacent normal RR intervals), and SDNN (the standard deviation of all normal RR intervals).55 Vanderlei LCM, PastreCM, Hoshi RA, CarvalhoTD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc 2009;24(02):205–217 For the frequency domain, the fundamental oscillatory components of high frequency (HF/0.15 to 0.4 Hz), and low frequency (LF/0.04 to 0.15 Hz),55 Vanderlei LCM, PastreCM, Hoshi RA, CarvalhoTD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc 2009;24(02):205–217 expressed in milliseconds squared (ms2) and normalized units (nu), and the LF/HF ratio were considered. The Fast Fourier Transform was used as an algorithm for spectral analysis, with 50% overlap and window of 256 beats.
The qualitative analysis was performed through the evaluation of the figures generated by the Poincaré plot attractor55 Vanderlei LCM, PastreCM, Hoshi RA, CarvalhoTD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc 2009;24(02):205–217: 1) Figure in which an increase in the dispersion of RR intervals beat-to-beat can be verified; 2) Figure with a small dispersion of RR intervals, both in the short and long term.55 Vanderlei LCM, PastreCM, Hoshi RA, CarvalhoTD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc 2009;24(02):205–217 The plot figure was drawn from the union of RR intervals of all participants in the study.
All procedures followed the recommendations described in the literature1414 Catai AM, Pastre CM, Godoy MF, Silva ED, Takahashi ACM, Vanderlei LCM. Heart rate variability: are you using it properly? Standardisation checklist of procedures. Braz J Phys Ther 2020;24 (02):91–102 and the normality values for HRV indices have been described by other authors.2727 van den Berg ME, Rijnbeek PR, Niemeijer MN, et al. Normal values of corrected heart-rate variability in 10-second electrocardiograms for all ages. Front Physiol 2018;9:424,2828 Dantas EM, Kemp AH, Andreão RV, et al. Reference values for short-term resting-state heart rate variability in healthy adults: Results from the Brazilian Longitudinal Study of Adult Health-ELSA-Brasil study. Psychophysiology 2018;55(06):e13052
Data analysis
The normality of the data was tested by the Shapiro-Wilk test. For the sample characterization, the descriptive statistical method was used, and the results were presented as means and standard deviations (SD) for parametric data, median and interquartile range for non-parametric data, and absolute numbers and frequency for categorical data. The comparison of the sample profile, cardiorespiratory parameters, and HRV indices between groups, at rest, was made using the Student t-test or Mann-Whitney test. For the categorical data, the chi-square test was performed, considering the Yates continuity correction for 2 × 2 cross tables. The Cohen d effect size was calculated, and values over 0.2 and under 0.5 were considered as having small effect, between 0.5 and 0.8 a moderate effect, and higher than 0.8 a high effect.
To compare the effect of the active tilt test on the cardiorespiratory parameters and HRV indices, considering groups and moments, analysis of variance for repeated measures was used. Possible differences were identified by the Bonferroni post-hoc test. The effect size was calculated using Eta-squared (small effect: > 0.01 to < 0.06; moderate effect: ≥ 0.06 to < 0.14; high effect: ≥ 0.14). The level of significance adopted was < 5% and the statistical software used was the Statistical Package Social Sciences (SPSS Inc., Chicago, IL, USA), version 15.0.
RESULTS
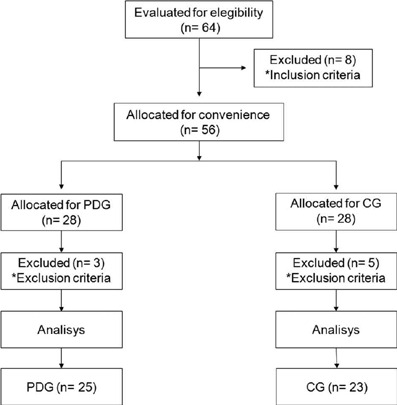
We analyzed 48 participants. The distribution and sample losses are described in ►Figure 1.
Sample loss flowchart. Representation of distribution and sample losses during study execution.
The characteristics of the groups are shown in ►Table 1. In both groups there was a male predominance (PDG: 84%, n = 21; CG: 82.6%, n = 19), and significant differences were found to BMI and MMSE (p < 0.001). Regarding the use of medication, significant differences were found for Levodopa and Dopamine agonist (►Table 2).
