Abstracts
The immune modulatory activity of diet long chain polyunsaturated fatty acids in fish has been previously demonstrated, although results for freshwater species are controversial. This study evaluates the effect of different dietary lipids on freshwater catfish jundiá, Rhamdia quelen, survival and its non-specific response (phagocytosis) after being inoculated with the pathogen Aeromonas hydrophila. Five diets were offered to jundiá fingerlings during 150 days prior to challenge: cod liver oil (FO), sunflower oil (SO), linseed oil (LO), canola oil (CO) and coconut oil (CNO). Accumulated mortality was significantly lower in fish fed FO and CNO diets and higher in fish fed LO. In spite of the highest values observed for phagocytotic activity in CNO-fed fish (50.0 + 12.7%) and in FO-fed fish (45.0 + 10.5%) when compared to those fed other diets, no significant differences in phagocytosis were reported. Results show the relevance of a balanced dietary lipid source with adequate concentrations of n-3 and n-6 series of fatty acids to prevent mortality after pathogen challenge.
mortality; phagocytosis; oils; immune response
A atividade moduladora dos ácidos graxos de cadeia longa no sistema imunológico de peixes já foi demonstrada, embora os resultados para espécies de água doce sejam controversos. O objetivo do estudo foi avaliar a sobrevivência e resposta imune não específica (fagocitose) de alevinos de jundiá, Rhamdia quelen, previamente alimentados com diferentes fontes lipídicas na dieta, após serem desafiados com o patógeno Aeromonas hydrophila. Cinco dietas com diferentes fontes lipídicas foram oferecidas a alevinos de jundiá durante os 150 dias antes do desafio: óleo de fígado de bacalhau (FO), óleo de girassol (SO), óleo de linhaça (LO), óleo de canola (CO) e gordura de coco (CNO). A mortalidade acumulada foi menor nos peixes alimentados com as dietas FO e CNO e maior nos que receberam a dieta LO. A fagocitose não apresentou diferenças significativas entre os tratamentos, porém os peixes que consumiram as dietas CNO (50,0 ± 12,7%) e FO (45,0 ± 10,5%) apresentaram valores mais elevados. Os resultados do estudo mostram a relevância de utilizar fontes lipídicas com adequadas quantidades de ácidos graxos das séries n-3 e n-6 na prevenção da infecção por patógenos em jundiá.
mortalidade; fagocitose; óleos; resposta imune
AQUACULTURE
Dietary lipid sources affect freshwater catfish jundiá, Rhamdia quelen, survival, when challenged with Aeromonas hydrophila
Fontes lipídicas da dieta afetam a sobrevivência do bagre de água doce jundiá, Rhamdia quelen, quando desafiados com Aeromonas hydrophila
Rodrigo Javier Vargas* * Author for correspondence: E-mail: roduruguaio@gmail.com ; Geovana Dotta; José Luiz Mouriño; Bruno Corrêia da Silva; Débora Machado Fracalossi
Departamento de Aquicultura, Universidade Federal de Santa Catarina. Rod. Admar Gonzaga 1346, 88034-001, Florianópolis, Santa Catarina, Brazil
ABSTRACT
The immune modulatory activity of diet long chain polyunsaturated fatty acids in fish has been previously demonstrated, although results for freshwater species are controversial. This study evaluates the effect of different dietary lipids on freshwater catfish jundiá, Rhamdia quelen, survival and its non-specific response (phagocytosis) after being inoculated with the pathogen Aeromonas hydrophila. Five diets were offered to jundiá fingerlings during 150 days prior to challenge: cod liver oil (FO), sunflower oil (SO), linseed oil (LO), canola oil (CO) and coconut oil (CNO). Accumulated mortality was significantly lower in fish fed FO and CNO diets and higher in fish fed LO. In spite of the highest values observed for phagocytotic activity in CNO-fed fish (50.0 + 12.7%) and in FO-fed fish (45.0 + 10.5%) when compared to those fed other diets, no significant differences in phagocytosis were reported. Results show the relevance of a balanced dietary lipid source with adequate concentrations of n-3 and n-6 series of fatty acids to prevent mortality after pathogen challenge.
