Abstracts
Reduction of excitability of the dominant primary motor cortex (M1) improves ipsilateral hand function in healthy subjects. In analogy, inhibition of non-dominant M1 should also improve ipsilateral performance. In order to investigate this hypothesis, we have used slow repetitive transcranial magnetic stimulation (rTMS) and the Purdue Pegboard test. Twenty-eight volunteers underwent 10 minutes of either 0.5Hz rTMS over right M1 or sham rTMS (coil perpendicular to scalp). The motor task was performed before, immediately after, and 20 minutes after rTMS. In both groups, motor performance improved significantly throughout the sessions. rTMS inhibition of the non-dominant M1 had no significant influence over ipsilateral or contralateral manual dexterity, even though the results were limited by unequal performance between groups at baseline. This is in contrast to an improvement in left hand function previously described following slow rTMS over left M1, and suggests a less prominent physiological transcallosal inhibition from right to left M1.
low-frequency transcranial magnetic stimulation; rTMS; Purdue Pegboard; primary motor cortex; M1
A redução da excitabilidade do córtex motor primário (M1) dominante melhora o desempenho manual ipsilateral: a inibição do M1 não-dominante poderia, analogamente, aprimorar a função manual direita. Para investigar esta hipótese, utilizou-se a estimulação magnética transcraniana repetitiva (EMTr) de baixa frequência e o teste Purdue Pegboard. Submetemos 28 voluntários a 10 minutos de EMTr sobre o M1 direito (0,5 Hz) ou a EMTr placebo (bobina perpendicular ao escalpo). O teste foi executado antes, imediatamente após e 20 minutos após a EMTr. Nos dois grupos, o desempenho manual mostrou significativa melhora entre as sessões. A inibição do M1 não-dominante não influenciou significativamente a destreza motora ipsi ou contralateral, apesar da conclusão limitada pelo desempenho discrepante dos grupos na primeira sessão. Este resultado contrasta com a melhora da função manual esquerda descrita após a EMTr sobre o M1 esquerdo e sugere uma inibição transcalosa fisiológica menos intensa do M1 direito para o esquerdo.
estimulação magnética transcraniana de baixa freqüência; EMTr; córtex motor primário; M1; Purdue Pegboard
Low frequency (0.5Hz) rTMS over the right (non-dominant) motor cortex does not affect ipsilateral hand performance in healthy humans
A inibição do córtex motor não-dominante por meio da estimulação magnética transcraniana a 0,5Hz não interfere no desempenho manual ipsilateral de sujeitos hígidos
Fernanda WeilerI; Pedro BrandãoI; Jairo de Barros-FilhoI; Carlos Enrique UribeII; Valdir Filgueiras PessoaIII; Joaquim Pereira Brasil-NetoIV
IUndergraduate medical student, Institutional Scientific Initiation Scholarship Program - UnB - Neurosciences and Behavior Laboratory, Physiological Sciences Department, Biology Institute, University of Brasília (UnB), Brasília DF, Brazil
IIMD, MSc, Neurosciences and Behavior Laboratory - UnB - Neurosciences and Behavior Laboratory, Physiological Sciences Department, Biology Institute, University of Brasília (UnB), Brasília DF, Brazil
IIIMD, PhD, Professor of Medical Neurophysiology, Neurosciences and Behavior Laboratory - UnB - Neurosciences and Behavior Laboratory, Physiological Sciences Department, Biology Institute, University of Brasília (UnB), Brasília DF, Brazil
IVMD, PhD, Professor of Medical Neurophysiology, Neurosciences and Behavior Laboratory - UnB. Financial support: CNPq's and UnB's Institutional Scientific Initiation Scholarship Program - Neurosciences and Behavior Laboratory, Physiological Sciences Department, Biology Institute, University of Brasília (UnB), Brasília DF, Brazil
ABSTRACT
Reduction of excitability of the dominant primary motor cortex (M1) improves ipsilateral hand function in healthy subjects. In analogy, inhibition of non-dominant M1 should also improve ipsilateral performance. In order to investigate this hypothesis, we have used slow repetitive transcranial magnetic stimulation (rTMS) and the Purdue Pegboard test. Twenty-eight volunteers underwent 10 minutes of either 0.5Hz rTMS over right M1 or sham rTMS (coil perpendicular to scalp). The motor task was performed before, immediately after, and 20 minutes after rTMS. In both groups, motor performance improved significantly throughout the sessions. rTMS inhibition of the non-dominant M1 had no significant influence over ipsilateral or contralateral manual dexterity, even though the results were limited by unequal performance between groups at baseline. This is in contrast to an improvement in left hand function previously described following slow rTMS over left M1, and suggests a less prominent physiological transcallosal inhibition from right to left M1.