►Table 3 shows the comparison between groups for cardiorespiratory parameters and HRV indices at rest. Lower values ofSDNN, rMSSD, LFms2 HFms2 SD1, and SD2 (p < 0.05) were observed for PDG compared with CG. Regarding cardiorespiratory parameters, significant differences were observed for DBP and SpO2 (p < 0.05). Higher SpO2 values were found in the PDG.
Comparison between groups for the HRV indexes and cardiorespiratory parameters assessed at rest
►Table 4 shows the comparison between groups for HRV indices evaluated in TT1 and TT2. Differences between groups were observed for the SDNN, rMSSD, LFms2 HFms2 SD1, and SD2 (p < 0.05). Differences within moments were observed for rMSSD, HFms2 and SD1 (p < 0.05), with lower values in the active tilt test period, when compared with rest in both groups, and for SDNN, LFun, LF/HF, and SD1/SD2 (p < 0.05) indices, however with higher values in the active tilt test when compared with rest. Furthermore, an interaction between groups and moments was observed for rMSSD, HFms2 and SD1 indices (p < 0.05).
►Table 5 shows the values of the cardiorespiratory parameters in CR1, CR2, and CR3. Differences between groups were found for DBP and SpO2 (p < 0.05). Differences within moments were observed for SBP and HR (p < 0.05). For PDG, SBP values were higher in CR3 compared with CR2. For HR, the values were higher in CR2 and CR3 compared with CR1 in both groups, and lower in CR3 compared with CR2 in the CG.
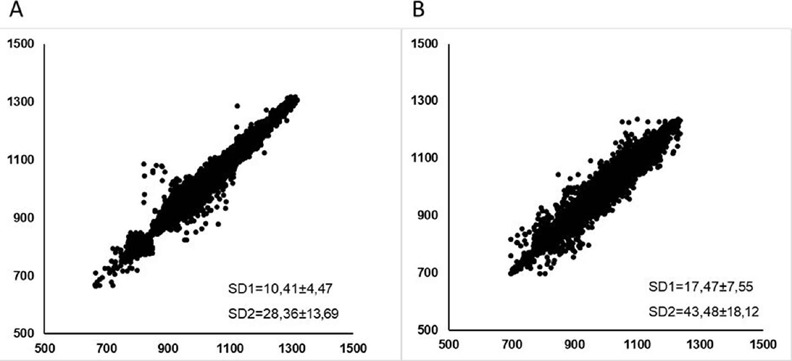
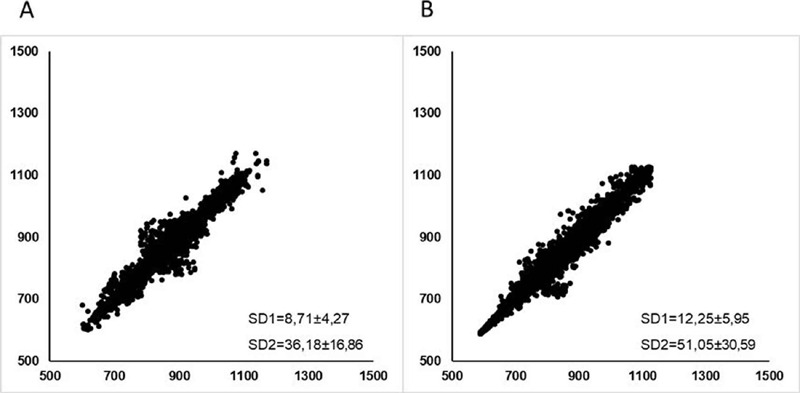
►Figures 2 and 3 correspond to the visual representation of the Poincaré Plot pattern of PDG and CG, at rest (2Aand2B) and during the active tilt test (3A and 3B), respectively. These representations required the RR intervals of all subjects examined in the study to plot the chart.
Visual representation of the Poincaré plot. Scatter plot that represents the qualitative analysis of Poincare plot in PDG (A) and CG (B) during rest.
Visual representation of the Poincaré plot. Scatter plot that represents the qualitative analysis of Poincare plot in PDG (A) and CG (B) during the active tilt test.