Keywords: mortality, phagocytosis, oils, immune response.
RESUMO
A atividade moduladora dos ácidos graxos de cadeia longa no sistema imunológico de peixes já foi demonstrada, embora os resultados para espécies de água doce sejam controversos. O objetivo do estudo foi avaliar a sobrevivência e resposta imune não específica (fagocitose) de alevinos de jundiá, Rhamdia quelen, previamente alimentados com diferentes fontes lipídicas na dieta, após serem desafiados com o patógeno Aeromonas hydrophila. Cinco dietas com diferentes fontes lipídicas foram oferecidas a alevinos de jundiá durante os 150 dias antes do desafio: óleo de fígado de bacalhau (FO), óleo de girassol (SO), óleo de linhaça (LO), óleo de canola (CO) e gordura de coco (CNO). A mortalidade acumulada foi menor nos peixes alimentados com as dietas FO e CNO e maior nos que receberam a dieta LO. A fagocitose não apresentou diferenças significativas entre os tratamentos, porém os peixes que consumiram as dietas CNO (50,0 ± 12,7%) e FO (45,0 ± 10,5%) apresentaram valores mais elevados. Os resultados do estudo mostram a relevância de utilizar fontes lipídicas com adequadas quantidades de ácidos graxos das séries n-3 e n-6 na prevenção da infecção por patógenos em jundiá.
Palavras-chave: mortalidade, fagocitose, óleos, resposta imune.
Introduction
Teleost fishes occupy a key evolutionary position in the development of innate and adaptive immune responses since they are the earliest class of vertebrates with innate and adaptive immunity (WHYTE, 2007). These responses are modulated by several intrinsic and extrinsic factors such as age (LU et al., 2008), temperature (BOWDEN et al., 2007), contaminants (KREUTZ et al., 2010) and others. Fish nutritional status is known to influence the immune system and its resistance to disease (XU et al., 2010). The immune system's development, maintenance and efficiency depend on a complete and balanced nutrition, where asseveral nutrients, such as vitamins and fatty acids or additives such as probiotics, have shown an immune-modulatory activity in fish (SARGENT et al., 2002). As a rule, diet fatty acids may affect the immune response by conditioning 1) plasmatic membrane fatty acid composition and its subsequent effects on the membrane's physical qualities (TOCHER, 1995), 2) cytokines and eicosanoid production, synthesized from the precursors eicosapentaenoic (20:5 n-3, EPA) and arachidonic (20:4 n-6, ARA) acids, which are the key cell messengers in the inflammation process (ROWLEY et al., 1995), and 3) transcription regulation of genes responsible for immune responses (MONTERO et al., 2008).
The stimulation or inhibition of the immune system by dietary fatty acids in freshwater fish depends directly on the quantity and quality of the dietary fatty acids and on their ratio, such as EPA: ARA ratio (LIN; SHIAU, 2003). This fact complicates mechanism elucidation and generates contradictory results since they depend on which fatty acids or fatty acid ratios are to be taken into account and which immune responses are to be measured. Rainbow trout, Oncorhychus mykiis, and channel catfish, Ictalurus punctatus, fingerlings fed diets rich in n-3 highly unsaturated fatty acids (n-3 HUFA) had a higher survival rate and macrophage activity, respectively, when inoculated with different bacterial strains (SHELDON; BLAZER, 1991; KIRON et al., 1995). Contrastingly, Atlantic salmon juveniles, Salmo salar, and channel catfish fed diets with increasing levels of n-3 HUFA (24.2% and 31.3%, respectively) had a mortality increase when inoculated with Yersinia ruckeri and Edwardsiella ictaluri (ERDAL et al., 1991; FRACALOSSI; LOVELL, 1994). Therefore, the modulatory effect of dietary fatty acids in fish should be further studied.
Rhamdia quelen, popularly known as jundiá, is a siluriform fish with a wide geographical distribution, featuring an emerging economical relevance in southern Brazil, Argentine and Uruguay (BICHUETTE; TRAJANO, 2003). Aeromonas hydrophila causes septicemia, a common disease in jundiá farming, causing high liabilities to fish farmers (BOIJINK; BRANDÃO, 2001). This study evaluates jundiá survival and a non-specific immune response to dietary lipid sources after being inoculated with A. hydrophila.
