Abstracts
OBJECTIVE: To compare voice and life quality of male patients with idiopathic Parkinson's disease, with individuals without disease (Control Group). METHODS: A cross-sectional study that evaluated the voice of individuals with Parkinson's disease, the group was composed of 27 subjects, aged from 39 to 79 years-old (average 59.96). The Control Group was matched on sex and age. Participants underwent voice recording. Perceptual evaluation was made using GRBASI scale, which considers G as the overall degree of dysphonia, R as roughness, B as breathiness, A as asthenia, S as strain and I as instability. The acoustic parameters analyzed were: fundamental frequency, jitter, shimmer, and harmonic to noise ratio (NHR). For vocal self-perception analysis, we used the Voice Related Quality of Life protocol. RESULTS: Fundamental frequency and jitter presented higher values in the Parkinson's group. NHR values were higher in the Control Group. Perceptual analysis showed a deviation ranging. The vocal disorder self-perception demonstrated a worse impact on quality of life. CONCLUSIONS: Individuals with Parkinson's disease have an altered voice quality and a negative impact on quality of life.
Parkinson disease; voice; quality of life; dysphonia
OBJETIVO: Comparar a qualidade vocal e a qualidade de vida entre pacientes do sexo masculino com doença de Parkinson idiopática e indivíduos sem a doença (Grupo Controle). MÉTODOS: Estudo transversal que avaliou a voz de 27 indivíduos com doença de Parkinson, com idades entre 39 a 79 anos (média de 59,96). O Grupo Controle foi pareado em sexo e idade. Avaliação perceptiva foi feita usando escala GRBASI, que considera G como o grau global da disfonia, R como a rugosidade, B como soprosidade, A como astenia, S como tensão e I como instabilidade. Os parâmetros acústicos analisados foram: frequência fundamental, jitter, shimmer e harmonic to noise ratio (NHR). Para análise da autopercepção vocal, utilizou-se o protocolo Qualidade de Vida e Voz. RESULTADOS: As medidas frequência fundamental e jitter apresentaram valores mais altos no grupo com doença de Parkinson. Valores de NHR foram maiores no Grupo Controle. Análise perceptivo-auditiva mostrou desvio da qualidade vocal. O distúrbio de autopercepção vocal demonstrou impacto negativo na qualidade de vida. CONCLUSÕES: Indivíduos com doença de Parkinson idiopática apresentam qualidade vocal alterada e impacto negativo na qualidade de vida.
doença de Parkinson; voz; qualidade de vida; disfonia
ARTICLE
Idiopathic Parkinson's disease: vocal and quality of life analysis
Doença de Parkinson idiopática: análise vocal e da qualidade de vida
Luiza Furtado e SilvaI; Ana Cristina Côrtes GamaII; Francisco Eduardo Costa CardosoIII; César Augusto da Conceição ReisIV; Iara Barreto BassiV
ISpeech and Language Therapist, UFMG, Belo Horizonte MG, Brazil
IISpeech and Language Therapist, Full Professor of the Department of Speech and Language Therapy, UFMG, Belo Horizonte MG, Brazil
IIINeurologist, Full Professor of the Department of Medical Clinical, UFMG, Belo Horizonte MG, Brazil
IVFull Professor of Linguistics, Language and Arts, UFMG, Belo Horizonte MG, Brazil
VSpeech and Language therapist, PhD in progress in Public Health, UFMG, Belo Horizonte MG, Brazil
Correspondence Correspondence: Iara Barreto Bassi Universidade Federal de Minas Gerais Faculdade de Medicina Avenida Alfredo Balena 190 / sala 733 30130-100 Belo Horizonte MG - Brasil E-mail: iara.bassi@hotmail.com
ABSTRACT
OBJECTIVE: To compare voice and life quality of male patients with idiopathic Parkinson's disease, with individuals without disease (Control Group).
METHODS: A cross-sectional study that evaluated the voice of individuals with Parkinson's disease, the group was composed of 27 subjects, aged from 39 to 79 years-old (average 59.96). The Control Group was matched on sex and age. Participants underwent voice recording. Perceptual evaluation was made using GRBASI scale, which considers G as the overall degree of dysphonia, R as roughness, B as breathiness, A as asthenia, S as strain and I as instability. The acoustic parameters analyzed were: fundamental frequency, jitter, shimmer, and harmonic to noise ratio (NHR). For vocal self-perception analysis, we used the Voice Related Quality of Life protocol.