DISCUSSION
The main findings of this study suggest that, when at rest, individuals with PD have reduced global and parasympathetic modulation, lower DBP, and higher SpO2 when compared with healthy individuals. The autonomic responses to the active tilt test were characterized by reduced parasympathetic and increasedglobalmodulationinbothgroups; however,thePDG presented a lower parasympathetic responsetothe test, when compared with the CG. The cardiorespiratory responses were characterizedbyincreasedHRandreducedSBPinboth groups. Furthermore, the qualitative analysis of the Poincaré plot showed the PDG presents a lower dot dispersion at rest and in the active tilt test, when compared with the CG.
In the HRV analysis, at rest, the SDNN, LFms2 and SD2 indices, which represent a global modulation,55 Vanderlei LCM, PastreCM, Hoshi RA, CarvalhoTD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc 2009;24(02):205–217 were decreased in the PDG compared with the CG. These results, together with the reduction observed in the rMSSD, HFms2 and SD1, might indicate that individuals with PD present increased sympathetic and reduced parasympathetic modulation, suggesting a reduction in the complexity of the RR intervals series of the PDG. Similar results have been found by Rocha et al.,88 Rocha RSB, De Oliveira Rocha LS, Pena ESM, Caldas LCP, Moreno MA. Analysis of autonomic modulation of heart rate in patients with Parkinson’s disease and elderly individuals submitted to game therapy training. Geriatr Gerontol Int 2018;18(01):20–25 Stoco-Oliveira et al.,2929 Stoco-Oliveira MC, Ricci-Vitor AL, Vanzella LM, et al. Parkinson’s disease effect on autonomic modulation: an analysis using geometric indices. Arq Neuropsiquiatr 2021;79(02):114–121 and Harnold et al.3030 Harnod D, Wen SH, Chen SY, Harnod T. The association of heart rate variability with parkinsonian motor symptom duration. Yonsei Med J 2014;55(05):1297–1302
Cardiovascular autonomic dysregulation in PD has been attributed to involvement of both the central as well as the peripheral postganglionic autonomic nervous system.3131 Jost WH. Autonomic dysfunctions in idiopathic Parkinson’s disease. J Neurol 2003;250(Suppl 1):I28–I30 Solla et al.3232 Solla P, Cadeddu C, Cannas A, et al. Heart rate variability shows different cardiovascular modulation in Parkinson’s disease patients with tremor dominant subtype compared to those with akinetic rigid dominant subtype. J Neural Transm (Vienna) 2015;122(10):1441–1446 have suggested that the autonomic dysfunction present in PD is associated with significant increase in morbidity and mortality. Moreover, it is important to highlight that individuals with PD with impaired autonomic function may present impaired functional performance more quickly,3333 Lucetti C, Gambaccini G, Del Dotto P, et al. Long-term clinical evaluation in patients with Parkinson’s disease and early autonomic involvement. Parkinsonism Relat Disord 2006;12(05): 279–283 which highlights the importance of the autonomic assessment.
Inresponsetothe active tilt test,reduced rMSSD, HFms2and SD1 were observed in both groups, but the magnitude of the reduction was smaller for the PDG. These results are in accordance with Barbic et al.,3434 Barbic F, Perego F, Canesi M, et al. Early abnormalities of vascular and cardiac autonomic control in Parkinson’s disease without orthostatic hypotension. Hypertension 2007;49(01):120–126 demonstrating the impaired ANS adjustments in individuals with PD and indicating a diminished response of the parasympathetic modulation55 Vanderlei LCM, PastreCM, Hoshi RA, CarvalhoTD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc 2009;24(02):205–217 of these individuals in comparison to the CG. The impairment of ANS autonomic adjustments is harmful to the organism because it reveals its inability to provide adequate adaptive responses to stress, which removes the organism from homeostasis.55 Vanderlei LCM, PastreCM, Hoshi RA, CarvalhoTD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc 2009;24(02):205–217
Regarding the indices which represent the global modulation, our results show that individuals with PD presented increased SDNN, LFms2, and SD2 with the execution of the test. This increase is likely due to the SNA adaptation to the test. Alterations in HR are promoted by adjustments that occur in the sympathetic and parasympathetic SNA components. These alterations induce increased RR interval variations, justifying the increased global variability.