Key words: low-frequency transcranial magnetic stimulation, rTMS, Purdue Pegboard, primary motor cortex, M1.
RESUMO
A redução da excitabilidade do córtex motor primário (M1) dominante melhora o desempenho manual ipsilateral: a inibição do M1 não-dominante poderia, analogamente, aprimorar a função manual direita. Para investigar esta hipótese, utilizou-se a estimulação magnética transcraniana repetitiva (EMTr) de baixa frequência e o teste Purdue Pegboard. Submetemos 28 voluntários a 10 minutos de EMTr sobre o M1 direito (0,5 Hz) ou a EMTr placebo (bobina perpendicular ao escalpo). O teste foi executado antes, imediatamente após e 20 minutos após a EMTr. Nos dois grupos, o desempenho manual mostrou significativa melhora entre as sessões. A inibição do M1 não-dominante não influenciou significativamente a destreza motora ipsi ou contralateral, apesar da conclusão limitada pelo desempenho discrepante dos grupos na primeira sessão. Este resultado contrasta com a melhora da função manual esquerda descrita após a EMTr sobre o M1 esquerdo e sugere uma inibição transcalosa fisiológica menos intensa do M1 direito para o esquerdo.
Palavras-chave: estimulação magnética transcraniana de baixa freqüência, EMTr, córtex motor primário, M1, Purdue Pegboard.
Transcranial magnetic stimulation, first described by Barker et al. in 1985, is a non-invasive painless technique for central nervous system stimulation. It involves a rapidly changing magnetic field, generated by a special coil placed close to the scalp, which induces a secondary and focal electric current at the cortical level, according to Faraday's law1. It may be used to apply single pulses of cortical stimulation, paired stimuli at variable intervals or trains of repetitive pulses at various frequencies. The first two are commonly used to study electrophysiological parameters such as cortico-spinal tract excitability, motor evoked potentials, silent periods, central motor conduction time, transcallosal conduction2. They have been increasingly used for clinical diagnostic purposes3. The latter, namely repetitive transcranial magnetic stimulation (rTMS), produces cortical modulation of excitability associated to measurable effects that usually last beyond the period of stimulation. It has been widely studied as therapy for neurological and psychiatric diseases, such as epilepsy4, depression5,6, chronic pain7, stroke8,9, dystonia and other movement disorders10. rTMS is also a useful tool to investigate active neuroplasticity, by altering excitability and function of the stimulated cortex or its connections11. These effects are, however, determined by the frequency of stimuli. Low frequencies (slow rTMS), up to 1 Hz, promote long lasting suppression of motor cortical excitability, while high frequencies (fast rTMS), above 1 Hz, lead to transient increases in cortical excitability2,12. The primary motor cortex (M1) is fundamental for short-term motor learning13. Functional studies with single and paired pulse TMS13,14 have shown changes in M1 excitability during the acquisition phase of learning complex motor skills, indicating a reorganization process over M1.