RESULTS: Fundamental frequency and jitter presented higher values in the Parkinson's group. NHR values were higher in the Control Group. Perceptual analysis showed a deviation ranging. The vocal disorder self-perception demonstrated a worse impact on quality of life.
CONCLUSIONS: Individuals with Parkinson's disease have an altered voice quality and a negative impact on quality of life.
Key words: Parkinson disease, voice, quality of life, dysphonia.
RESUMO
OBJETIVO: Comparar a qualidade vocal e a qualidade de vida entre pacientes do sexo masculino com doença de Parkinson idiopática e indivíduos sem a doença (Grupo Controle).
MÉTODOS: Estudo transversal que avaliou a voz de 27 indivíduos com doença de Parkinson, com idades entre 39 a 79 anos (média de 59,96). O Grupo Controle foi pareado em sexo e idade. Avaliação perceptiva foi feita usando escala GRBASI, que considera G como o grau global da disfonia, R como a rugosidade, B como soprosidade, A como astenia, S como tensão e I como instabilidade. Os parâmetros acústicos analisados foram: frequência fundamental, jitter, shimmer e harmonic to noise ratio (NHR). Para análise da autopercepção vocal, utilizou-se o protocolo Qualidade de Vida e Voz.
RESULTADOS: As medidas frequência fundamental e jitter apresentaram valores mais altos no grupo com doença de Parkinson. Valores de NHR foram maiores no Grupo Controle. Análise perceptivo-auditiva mostrou desvio da qualidade vocal. O distúrbio de autopercepção vocal demonstrou impacto negativo na qualidade de vida.
CONCLUSÕES: Indivíduos com doença de Parkinson idiopática apresentam qualidade vocal alterada e impacto negativo na qualidade de vida.
Palavras-Chave: doença de Parkinson, voz, qualidade de vida, disfonia.
Parkinson's disease (PD) is a degenerative and chronic disease of the central nervous system that affects, specifically, the neurons in substantia nigra mesencephalic, which are responsible for the release of the dopamine neurotransmitter1-4. The reduction of dopamine in the region of striatum leaves it overly active, leading to motor abnormalities typical of PD, such as tremor at rest, bradykinesia, rigidity, and postural instability1-6.
PD happens typically from 50 to 75 years-old in both sexes and is considered the second most common neurodegenerative disease in the elderly. About 3.4% of Brazilians over 64 years-old have the disease7.
The combination of PD motor signs leads to phonoarticulatory changes that result in voice, articulation, and swallowing disorders. It is estimated that 70% of individuals with PD have voice disorders characterized by: decreasing in the intensity of voice, monotone, imprecise articulation, changes in the speech speed, change of pitch, qualitative changes such as tremors, hoarseness, and breathiness. This group of amendments is called hypokinetic dysarthria3,5,8,9. The vocal disorders in PD commonly affect the standard communication, making diagnosis important for treatment and health promotion of individuals with PD.
PD negatively affects the individual's quality of life, since it is characterized by a set of motor abnormalities that may be accompanied by cognitive changes, memory deficits, freezing, and slow physiological responses, decreased in voice volume and other complications in both speech and swallowing, which directly interfere with the individual's level of disability4. As soon as the communication is understood as a human need, it is possible to conclude that changes that limit this process affect the individual's quality of life in a negative way.
Although several studies have analyzed the impact of PD on individual's quality of life, few studies have evaluated the quality of life related to the voice.
A multidimensional analysis of the PD patient's voice, involving aspects of perceptual and acoustic analysis of voice, beyond the vocal self-perception of the patient, can improve the ability of the speech and language therapist in describing voice quality in PD as well as can aid the diagnosis of laryngeal changes in PD10.
This research aimed at analyzing the voice quality and vocal self-perception of bearers of idiopathic PD that were male, compared with those without the disease (Control Group).
METHODS
This was a cross-sectional study, in which voices from bearers of idiopathic PD and those without it were analysed by means of a vocal quality of life assessment11.