The visual (qualitative) analysis of the Poincaré plot (►Figures 2 and 3) shows a lower dispersion of the beat-to-beat intervals in the PDG, at rest and during the test, which can be noted by the shape of the plots, indicating impaired autonomic modulation at rest and reduced response capacity in the active tilt test. To our knowledge, this is the first study to assess autonomic test responses of individuals with PD using the qualitative and quantitative analysis of the Poincaré plot, which can assist the interpretation and analysis of the results, since in biological systems, non-linear behavior prevails.55 Vanderlei LCM, PastreCM, Hoshi RA, CarvalhoTD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc 2009;24(02):205–217
Regarding cardiorespiratory parameters, lower DBP values and higher SpO2 values for PDG were observed, at rest. Despite the difference found between PDG and controls, this result corresponds to normal values of DBP,2222 Malachias M, Plavnik FL, Machado CA, Malta D, Scala LCN, Fuchs S. 7th Brazilian Guideline of Arterial Hypertension: Chapter 1 -Concept, Epidemiology and Primary Prevention. Arq Bras Cardiol 2016;107(3, Suppl 3)1–6 suggesting individuals with PD do not have impairment in the PA regulation when at rest. This is the first study to consider the SpO2, and our results, when analyzed together with the information that none of the participants reported to have respiratory diseases, suggest the respiratory system of individuals with PD is not impaired. Furthermore, our findings are in accordance with previous studies, which have found normal results on respiratory spirometry assessments in individuals diagnosed with PD.3535 Strano S, Fanciulli A, Rizzo M, et al. Cardiovascular dysfunction in untreated Parkinson’s disease: A multi-modality assessment. J Neurol Sci 2016;370:251–255
Both groups showed increase HR immediately after the active tilt test and, after 10 minutes, the participants had not returned to the initial condition yet. However, in the CG the HR value after 10minutes was significantly lower than the HR value obtained after participants stood up, which did not occur in the PDG, suggesting a delay in HR recovery in individuals with PD. According to Roberson et al.,3636 Roberson KB, Signorile JF, Singer C, et al. Hemodynamic responses to an exercise stress test in Parkinson’s disease patients without orthostatic hypotension. Appl Physiol Nutr Metab 2019;44(07): 751–758 individuals with PD have impaired capacity to regulate HR during the recovery period. Our results corroborate with this statement and may be related to impairments in autonomic modulation caused by autonomic dysfunction, as evidenced by HRV results found in our study.
Both groups presented non-significant, reduced SBP values in the test. However, 10 minutes after the postural change, the PDG showed increased SBP. These results represent normal physiological changes of individuals with PD in the test, since the reduction of PA in the baroreceptors promotes an immediate reflex, which results in increased sympathetic activity and in increased PA.3737 Hall JE. Regulação nervosa da circulação e o controle rápido da pressão arterial. In: Hall JE. Tratado de Fisiologia Médica. 12.ed. Rio de Janeiro: Elsevier Ltd; 2011:219 Furthermore, greater SpO2 values were found in the PDG, and no significant changes were observed in both groups for RR, which represents a normal response, and it demonstrates the integrity of the participants’ respiratory system.
Some strengths of our study include the analysis of the autonomic modulation of individuals with PD in response to the active tilt test, an autonomic test that is more reproducible in clinical practice than the passive tilt test. Moreover, we measured respiratory parameters and utilized the qualitative and quantitative analysis of the Poincaré plot to assess autonomic modulation.
However, our study has some limitations. Due to the possible medication influence on autonomic regulation, the data shouldbeinterpreted with caution, since theparticipants used their habitual medication during the data collection (►Table 2). Furthermore, our study has not enabled the assessment of the influence of levodopa and dopamine agonists on the autonomic modulation of PD participants. Finally, diabetic participants were included in both groups. However, the number of diabetic participants was the same in both groups (n = 4), and significant differences for hypoglycemic values between groups were not observed (►Table 2).