There is growing evidence for asymmetries in the role of the dominant vs. the non-dominant M1 for unimanual tasks performed with either the left or the right hand, especially among right-handers15-17. Hanna-Pladdy et al.18, while investigating precise coordinated finger movements in patients with damaged right or left hemisphere, showed more substantial impairment of ipsilateral deftness in those subjects that had lesion in the left hemisphere. Slow rTMS above motor threshold decreases transcallosal interhemispheric inhibition19, in an effect thought to be bidirectional, more significant from the stimulated to the non-stimulated side. Some authors suggest that there is an interhemispheric rivalry, demonstrated by improvement in performance of the ipsilateral hand (left) following 10 minutes of slow rTMS over the dominant motor cortex20. The improved performance is explained by a disinhibition of the contralateral M1 (non-dominant), due to a transient decrease in transcallosal inhibition. However, most studies dealing with interhemispheric motor physiology have evaluated the influence of dominant (left) M1 over ipsilateral hand function and there are still uncertainties concerning the exact dynamics of the physiological balance between the cerebral hemispheres21,22. Here we intended to investigate, then, functional consequences of manipulating the excitability of the non-dominant, right, cerebral hemisphere of normal human subjects. Specifically, we verified whether inhibition of the non-dominant M1 with repetitive transcranial magnetic stimulation (rTMS) affected ipsilateral fine motor performance in a classical hand coordination task (Purdue Pegboard test), compared to the effect over contralateral performance.
We expected that slow rTMS would impair performance gains of the contralateral hand it should be able to decrease cortical excitability during motor learning, competing with the practice-dependent increase in M1 excitability that usually occurs. We also hypothesized that ipsilateral hand performance would be improved by rTMS possibly by releasing the left hemisphere from transcallosal inhibition exerted by right M1.
METHOD
Subjects
Twenty-eight healthy male volunteers, ranging in age from 18 to 29 years (mean age=21.93±2.29 years), all right-handed according to the Edinburgh Handedness Inventory23 (Mean laterality quotient=+78.87±18.06), were recruited. Just dextrals were included because of higher heterogeneity for hemisphere dominance in sinisters: 9095% of the right-handers have left-hemisphere dominance while right-hemisphere dominance is found in 27% of left-handers24. No subject had a history of neurological or psychiatric disease or contraindications for TMS, such as intracranial metallic or magnetic implants, pacemakers, or any other implanted devices25. They gave written informed consent prior to the study and were naïve as to the task and to the TMS. The protocol was approved by the local institutional ethics committee.
Apparatus
Subjects were submitted to a fine motor learning task, using the Purdue Pegboard (Lafayette Instruments model 32020, Lafayette, IN, USA). The height of the chair and position of the pegboard were adjusted to allow unrestricted, comfortable movement of the arm over the entire surface of the pegboard. The task was performed in a quiet room, free of noise. Only one of the authors, present in the room at the moment of the task, gave instructions and recorded task scores. This investigator was blind to the group (experimental or sham) to which each subject belonged.
rTMS procedure
For the rTMS paradigm, subjects were sitting comfortably in a chair, in another quiet room. A Dantec MagLite magnetic stimulator (Skovlunde, Denmark) connected to a figure-of-eight shaped coil, placed tangentially to the subject's scalp with the handle pointing backward and laterally at an approximate angle of 45º to the midsagittal line, was used to deliver stimuli at an intensity of 80% of the subject's resting motor threshold (MT). The volunteers were instructed to maintain muscle relaxation throughout the stimulation period. The MT was defined as the lowest stimulus intensity capable of producing at least 5 visible contractions of the relaxed first dorsal interosseus (FDI) muscle, in a total of 10 stimuli.
Experimental group
Focal 0.5 Hz rTMS was performed, according to current safety recommendations25. The rTMS train consisted of 300 pulses, during 10 minutes, and was delivered to the right M1 (Fig 1), defined as the optimal scalp position to activate the FDI muscle of the contralateral hand.
Sham (Placebo) group
The coil was oriented in a perpendicular position over the scalp, for ten minutes, with a stimulus intensity of 10% of MT. This position and intensity were chosen because they maintain the "click" noise, although they are devoid of neurophysiologic effect26.
Assessment of motor performance
Each subject was submitted to 3 sessions of testing in the pegboard (Fig 2), at 3 different times: before rTMS (Pre or baseline), immediately after rTMS (+0), and 20 minutes after rTMS (+20).