The Movement Disorders Clinic at the Hospital das Clínicas has 450 registered patients diagnosed with idiopathic PD. According to sample calculation with an estimation error of 10 and 95% of significance level, a sample of 27 individuals with PD was obtained. The inclusion criteria for for the PD group were: male individuals with PD diagnosis according to the United Kingdom PD Society Brain Bank Clinical Diagnostic Criteria and individuals at stages 1 to 3 of the Hoehn-Yahr scale of disability12. The exclusion criteria were: to have a history of vocal and laryngeal disorders; to be diagnosed with other associated neurological disease; to not understand the tasks required to assess vocal speech and present inability to respond to the Voice-Related Quality of Life (V-RQOL) protocol13.
The inclusion criteria for the Control Group were: male individuals without the disease and age matched with the group of individuals with PD. The exclusion criteria for the Control Group were: to be diagnosed with neurological disease and/or history of voice and laryngeal disorders; to not understand the tasks required and to present inability to respond to the V-RQOL protocol.
The group of individuals with PD was composed of 27 male individuals, with ages ranging from 39 to 79 years-old and a 59.96 average of age, who attended the service for neurological consultation. All participants were using one or more medications for PD, such as Sifrol, Amantadine, Levodopa, Entacapone, Sinemet, Biperiden, Bromocriptine, Artane, and Niar. The Control Group consisted of 27 male individuals, with ages ranging from 42 to 82 years-old and 59.48 average of age, who fit the inclusion and exclusion criteria of the study, being caregivers of patients.
The patients underwent a voice and speech recording by: sustained vowel /a/, counting 1 to 20, and days of week. To perform the data analysis and recording, a Dell® computer, Optiplex GX260 model, with Direct Sound® professional sound board was used, which is available in the Speech and Language Therapy Clinic of Hospital das Clínicas. The recording was performed in a soundproof booth, and the individuals were standing, using a headset microphone AKG 170®(Austria). Voice capture was performed using the CSL, Kay Elemetrics®, MDVP module and the Audacity program, version 1.3 Beta.
After recording the sound wave, the following parameters were selected for acoustic analysis: fundamental frequency in Hertz, jitter in percentage, shimmer in percentage, and harmonic-to-noise ratio (NHR) in dB.
The GRBAS, which considers G as the overall degree of dysphonia, R as roughness, B as breathiness, A as asthenia, and S as strain, parameters were used for perceptual evaluation, proposed by the Japanese Society of Logopedics and Phoniatrics14, which considers G as the overall degree of dysphonia, R as roughness, B as breathiness, A as asthenia, and S as strain. It was also analysed the instability parameter as I, which was later introduced15. On this scale, the appraiser must indicate in the changed parameters which are the degrees of deviation on a numerical scale ranging from 0 to 3. It was considered 0 as no change, 1 as mild change, 2 as moderate change, and 3 as an amendment intense.
The sustained vowels were presented to the evaluators separately from the connected speech, who did not have prior knowledge if the voice belonged to PD Group or to the Control one. Thus, voices presentation was random.
Four speech and language therapists were selected to perform the perceptual evaluation. In order to determine the intra-rater reliability, 20% of the voices were repeated randomly, totalizing 65 emissions of sustained vowels and 65 of connected speech.
Professionals who had intra-rater reliability over 70%, as measured by Spearman's correlation, had their responses accounted. Four evaluators were selected for the sustained vowel and two others for connected speech.
It was applied the V-RQOL to analyze the quality of life, which is a vocal self-evaluation protocol that analyses aspects from quality of life related to voice. It is composed of ten items, being six of physical domain and four of socioemotional domain. The protocol provides a total score (ranging from 0 to 100, in which 0 indicates worse quality of life and 100 better quality of life) and a score for each domain. The participants answered a Brazilian validated version of V-RQOL13, which was read by the participants with one of the researchers together.
The statistical analysis was performed by means of the Wilcoxon's test, and p-values were less than 0.05.
All patients were duly informed about their participation in the research, and allowed the use of their voices after the informed consent had been signed. This research was approved by the Research Ethics Committee, on the report number ETIC 676/07.
RESULTS
Table 1 shows the comparison of acoustic measurements between the groups of individuals with and without the disease. The measures of fundamental frequency and jitter are higher in the group with PD. The values of NHR were higher in the Control Group.