Material and methods
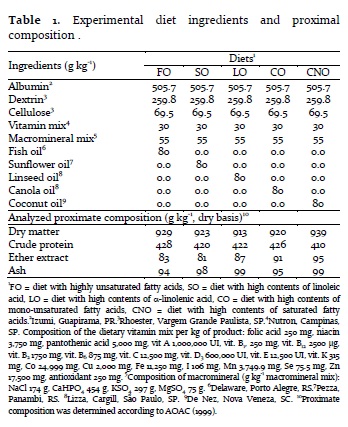
A basal semipurified and isonitrogenous diet (42% crude protein) was mixed to five lipid sources to obtain five isoenergetic diets (17.2 kJ g-1) with different fatty acid composition. The lipid sources were cod liver oil (highly unsaturated fatty acid rich diet, FO), sunflower oil (linoleic rich diet, SO diet), linseed oil (linolenic rich diet, LO), canola oil (monounsaturated rich diet, CO) and coconut oil (saturated rich diet, CNO) (Table 1).
Ingredients were mixed with distilled water at a proportion of 3:1, and the mixture was passed through a commercial meat grinder fitted with a 2 mm die. Diets were dried in a forced-air oven at 60°C for 12h, and kept at -20°C prior to feeding. Dry matter, crude protein, ether extract and ash contents were determined according to AOAC standardized procedures (AOAC, 1999).
Jundiá fingerlings from a single spawn were obtained at a commercial farm (Girassol Farm, Joinville, SC). They were adapted to experimental conditions during one month, and fed a commercial diet (40% crude protein and 8% ether extract). After this period, fingerlings (initial weight = 2.1 ± 0.02 g) were distributed into five 1,000 L-tanks (311 fingerlings per tank) connected to a closed water circulating system. Fish were fed twice a day (9 am. and 5 pm.) to apparent satiation for 150 days. The growth (average final weight = 33.5 ± 1.2 g) did not differ among diet treatments in this period. Water quality was monitored daily and did not differ among types of treatments: temperature = 27.5 ± 0.5 °C, pH = 6.6 ± 0.3, dissolved oxygen = 6.4 ± 0.7 mg L-1, and ammonium = 0.0013 ± 0.001 mg L-1. Fish handling was performed according to PP00815 protocol, approved by the Ethics Committee on Animal Welfare, Federal University of Santa Catarina (CEUA, UFSC).
A virulent strain of Aeromonas hydrophila (CPQBA228-08DRM) was grown on triptone soy agar medium (DIFCO) and then cultured in a brain-heart infusion broth (DIFCO) for 24h at 30°C. Bacterial concentration was determined at 630 nm absorbance by comparison with a standard curve (y = 9.5 x 10-9 x + 4.6 x 10-2). Bacterial culture was centrifuged at 1,800 x g for 30 min.; the supernatant was discarded and the pellet was re-suspended in a sterile saline solution (NaCl, 0.85%). The final bacterial concentration was adjusted to 2 x 108 colony-forming unit (CFU) mL-1 by serial dilution. This solution was utilized for the experimental challenge with A. hydrophila.
Two trials were performed in the present study: Trial I evaluated the accumulated mortality after challenge with A. hydrophila and Trial II, the non-specific immune response phagocytosis of jundiá's leukocytes. In Trial I, a completely randomized experimental design was adopted, with three replicates. Ten jundiá fingerlings (10.0 ± 0.3 g) from each dietary treatment were allocated into each experimental unit (15-L tank), equipped with aeration and individual heaters, and kept at a constant water temperature of 27°C. Eighty percent of the water volume in each tank was changed daily. After an acclimation period of 24h, fish were anaesthetized with clove oil at 0.5 mL L-1 (EUGENOL®) and inoculated by intraperitoneal injection (100 μL g-1 fish) with 2 x 108 CFU A. hydrophila mL-1 solution. A pilot trial (data not shown) verified the pathogenicity of the bacterial strain and the appropriate dose for injection. Fish mortality in each experimental unit was recorded 24, 48, 72 and 96h after inoculation.