Each session consisted of nine 30 seconds trials of the Purdue Pegboard test, assessing fine movements of the right hand (RH), left hand (LH) and both hands (BH). RH, LH and BH were combined in a random sequence and, in each session, the sequence was repeated 3 times, i.e. the subject had to perform a total of 9 trials. The intertrial interval (ITI) was approximately 30 s, to minimize fatigue. The procedure was carried out as follows: the subject picked individual pegs from a well using the thumb and index finger and placed them in individual holes in the pegboard. Subjects were encouraged to place as many pegs as possible and the number of pegs placed on each trial was recorded. The order of the hand tested was counterbalanced across subjects. One minute of practice was allowed for each subject before the first session, in order to make him acquainted with the experimental setup.
Statistical analysis
The mean score of the three trials for each hand during each session was calculated for each subject. These data were analyzed in a mixed-design three-way ANOVA (session × hand × group). Baseline for each condition was calculated as the mean score in the Pegboard of the three trials for each hand before rTMS. Significance level for all tests was set at p<0.05. Bonferroni method for adjustment of the significance level was used where applicable.
RESULTS
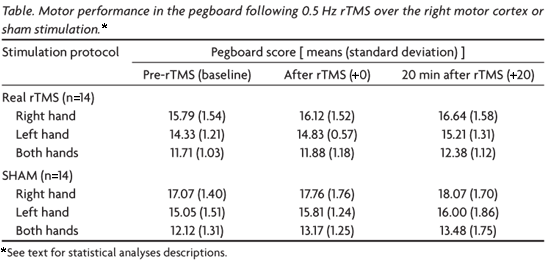
None of the subjects experienced any adverse effects during or after the rTMS procedure. The means and standard deviation of the pegboard scores for each intervention are summarized, in Table, and divided by session and hand used.
We found a significant effect for the session factor (p<0.001) over performance. The multiple comparisons procedure showed significant differences between all sessions, being the performance in (Pre-rTMS) worse than that at [+0], which, in turn, was worse than that at (+20) (p<0.004 for all comparisons).
The hand factor (right, left or both) also influenced performance (p<0.001). The multiple comparisons procedure showed significant statistical difference between the three conditions; the mean pegboard score with the right hand was better than that for the left hand, which in turn was better than that for both hands (p<0.001 for all comparisons).
The group factor also had a significant influence on overall performance. It was found that the group submitted to the slow rTMS paradigm had a worse performance when compared to the sham group (p=0.028). However, it was noted that baseline performance had been better in the sham group, and this was a potentially confounding factor.
Interactions between session × group, hand × group, session × hand, session × hand × group did not exert any significant effect over the pegboard performance (p<0.107 for all comparisons).
A second analysis was, then, performed: a mixed design ANOVA 2 × 3 × 2 (session × hand × group), including the performance with the right hand in the baseline session (RH-s1) as a confounding variable.
There was a significant effect of the session factor over performance (p=0.033). The multiple comparisons procedure showed that there was an increase in performance from time (+0) to (+20) (p=0.001).
As in the previous analysis, we also encountered an effect of the hand factor over performance (p=0.042), scores with the right hand being better than those with the left hand, which were better than when both hands were used (p<0.001).
The variable RH-s1 exerted a statistically significant influence (p<0.001), meaning that the difference seen in the baseline results between groups might be affecting the results in an independent manner.
After including the control for the confounding factor (RH-s1) in the analysis, the group factor no longer had a significant influence over the pegboard score, i.e., the general performance of the sham group did not significantly differ from that of the real rTMS group (p=0.347).
DISCUSSION
Subjects improved their performance on the pegboard task progressively over the three sessions. This result indicates that simple practice alone improves performance on the pegboard task, i.e. there is a discernible motor learning effect.
We have also shown an overall better performance with the right hand than with the left or both hands. This might be expected given the right-handedness of the subjects.
It is reasonable to assume that the task involved predominantly "short-term learning", as the subjects had no intensive practice before the experiment and it was not long enough to produce a motor learning stagnation, i.e. the pegboard score did not reach a plateau. Our main hypothesis was that 0.5Hz rTMS might hinder performance gains by impairing the already proven practice-dependent increase in M1 excitability associated with learning of new motor skills13. On the other hand, a decrease in transcallosal inhibition might result in performance improvements of the ipsilateral (right) hand.