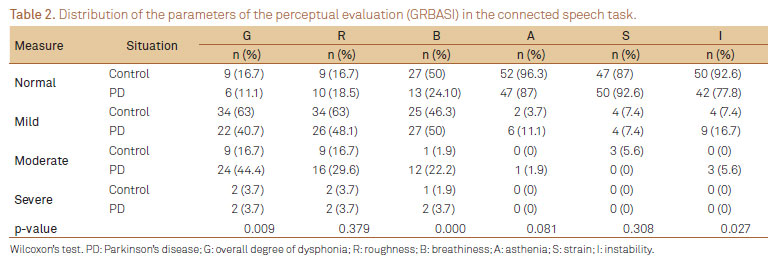
Tables 2 and 3 indicate the parameters distribution of the perceptual evaluation (GRBASI) for the tasks of speech and sustained vowel, respectively.
Table 4 indicates the comparison of the V-RQOL values between the group of individuals with PD and the Control one, showing a greater negative impact on quality of life in all domains of the questionnaire, in the group with PD.
DISCUSSION
PD has been studied since the beginning of 19th century and much has been discovered about the clinical symptoms of the disease, as well as treatments to be applied. With regards to speech and language therapy, the disease can lead to vocal and orofacial disorders with an impact on swallowing. It is estimated that 70% of individuals with PD have vocal disorders, such as decreasing in the intensity of voice, monotone, imprecise articulation, changes in the speech speed, change of pitch, qualitative changes such as tremors, hoarseness, and breathiness8,9.
This research has analysed acoustically the voice of 27 males with PD and 27 without it, and it was found that measures of fundamental frequency (F0) and jitter showed a statistical difference between groups with higher values in the PD Group.
The higher values of F0 in PD patients treated with dopaminergic drugs, when compared to the Control Group, are similar to those found in the literature16-19, which may be the rigidity result of the laryngeal and respiratory muscles20, besides the laryngopharyngeal tract hypomobility19. Therefore, stiffness and hypokinesia seem to be responsible for the increase in the F0 in men with PD19.
The jitter values were higher in men with PD in several studies9,10,16-18,20-22, when compared to the controls, corroborating the present study. The jitter increase may be associated with the loss of muscles motor control, which holds the function of the vocal folds, increasing the aperiodicity in the acoustic signal and the values of such measure in the PD Group10.
The shimmer values were not different in the groups and this result is similar to that found in the literature10,17,23.
Measures of NHR showed statistical differences between the PD Group and the Control Group in this study, being higher in the latter. These results are consistent with the literature17,23, and this finding may reflect on the aging process of the larynx, which can compromise the quality of the voice, being more evident in males, especially from the sixth decade of life, and may or may not be aggravated by PD20.
The anatomic and/or physiological features that may be associated with the changes in the NHR measures, in cases of hypokinetic dysarthria, as patients with PD, are still not clear.
In the perceptual evaluation of connected speech (Table 2), the parameters that showed statistically significance difference between PD patients and controls were G (p=0.009), B (p=0.000), and I (p=0.027). With regards to the perceptual evaluation of sustained vowel (Table 3), the parameters that showed statistically significance difference between PD patients and controls were B (p=0.026), A (p=0.000), S (p=0.002), and I (p=0.006) and such findings are consistent with the literature8,16,18,24.
One study found 79.2% of vocal deviation in the group with PD, ranging from mild (29.2%), moderate (45.8%), and intense degrees (4.2%)16, showing a prevalence of dysphonia in PD similar to the present study. The authors found that the parameters hoarseness and breathiness were the most altered ones16. These findings are similar to the present study, in which the most altered perceptual parameters of connected speech and sustained vowel were breathiness (B) and instability (I). These findings support the hypothesis that the hypokinetic dysarthria, present in PD, by impairing glottic closure20, can therefore generate the presence of air that is not sounded, auditorily perceived as breathiness.
Regarding the presence of instability, a research that correlated the perceptual evaluation with the contractile pattern of the intrinsic muscles of the larynx in PD, by means of electromyography, found that the vocal tremor auditory rated did not correlate with the electromyography findings24. This suggests that the vocal instability, perceived in the perceptual evaluation, does not have a direct correlation with the standard laryngeal.
The perceptual evaluation parameter of asthenia, observed in PD, has been associated with two distinct mechanisms, which, however, are coincident in their clinical expression: respiratory support and limitation of adduction of the vocal folds19. The presence of weakness also appears in other studies8, while the strain parameter found in this research can be the result of a patient's compensation mechanism facing the frame stiffness, present in PD.