Trial II evaluated the phagocytosis of jundiá's leukocytes after a 24h-period challenge with a sub-lethal dose of A. hydrophila. Fingerlings were acclimated to the experimental units for 24h, anaesthetized with clove oil at 0.5 mL L-1 (EUGENOL®), and inoculated with 50 μL g-1 fish of 2 x 108 CFU mL-1 (approximately 0.2 mL) solution by intraperitoneal injection. Additionally, two groups of fish were either inoculated with a sterile saline solution (0.2 mL per fish) or not inoculated at all. These two groups represented controls of bacterial challenge and injection procedures, respectively. Each diet and challenge procedure were done in triplicate, totalizing 45 experimental units. Ten fingerlings (average weight 33.5 ± 9.8 g) were allocated to each unit, similar to the previous challenge trial. Water was periodically changed to maintain water quality at acceptable levels, with temperature at 27.0 ± 0.2°C, dissolved oxygen at 6.5 ± 0.7 mg L-1 and pH at 7.2 ± 0.4. After 24h, three fish from each experimental unit (27 fish per dietary treatment) were randomly sampled and euthanized with an overdose (2.5mL L-1) of clove oil (EUGENOL®). Blood and liver samples were immediately collected to evaluate phagocytosis and liver fatty acid composition respectively. Leukocyte phagocyte percentage was evaluated by using a procedure adaptation described by Martins et al. (2008). Briefly, 120 μL of blood were placed into the microplate wells and 60 μL of A. hydrophila, previously inactivated with formalin (1 x 106 UFC mL-1), were added, mixed and incubated at 28°C for 30 min. Microplates were shaken at 10-min. intervals. A blood aliquot was used to prepare blood slides (two for fish). Slides were air dried and then stained with Giemsa-MayGrunwald (ROSENFELD, 1947). Phagocytotic and un-phagocytotic leukocytes were counted under the microscope (at least 100 leukocytes per slide) and phagocytosis percentage was calculated by the equation below:
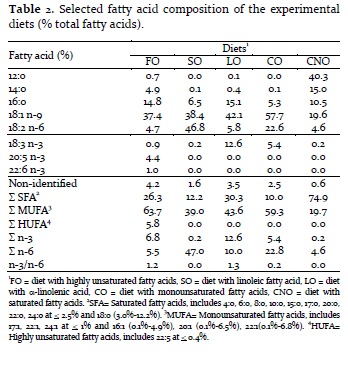
Diets and liver of three fish per experimental unit were pooled for lipid and fatty acid analyses. Liver lipids were extracted in chloroform / methanol (BLIGH; DYER, 1959) and fatty acid methyl esters (FAMEs) were prepared with sulfuric acid (HARTMAN; LAGO, 1973). Fatty acid was determined by gas chromatograph (VARIAN 3900) and identified by comparison with a known standard Supelco 37 FAME Mix 47885-U (SIGMA). Fatty acids were quantified by area normalization and results were expressed in percentage of total fatty acids (Table 2).
All data were tested for normality, homogeneity and independence. Since the distribution of accumulated mortality data was not normal for evaluated period, a LOGIT model was adopted. Data for phagocyte percentage were compared by Analyses of Variance and the means was compared by Tukey's test when required, significance level was set at 5%.
Results and discussion
As expected, diet fatty acid composition was influenced by the added lipid source. FO diet showed high contents of long chain n-3 and n-6 fatty acids, fundamentally EPA and docosahexaenoic acid (22:6 n-3, DHA). SO and LO diets showed high contents of linoleic acid (LOA = 46.8%) and linolenic acid (LNA = 12.6%) respectively. CO diet provided intermediate values of LOA (22.6%) and LNA (5.4%), whereas CNO diet presented high contents of saturated fatty acids (SFA = 74.9%). The n-3/n-6 ratio was also influenced by the diet lipid source (Table 2).
Jundiá's liver fatty acid composition was significantly affected by the different lipid diet sources and reflected diet fatty acid composition. In this way, the FO, CNO and SO diets produced the highest levels of DHA, SFA and eicosatrienoic acid (20:3 n-6, DHLA) in jundiá's liver, respectively (Table 3).