Our results show, however, that slow rTMS over the right M1 did not significantly influence manual dexterity in the Purdue Pegboard test; moreover, it did not seem to affect short-term motor learning.
This result raises the question: does the concept of inter-hemispheric rivalry also apply when we analyze interference from the non-dominant cerebral hemisphere upon the dominant one?
Previous studies have shown that transcallosal inhibition of the contralateral M1 plays a more relevant role when exerted by the left, dominant, hemisphere, which inhibits the right hemisphere and prevents mirror activity and overflow of movements20,27,28. Our 0.5Hz rTMS protocol may have failed to reveal changes in ipsilateral performance because the non-dominant M1 exerts a much weaker transcallosal inhibitory effect29,30, especially if in association with performance of skilled movements31. Stronger transcallosal excitability transfer from the dominant M1 to the non-dominant M1 may be also noticed when exploring post-exercise ipsilateral facilitation of motor evoked potentials, a phenomenon that is present if the exercised hand is the right and absent if it is the left hand28,30.
Our results differ from those reported by Kobayashi et al.20, who reported improvement in performance of the left hand after slow rTMS over the left M1. This again seems to confirm the hypothesis that it is the left hemisphere that exerts a significant degree of inhibition upon the non-dominant (right) motor cortex.
High frequency rTMS at 80% of MT, applied to the contralateral motor cortex (right), significantly improved motor learning and facilitated complex movements of the left hand32. It could be expected that, in analogy, low frequency rTMS might have the inverse effect, but no changes in performance gains were produced in our study.
Previous investigations have demonstrated an improvement in hand function of hemiparetic stroke patients when the unaffected hemisphere was inhibited by low-frequency rTMS, even when the deficits were on the right side of the body8,9. It is possible that, when there is damage to the left cerebral hemisphere, the normally weak transcallosal inhibitory effects exerted by the right hemisphere become much more significant.
In summary, slow rTMS applied over the right M1 did not lead to impairments or improvements in hand dexterity in normal human volunteers, as assessed by the Purdue Pegboard test. We suggest that, in normal subjects, transcallosal inhibitory phenomena are much more prominent from the left to the right M1, but that this may change in neurological patients. It would be interesting to see if these results also hold when only left-handers are studied; the dominant hemisphere, however, would have to be ascertained by methods such as high-frequency rTMS over Broca's area, which is able to call speech arrest33. A more detailed knowledge of inter-hemispheric relationships will be very important for the design of new therapeutic interventions for stroke and other hemispheric pathologies.
Received 18 February 2008, received in final form 12 June 2008. Accepted 11 July 2008.
Dr. Joaquim Pereira Brasil-Neto Neurosciences and Behavior Laboratory / Physiological Sciences Department / Biology Institute / University of Brasília / Asa Norte - 70919-970 Brasília DF - Brasil. E-mail: jbrasil@unb.br
- 1. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985;1:1106-1107.
- 2. Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol 2003;2:145-156.
- 3. Chen R, Cros D, Curra A, et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 2008;119:504-532.
- 4. Brasil-Neto JP, Araújo DP, Teixeira WA, Araújo VP, Boechat-Barros R. Experimental therapy of epilepsy with transcranial magnetic stimulation: lack of additional benefit with prolonged treatment. Arq Neuropsiquiatr. 2004;62:21-25.
- 5. Boechat-Barros R, Brasil-Neto JP. Transcranial Magnetic Stimulation in depression: results of bi-weekly treatment. Rev Bras Psiquiatr 2004;26:100-102.
- 6. Pascual-Leone A, Rubio B, Pallardó F, Catalá MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 1996;348:233-237.
- 7. Fregni F, Freedman S, Pascual-Leone A. Recent advances in the treatment of chronic pain with non-invasive brain stimulation techniques. Lancet Neurol. 2007;6:188-191.
- 8. Mansur CG, Fregni F, Boggio PS, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology 2005;64:1802-1804.
- 9. Fregni F, Boggio PS, Valle AC, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke 2006;37:2115-2122.