If you consider that speech is the mirror of our personality, unique in its vibrations, tones and musicality, it is easy to understand how these changes interfere in patient's daily activities and in their social lives3. Several studies25-27 have evaluated the quality of life in patients with PD, using different types of protocols, but there are few ones that assess the quality of life related do voice.
A research that evaluated the relationship between depressive symptoms and quality of life in individuals with PD found that the worst scores on the Beck Depression Inventory, the worse is the perception of quality of life28.
The present study evaluated the impact of vocal disorders in the quality of life through the Voice-Related Quality of Life protocol, and found a worse perception of quality of life related to voice, in all domains, in the PD Group.
It was found in a literature review25 that the physical and motor aspects present in PD have negative impact on quality of life, since rigidity, bradykinesia and tremor cause physical limitations at an early stage of the disease.
Another study observed how changes in communication impact the lives of individuals with PD from the application of protocols, such as the Unified PD Rating Scale (UPDRS), speech tests, GRBASI scale, and PDQ-39, which is a protocol that assess the dimensions of mobility, activities of daily living (ADLs), emotional well-being, stigma, social support, cognition, communication, and bodily discomfort. The authors of such study found that the physical aspects of PD are the ones that cause the greatest negative impact on quality of life, which, although patients perceive the voice disorders, is only affected by changes in communication.
Another research28 that applied the same protocol, the PDQ-39, found no relationship between worsening in the communication aspect and poor quality of life.
The vocal assessment performed revealed that individuals with PD have vocal disorders, as evaluated by perceptual and acoustic analyses, resulting from the disease. These disorders negatively impact the quality of life, when compared to the Control Group.
In conclusion, individuals with idiopathic PD show an altered voice quality and a negative impact on quality of life.
In the perceptual evaluation, it was observed the overall degree of dysphonia (G) ranging from mild to severe and the changed parameters were, respectively, roughness, breathiness, instability, tension, and asthenia. In the acoustic analysis, F0 and jitter were increased.
The global, physical and socioemotional domains of quality of life related do voice are increased, which represents a negative impact on quality of life.
These results demonstrate the need of speech and language therapy in order to encourage improvements in the voice and in the quality of life of individuals.
Received 16 April 2012
Received in final form 15 May 2012
Accepted 22 May 2012
Conflict of interest: There is no conflict of interest to declare.
Study carried out at the Universidade Federal de Minas Gerais (UFMG), Belo Horizonte MG, Brazil:
- 1. Goulart F, Santos CC, Teixeira-Salmela LF, Cardoso F. Análise do desempenho funcional em pacientes portadores de doença de Parkinson. Acta Fisiatr 2004;11:12-16.
- 2. Goulart FRP, Barbosa CM, Silva CM, Teixeira-Salmela L, Cardoso F. O impacto de um programa de atividade física na qualidade de vida de pacientes com doença de Parkinson. Rev Bras Fisioter 2005;9:49-55.
- 3. Quedas A, Duprat AC, Gasparini G. Implicações do efeito Lombard sobre a intensidade, frequência fundamental e estabilidade da voz de indivíduos com doença de Parkinson. Rev Bras Otorrinolaringol 2007;73:675-683.
- 4. Lana RC, Álvares LMRS, Nasciutti-Prudente C, Goulart FRP, Teixeira-Salmela LF, Cardoso FE. Percepção da qualidade de vida de indivíduos com doença de Parkinson através do PDQ-39. Rev Bras Fisioter 2007;11:397-402.
- 5. Bigal A, Harumi D, Luz M, De Luccia G, Bilton T. Disfagia do idoso: estudo videofluoroscópico de idosos com e sem doença de Parkinson. Distúrb Comum 2007;19:213-223.
- 6. Azevedo LL, Cardoso F. Ação da levodopa e sua influência na voz e na fala de indivíduos com doença de Parkinson. Rev Soc Bras Fonoaudiol 2009;14:136-141.
- 7. Barbosa MT, Caramelli P, Maia DP, et al. Parkinsonism and Parkinson's disease in the elderly: a community-based survey in Brazil (the Bambui study). Mov Disord 2006;21:800-808.