The onset of mortality in Trial I was observed at 12h after challenge. Loss of balance, lethargy, ascites and injuries at the base of the fins were the clinical signs observed; internally, pale organs were also manifested. The above are typical signs associated with A. hydrophila infection and their presence was biochemically identified by an API 20E kit (BIOMERIEUX). The highest mortality was observed at 72h after challenge and accumulated mortality was significantly affected by the diet lipid source (Figure 1). After 24h, fish fed FO showed lower mortality when compared to other diet treatments (p = 0.0228). LO-fed fish showed a significantly higher accumulated mortality (p = 0.0199) after 72h and 96h, when compared to FO- or CNO-fed fish. On the other hand, SO- and CO-fed fish showed an intermediate mortality rate. No mortality was recorded in fish submitted to control protocols which consisted of saline injection or no injection.
Jundiá survival after A. hydrophila challenge was affected by dietary lipid source. Thus, accumulated mortality after 24h challenge was significantly lower in FO-fed fish, namely, the only lipid source with high unsaturated fatty acids (5.8%, Table 2). The rapid response from FO-fed fish may be due to the more balanced fatty acid composition of this lipid source. After 48h, the accumulated mortality was lower in FO-fed and CNO-fed fish (rich in saturated fatty acids) and remained stable until 96h. This result was unexpected, since CNO lacked essential fatty acids. However, recent studies in mammals and fish showed that the presence of SFA in the membranes of the response cells may form insoluble areas in the plasmatic membrane with greater stability, called 'lipid rafts', which are fundamental processes in vertebrates' immune response (LENNARTZ, 2005; GYLFASON et al., 2010). In fact, channel catfish fed on diets high in SFA (beef tallow) and LOA (corn oil) or with a mixed diet (beef tallow: corn oil: menhaden oil) also showed lower mortality when inoculated with E. ictaluri (FRACALOSSI; LOVELL, 1994). Current study shows that the highest cumulative mortality after 96h challenge was observed in jundiá fed on LO, which contained 12% LNA. Additionally, LO-fed fish have ten times more LNA in their livers when compared with other treatments (Table 3).
The immunosuppression caused by high LNA inclusion or the unbalanced n-3/n-6 ratio in this diet has already been reported in other fish species and may have been caused by changes in eicosanoid production (SARGENT et al., 2002). Also, jundiá fingerlings fed on LNA rich diet (14.3%) for seven weeks showed higher mortality than fish fed diets containing fish oil as the main lipid source when inoculated with the protozoan ich, Ichthyophthirius multifiliis (VARGAS et al., 2008).
In Trial II, leukocyte phagocytosis activity ranged between 23% and 59% for all diets and control protocols. No statistical difference among dietary treatments for phagocytosis was detected in spite of the highest values observed for phagocytotic activity in CNO-fed fish (50.0 ± 12.7%) and in FO-fed fish (45.0 ± 10.5%) when compared to those fed other diets (Figure 2). Pathogen infection causes a complex inflammatory response in fish and cellular responses, such as phagocytosis, are activated (SECOMBES, 1996). Interestingly, in this study FO and CNO-fed fish showed the highest values of leukocyte's phagocytosis and the lowest values of mortality. It may probably indicate that phagocytosis is a key response to prevent fish mortality after challenge. In vitro studies with macrophages of channel catfish also showed an increase in the bactericidal activity when menhaden oil was included in this species' diet (SHELDON; BLAZER, 1991). Moreover, eicosanoids are lipid mediators produced from 20-C fatty acids, especially ARA, EPA and DHLA, present in the plasmatic membrane of the cells that compose the affected tissues and immune cells (ROWLEY et al., 1995). These molecules have different biological activities depending on the fatty acid from which they were derived: eicosanoids generated from ARA provide a more potent response that those from EPA or DHLA (TOCHER, 2003).