The changes observed in the autonomic modulation and cardiorespiratory parameters in individuals with PD highlight the relevance of monitoring and evaluating the autonomic modulation in PD, which can be done by the active tilt test, as demonstrated in our study. Our results may help consolidate the active tilt test as a screening method, which can assist the choice of interventional therapies that require less postural changes. Studies comparing the autonomic modulation in response to the active tilt test and the passive tilt test can be also interesting in clinical practice.
In summary,our findings suggest that individuals with PD show reduced global variability and parasympathetic modulation when at rest, and reduced parasympathetic response and damage in HR regulation during the active tilt test, when compared with healthy individuals.
-
SupportThe present study was supported by the Programa Institucional de Bolsas de Iniciação Científica - PIBIC/Reitoria/ CNPq/UNESP.
References
-
1Kalia LV, Lang AE. Parkinson’s disease. Lancet 2015;386 (9996):896–912
-
2Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 2017;124(08):901–905
-
3Poewe W, Seppi K, TannerCM, etal. Parkinson disease. Nat Rev Dis Primers 2017;3:17013
-
4De Pablo-Fernandez E, Tur C, Revesz T, Lees AJ, Holton JL, Warner TT. Association of autonomic dysfunction with disease progression and survival in Parkinson disease. JAMA Neurol 2017;74(08): 970–976
-
5Vanderlei LCM, PastreCM, Hoshi RA, CarvalhoTD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc 2009;24(02):205–217
-
6Giacon TR, Vanderlei FM, Christofaro DGD, Vanderlei LCM. Impact of diabetes type 1 in children on autonomic modulation at rest and in response to the active orthostatic test. PLoS One 2016;11 (10):e0164375
-
7Jones PK, Gibbons CH. Autonomic function testing: an important diagnostic test for patients with syncope. Pract Neurol 2015;15 (05):346–351
-
8Rocha RSB, De Oliveira Rocha LS, Pena ESM, Caldas LCP, Moreno MA. Analysis of autonomic modulation of heart rate in patients with Parkinson’s disease and elderly individuals submitted to game therapy training. Geriatr Gerontol Int 2018;18(01):20–25
-
9Ke JQ, Shao SM, Zheng YY, Fu FW, Zheng GQ, Liu CF. Sympathetic skin response and heart rate variability in predicting autonomic disorders in patients with Parkinson disease. Medicine (Baltimore) 2017;96(18):e6523
-
10Bouhaddi M, Vuillier F, Fortrat JO, et al. Impaired cardiovascular autonomic control in newly and long-term-treated patients with Parkinson’s disease: involvement of L-dopa therapy. Auton Neurosci 2004;116(1-2):30–38
-
11Watano C, Shiota Y, Onoda K, et al. Evaluation of autonomic functions of patients with multiple system atrophy and Parkinson’s disease by head-up tilt test. J Neural Transm (Vienna) 2018; 125(02):153–162
-
12Moura-Tonello SCG, Takahashi ACM, Francisco CO, et al. Influence of type 2 diabetes on symbolic analysis and complexity of heart rate variability in men. Diabetol Metab Syndr 2014;6(01):13
-
13de Rezende Barbosa MPC, Júnior JN, Cassemiro BM, et al. Effects of functional training on geometric indices of heart rate variability. J Sport Health Sci 2016;5(02):183–189
-
14Catai AM, Pastre CM, Godoy MF, Silva ED, Takahashi ACM, Vanderlei LCM. Heart rate variability: are you using it properly? Standardisation checklist of procedures. Braz J Phys Ther 2020;24 (02):91–102
-
15Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17(05):427–442
-
16Bertolucci PHF, Brucki SMD, Campacci SR, Juliano Y. O Mini-Exame do Estado Mental em uma população geral. Impacto da escolaridade. Arq Neuropsiquiatr 1994;52(01):1–7
-
17Brisinda D, Sorbo AR, Di Giacopo R, Venuti A, Bentivoglio AR, Fenici R. Cardiovascular autonomic nervous system evaluation in Parkinson disease and multiplesystem atrophy. JNeurol Sci 2014; 336(1-2):197–202
-