- 8. Gasparini G, Diaféria G, Behlau M. Queixa vocal e análise perceptivo-auditiva de pacientes com doença de Parkinson. R Ci Med Biol 2003;2:72-76.
- 9. D'Alatri L, Paludetti G, Contarino MF, Galla S, Marchese MR, Bentivoglio AR. Effects of bilateral subthalamic nucleus stimulation and medication on Parkinsonian speech impairment. J Voice 2008;22:365-372.
- 10. Rahn DA, Chou M, Jiang JJ, Zhang Y. Phonatory impairment in Parkinson's disease: evidence from nonlinear dynamic analysis and perturbation analysis. J Voice 2007;21:64-71.
- 11. Bussab WO, Miazaki ES, Andrade DF. Introdução à análise de agrupamentos. São Paulo: Associação Brasileira de Estatística; 1990.
- 12. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427-442.
- 13. Gasparini G, Behlau M. Quality of life: validation of the Brazilian version of the Voice-Related Quality of Life (V-RQOL) measure. J Voice 2009;23:76-81.
- 14. Hirano M. Clinical examination of voice. New York: Springer Verlag; 1981.
- 15. Dejonckere PH, Remacle M, Fresnel-Elbaz E, Woisard V, Crevier-Buchman L, Millet B. Differentiated perceptual evaluation of pathological voice quality: reliability and correlations with acoustic measurements. Rev Laryngol Otol Rhinol 1996;117:219-224.
- 16. Carrara-de Angelis E. Deglutição, Configuração Laríngea, Análise Clínica e Acústica Computadorizada da Voz de Pacientes com Doença de Parkinson. [tese] São Paulo: Unifesp, 2000.
- 17. Gamboa J, Jiménez-Jiménez FJ, Nieto A, et al. Acoustic voice analysis in patients with Parkinson's disease treated with dopaminergic drugs. J Voice 1997;11:314-332.
- 18. Santos LL, Reis LO, Bassi I, et al. Acoustic and hearing-perceptual voice analysis in individuals with idiopathic Parkinson's disease in "on" and "off" stages. Arq Neuropsiquiatr 2010;68:706-711.
- 19. Skodda S, Visser W, Schlegel U. Gender-related patterns of dysprosody in parkinson disease and correlation between speech variables and motor symptoms. J Voice 2011;25:76-82.
- 20. Ferreira FV, Cielo CA, Trevisan ME. Medidas vocais acústicas na doença de Parkinson: estudo de casos. Rev CEFAC 2010;12:889-898.
- 21. Carrillo L, Ortiz KZ. Análise vocal (auditiva e acústica) nas disartrias. Pró-Fono 2007;19:381-386.
- 22. Rosa JC, Cielo CA, Cechella C. Função fonatória em pacientes com doença de Parkinson: uso de instrumento de sopro. Rev CEFAC 2009;11:305-313.
- 23. Xie Y, Zhang Y, Zheng Z, et al. Changes in speech characters of patients with Parkinson's disease after bilateral subthalamic nucleus stimulation. J Voice 2011;25:751-758.
- 24. Zarzur AP, Duarte IS, Gonçalves GNH, Martins MAUR. Eletromiografia laríngea e análise vocal em pacientes com mal de Parkinson: estudo comparativo. Braz J Otorhinolaryngol 2010;76:40-43.
- 25. Camargos ACR, Cópio FCQ, Sousa TRR, Goulart F. O impacto da doença de Parkinson na qualidade de vida: uma revisão de literatura. Rev Bras Fisioter 2004;8:267-272.
- 26. Schestatsky P, Zanatto VC, Margis R, et al. Quality of life in a Brazilian sample of patients with Parkinson's disease and their caregivers. Rev Bras Psiquiatr 2006;28:209-211.
- 27. McCabe MP, Firth L, O'Connor E. A comparison of mood and quality of life among people with progressive neurological illnesses and their caregivers. J Clin Psychol Med Settings 2009;16:355-362.
- 28. Scalzo P, Kummer A, Cardoso F, Teixeira AL. Depressive symptoms and perception of quality of life in Parkinson's disease. Arq Neuropsiquiatr 2009;67:203-208.
Correspondence:
Publication Dates
-
Publication in this collection
12 Sept 2012 -
Date of issue
Sept 2012
History
-
Received
16 Apr 2012 -
Accepted
22 May 2012 -
Reviewed
15 May 2012