Recently, new lipid mediator families, called resolvins and protectins, have been discovered. They are generated from highly unsaturated fatty acids of n-3 family, fundamentally from DHA. These lipid mediators are important in the inflammatory process resolution. Some authors suggest that the eicosanoids and resolvins may act jointly, albeit at different times within the inflammatory response; the former would act first, while the second, to activate the resolution of the inflammatory proccess (SHERAN, 2008). Resolvins and protectins biosynthesis in fish was detected for the first time in rainbow trout brain cells and demonstrated highly conserved molecules (HONG et al., 2005). Eicosanoids and resolvins were not measured in the present study, although, taking into consideration their fatty acid precursors, the joint action of these mediators may partially explain FO- and CNO-fed fish high survival. Although these fish had ARA in their membranes for eicosanoid synthesis, they also contained DHA to generate resolvins for an inflammation process better resolution. However, SO-fed fish showed high values of ARA and DHA in the liver fatty acid composition but had very poor survival. Interestingly, these fish had high DHLA contents (Table 3), which is known to compete with ARA for binding the enzyme site in eicosanoid production (PETERSON et al., 1999). In turbot, Scophthalmus maximus, diet 18:3 n-6, a DHLA precursor, suppressed ARA-derived eicosanoid production (TOCHER et al., 1997). In the current study, DHLA may have had suppressor effect in eicosanoids in SO-fed fish production, which may explain their poor survival.
Conclusion
FO diet presents an adequate balance between n-3 and n-6 fatty acids, and provides the lowest jundiá accumulated mortality after challenge with pathogen A. hydrophila. This demonstrates the importance of incorporating fatty acids of both series in adequate concentrations in diets of freshwater fishes for an optimal immune response.
Acknowledgements
The authors are grateful to the Brazilian National Council for Technological and Scientific Development (CNPq) for funding this study and to the Food Technology Institute (ITAL, São Paulo State) for the fatty acids analyses. The authors would also like to thank Fernando H. Gomes, Patricia Olivera, Ana Paula R. Oeda and Giselle Speck for their technical assistance during trials.
References
AOAC-Association of Official Analytical Chemists. Official Methods of Analysis of the AOAC. 17th ed. Gaithersburg: AOAC International, 1999.
BICHUETTE, M. E.; TRAJANO, E. Epigean and subterrean ichthyofauna from São Domingos karts area, upper Tocantins river basin, Central Brazil. Journal of Fish Biology, v. 63, n. 5, p. 1100-1121, 2003.
BLIGH, E. G.; DYER, W. J. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, v. 37, n. 8, p. 911-917, 1959.
BOIJINK, C. L.; BRANDÃO, D. A. Inoculação bacteriana de Aeromonas hydrophila e a sobrevivência de juvenis de jundiá, Rhamdia quelen (Teleostei: Pimelodidae). Ciência Rural, v. 31, n. 3, p. 503-507, 2001.
BOWDEN, T. J.; THOMPSON, K. D.; MORGAN, A. L.; GRATACAP, R. M. L.; NIKOSKELAINEN, S. Seasonal variation and the immune response: a fish perspective. Fish and Shellfish Immunology, v. 22, n. 6, p. 695-706, 2007.
ERDAL, J. I.; EVENSON, O.; KAURSTAD, O. K.; LILLEHAUG, A.; SOLBAKKEN, R.; THORUD, K. Relationship between diet and immune response in Atlantic salmon (Salmo salar L.) after feeding various levels of ascorbic acid and omega-3 fatty acids. Aquaculture, v. 98, n. 4, p. 363-379, 1991.
FRACALOSSI, D. M.; LOVELL, R. T. Dietary lipid sources influence responses of channel catfish (Ictalurus punctatus) to inoculate with the pathogen Edwardsiella ictaluri. Aquaculture, v. 119, n. 2, p. 287-298, 1994.
GYLFASON, G. A.; KNÚTSDÓTTIR, E.; ÁSGEIRSSON, B. Isolation and biochemical characterisation of lipid rafts from Atlantic cod (Gadus morhua) intestinal enterocytes. Comparative Biochemistry and Physiology, v. 155, n. 1, p. 86-95, 2010.
HARTMAN, L.; LAGO, R. C. A. Rapid preparation of fatty acid methyl esters from lipids. Laboratory Practice, v. 22, n. 3, p. 475-476, 1973.