18Associação Brasileira para o Estudo da Obesidade e da Síndrome Metabólica. Diretrizes brasileiras de obesidade.4°ed. São Paulo, SPABESO2016
-
19Ferreira MG, Valente JG, Gonçalves-Silva RMV, Sichieri R. [Accuracyof waist circumference and waist-to-hipratioaspredictors of dyslipidemia in a cross-sectional study among blood donors in Cuiabá, Mato Grosso State, Brazil]. Cad Saude Publica 2006;22 (02):307–314
-
20Goetz CG, Tilley BC, Shaftman SR, et al; Movement Disorder Society UPDRS Revision Task Force. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23(15):2129–2170
-
21Carpenter MG, Allum JHJ, Honegger F, Adkin AL, Bloem BR. Postural abnormalities to multidirectional stance perturbations in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2004;75 (09):1245–1254
-
22Malachias M, Plavnik FL, Machado CA, Malta D, Scala LCN, Fuchs S. 7th Brazilian Guideline of Arterial Hypertension: Chapter 1 -Concept, Epidemiology and Primary Prevention. Arq Bras Cardiol 2016;107(3, Suppl 3)1–6
-
23Humberstone N, Tecklin JS. Avaliação Respiratória. Irwin S, Tecklin JS, organizadores. Fisioterapia cardiopulmonar. 3.ed. São Paulo: Manole; 2003. p. 334–55
-
24Kiviniemi AM, Hautala AJ, Kinnunen H, Tulppo MP. Endurance training guided individually by daily heart rate variability measurements. Eur J Appl Physiol 2007;101(06):743–751
-
25Barreto GSC, Vanderlei FM, Vanderlei LCM, Leite AJM. Impact of malnutrition on cardiac autonomic modulation in children. J Pediatr (Rio J) 2016;92(06):638–644
-
26Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV–heart rate variability analysis software. Comput Methods Programs Biomed 2014;113(01):210–220
-
27van den Berg ME, Rijnbeek PR, Niemeijer MN, et al. Normal values of corrected heart-rate variability in 10-second electrocardiograms for all ages. Front Physiol 2018;9:424
-
28Dantas EM, Kemp AH, Andreão RV, et al. Reference values for short-term resting-state heart rate variability in healthy adults: Results from the Brazilian Longitudinal Study of Adult Health-ELSA-Brasil study. Psychophysiology 2018;55(06):e13052
-
29Stoco-Oliveira MC, Ricci-Vitor AL, Vanzella LM, et al. Parkinson’s disease effect on autonomic modulation: an analysis using geometric indices. Arq Neuropsiquiatr 2021;79(02):114–121
-
30Harnod D, Wen SH, Chen SY, Harnod T. The association of heart rate variability with parkinsonian motor symptom duration. Yonsei Med J 2014;55(05):1297–1302
-
31Jost WH. Autonomic dysfunctions in idiopathic Parkinson’s disease. J Neurol 2003;250(Suppl 1):I28–I30
-
32Solla P, Cadeddu C, Cannas A, et al. Heart rate variability shows different cardiovascular modulation in Parkinson’s disease patients with tremor dominant subtype compared to those with akinetic rigid dominant subtype. J Neural Transm (Vienna) 2015;122(10):1441–1446
-
33Lucetti C, Gambaccini G, Del Dotto P, et al. Long-term clinical evaluation in patients with Parkinson’s disease and early autonomic involvement. Parkinsonism Relat Disord 2006;12(05): 279–283
-
34Barbic F, Perego F, Canesi M, et al. Early abnormalities of vascular and cardiac autonomic control in Parkinson’s disease without orthostatic hypotension. Hypertension 2007;49(01):120–126
-
35Strano S, Fanciulli A, Rizzo M, et al. Cardiovascular dysfunction in untreated Parkinson’s disease: A multi-modality assessment. J Neurol Sci 2016;370:251–255
-
36Roberson KB, Signorile JF, Singer C, et al. Hemodynamic responses to an exercise stress test in Parkinson’s disease patients without orthostatic hypotension. Appl Physiol Nutr Metab 2019;44(07): 751–758
-
37Hall JE. Regulação nervosa da circulação e o controle rápido da pressão arterial. In: Hall JE. Tratado de Fisiologia Médica. 12.ed. Rio de Janeiro: Elsevier Ltd; 2011:219
Publication Dates
-
Publication in this collection
21 Nov 2022 -
Date of issue
July 2022
History
-
Received
12 Aug 2021 -
Accepted
07 Nov 2021