- 10. Cantello R. Applications of transcranial magnetic stimulation in movement disorders. J Clin Neurophysiol 2002;19:272-293.
- 11. Ziemann U. TMS induced plasticity in human cortex. Rev Neurosci 2004;15:253-266.
- 12. Valero-Cabré A, Pascual-Leone A. Impact of TMS on the primary motor cortex and associated spinal systems. IEEE Eng Med Biol Mag 2005; 24:29-35.
- 13. Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res 2001; 136:431-438.
- 14. Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 1995;74:1037-1045.
- 15. Triggs WJ, Subramanium B, Rossi F. Hand preference and transcranial magnetic stimulation asymmetry of cortical motor representation. Brain Res 1999;835:324-329.
- 16. Civardi C, Cavalli A, Naldi P, Varrasi C, Cantello R. Hemispheric asymmetries of cortico-cortical connections in human hand motor areas. Clin Neurophysiol 2000;111:624-629.
- 17. Ghacibeh GA, Mirpuri R, Drago V, Jeong Y, Heilman KM, Triggs WJ. Ipsilateral motor activation during unimanual and bimanual motor tasks. Clin Neurophysiol 2007;118:325-332.
- 18. Hanna-Pladdy B, Mendoza JE, Apostolos GT, Heilman KM. Lateralised motor control: hemispheric damage and the loss of deftness. J Neurol Neurosurg Psychiatry 2002;73: 574-577.
- 19. Pal PK, Hanajima R, Gunraj CA, et al. Effect of low-frequency repetitive transcranial magnetic stimulation on interhemispheric inhibition. J Neurophysiol 2005;94:1668-1675.
- 20. Kobayashi M, Hutchinson S, Theoret H, Schlaug G, Pascual-Leone A. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology 2004;62:91-99.
- 21. Kobayashi M, Hutchinson S, Schlaug G, Pascual-Leone A. Ipsilateral motor cortex activation on functional magnetic resonance imaging during unilateral hand movements is related to interhemispheric interactions. Neuroimage 2003;20:2259-2270.
- 22. Carey JR, Fregni F, Pascual-Leone A. rTMS combined with motor learning training in healthy subjects. Restor Neurol Neurosci 2006;24:191-199.
- 23. Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971;1:97-113.
- 24. Knecht S, Dräger B, Deppe M, et al. Handedness and hemispheric language dominance in healthy humans. Brain 2000;123:2512-2518.
- 25. Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 57, 1996. Electroencephalogr Clin Neurophysiol 1998;108:1-16.
- 26. Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA. Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol Psychiatry 2001;49:460-463.
- 27. Chen R, Gerloff C, Hallett M, Cohen LG. Involvement of the ipsilateral motor cortex in finger movements of different complexities. Ann Neurol 1997;41:247-254.
- 28. Brasil-Neto JP, Araújo VP, Carneiro CR. Postexercise facilitation of motor evoked potentials elicited by ipsilateral voluntary contraction. Muscle Nerve 1999;22:1710-1712.
- 29. Netz J, Ziemann U, Hömberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res 1995;104:527-533.
- 30. Samii A, Caños M, Ikoma K, Wassermann EM, Hallett M. Absence of facilitation or depression of motor evoked potentials after contralateral homologous muscle activation. Electroencephalogr Clin Neurophysiol 1997;105:241-245.
- 31. Duque J, Murase N, Celnik P, et al. Intermanual differences in movement-related interhemispheric inhibition. J Cogn Neurosci 2007;19: 204-213.
- 32. Kim YH, Park JW, Ko MH, Jang SH, Lee PK. Facilitative effect of high frequency subthreshold repetitive transcranial magnetic stimulation on complex sequential motor learning in humans. Neurosci Lett 2004; 367:181-185.
- 33. Pascual-Leone A, Gates JR, Dhuna A. Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology 1991;41:697-702.
Publication Dates
-
Publication in this collection
15 Oct 2008 -
Date of issue
2008
History
-
Accepted
11 July 2008 -
Received
18 Feb 2008 -
Reviewed
12 June 2008