HONG, S.; TJONAHEN, E.; MORGAN, E. L.; YU, L.; SERHAN, C. N.; ROWLEY, A. F. Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins mediator lipidomic analysis. Prostaglandins and Other Lipid Mediators, v. 78, n. 1-4, p. 107-116, 2005.
KIRON, V.; FUKUDA, H.; TAKEUCHI, T; WATANABE, T. Essential fatty acid nutrition and defence mechanisms in rainbow trout O. mykiss. Comparative Biochemistry and Physiology, v. 111A, n. 3, p. 361-367, 1995.
KREUTZ, L. C.; BARCELLOS, L. J. G.; MARTENINGHE, A.; SANTOS, E. D.; ZANATA, R. Exposure to sublethal concentration of glyphosate or atrazine-based herbicides alters the phagocytic function and increases the susceptibility of silver catfish fingerlings (Aeromonas hydrophila hydrophila) to Aeromonas hydrophila challenge. Fish and Shellfish Immunology, v. 29, n. 4, p. 694-697, 2010.
LENNARTZ, M. Phospholipases and phagocytosis. In: ROSALES, C. (Org.). Molecular mechanisms of phagocytosis. New York: Springer Science, 2005. p. 97-116.
LIN, Y. H.; SHIAU, S. Y. Dietary lipid requirement of grouper, Epinephelus malabaricus, and effects on immune responses. Aquaculture, v. 225, n. 1-4, p. 243-250, 2003.
LU, M. W.; CHAO, Y. M.; GUO, T. C.; SANTI, N.; EVENSEN, O.; KASANI, S. K.; HONG, J. R.; WU, J. L. The interferon response is involved in nervous necrosis virus acute and persistent infection in zebra-fish infection model. Molecular Immunology, v. 45, n. 4, p. 1146-1152, 2008.
MARTINS, M. L.; MOURIÑO, J. L. P.; AMARAL, G. V.; VIEIRA, F. N.; DOTTA, G.; JATOBÁ, A. M. B.; PEDROTTI, F. S.; JERONIMO, G. T.; BUGLIONE NETO, C. C.; PEREIRA, G. Haematological changes in Nile tilapia experimentally infected with Enterococcus sp. Brazilian Journal of Biology, v. 68, n. 3, p. 631-637, 2008.
MONTERO, D.; GRASSO, V.; IZQUIERDO, M. S.; GANGA, R.; REAL, F.; TORT, L.; CABALLERO, M. J.; ACOSTA, F. Total substitution of fish oil by vegetable oils in gilthead sea bream (Sparus aurata) diets: effects on hepatic Mx expression and some immune parameters. Fish and Shellfish Immunology, v. 24, n. 2, p. 147-155, 2008.
PETERSON, L. D.; THIES, F.; CALDER, P. C. Dose-dependent effects of dietary γ-linolenic acid on rat spleen lymphocyte functions. Prostaglandins, Leukotrienes and Essential Fatty Acids, v. 61, n. 1, p. 19-24, 1999.
ROSENFELD, G. Corante pancrômico para hematologia e citologia clínica: nova combinação dos componentes do May-Grünwald e do Giemsa num só corante de emprego rápido. Memórias do Instituto Butantan, v. 20, n. 1, p. 329-334, 1947.
ROWLEY, A. F.; KNIGHT, J.; LLOYD-EVANS, P.; HOLLAND, J. W.; VICKER, P. J. Eicosanoids and their role in immune modulation in fish-a brief overview. Fish and Shellfish Immunology, v. 5, n. 8, p. 549-567, 1995.
SARGENT, J. R.; TOCHER, D. R.; BELL, J. G. The lipids. In: HALVER, J. E.; HARDY, R. (Ed.). Fish nutrition. San Diego: Academic Press, 2002. p. 182-246.
SECOMBES, C. J. The nonspecific immune system: cellular defense. In: IWAMA, G.; NAKANISHI T. (Ed.). The fish immune system. San Diego: Academic Press, 1996. p. 63-105.
SHELDON JR., W. M.; BLAZER, V. S. Influence of dietary lipid and temperature on bactericidal activity of channel catfish macrophages. Journal of Aquatic Animal Health, v. 3, n. 1, .p. 87-93, 1991.
SHERAN, C. N. Novel lipid mediators in resolution and their aspirin triggered epimers: lipoxins, resolvins, and protectins. In: ROSSI, A. G.; SAWATZKY, D. A. (Ed.). The resolution of inflammation. New York: Birkhäuser Verlag Basel, 2008. p. 93-117.
TOCHER, D. R. Glycerophospholipid metabolism. In: HOCHACHKA, P. W; MOMMSEN, T. P. (Org.). Biochemistry and molecular biology of fishes. Amsterdam: Elsevier Press, 1995. p. 119-157.
TOCHER, D. R. Metabolism and functions of lipids and fatty acids in teleost fish. Reviews in Fisheries Science, v. 11, n. 2, p. 107-184, 2003.
TOCHER, D. R.; BELL, J. G.; FARNDALE, B. M.; SARGENT, J. R. Effects of dietary γ-1inolenic acid-rich borage oil combined with marine fish oils on tissue phospholipid fatty acid composition and production of prostaglandins E and F of the 1-, 2- and 3- series in a marine fish deficient in Δ5 fatty acyl desaturase. Prostaglandins, Leukotrienes and Essential Fatty Acids, v. 57, n. 2, p. 125-134, 1997.
VARGAS, R. J.; GUIMARÃES DE SOUZA, S. M.; MABILIA, R.; CARLET, F.; BAGGIO, S. Resposta fisiológica à infestação experimental com Ichthyophthirius multifiliis (Fouquet, 1876) em alevinos de jundiá Rhamdia quelen (Quoy and Gaimard, 1824) previamente alimentados com diferentes fontes lipídicas. Revista Brasileira de Parasitologia Veterinária, v. 17, n. 2, p. 81-86, 2008.
WHYTE, S. The innate immune response of finfish - a review of current knowledge. Fish and Shellfish Immunology, v. 23, n. 6, p. 1127-1151, 2007.
XU, H.; AI, Q.; MAI, K.; XU, W.; WANG, J.; MA, H.; ZHANG, W.; WANG, X.; LIUFU, Z. Effects of dietary arachidonic acid on growth performance, survival, immune response and tissue fatty acid composition of juvenile Japanese seabass, Lateolabrax japonicas. Aquaculture, v. 307, n. 1, p. 75-82, 2010.
Received on January 24, 2013.
Accepted on May 27, 2013.
- AOAC-Association of Official Analytical Chemists. Official Methods of Analysis of the AOAC. 17th ed. Gaithersburg: AOAC International, 1999.
- BICHUETTE, M. E.; TRAJANO, E. Epigean and subterrean ichthyofauna from São Domingos karts area, upper Tocantins river basin, Central Brazil. Journal of Fish Biology, v. 63, n. 5, p. 1100-1121, 2003.
- BLIGH, E. G.; DYER, W. J. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, v. 37, n. 8, p. 911-917, 1959.
- BOIJINK, C. L.; BRANDÃO, D. A. Inoculação bacteriana de Aeromonas hydrophila e a sobrevivência de juvenis de jundiá, Rhamdia quelen (Teleostei: Pimelodidae). Ciência Rural, v. 31, n. 3, p. 503-507, 2001.
- BOWDEN, T. J.; THOMPSON, K. D.; MORGAN, A. L.; GRATACAP, R. M. L.; NIKOSKELAINEN, S. Seasonal variation and the immune response: a fish perspective. Fish and Shellfish Immunology, v. 22, n. 6, p. 695-706, 2007.
- ERDAL, J. I.; EVENSON, O.; KAURSTAD, O. K.; LILLEHAUG, A.; SOLBAKKEN, R.; THORUD, K. Relationship between diet and immune response in Atlantic salmon (Salmo salar L.) after feeding various levels of ascorbic acid and omega-3 fatty acids. Aquaculture, v. 98, n. 4, p. 363-379, 1991.
- FRACALOSSI, D. M.; LOVELL, R. T. Dietary lipid sources influence responses of channel catfish (Ictalurus punctatus) to inoculate with the pathogen Edwardsiella ictaluri Aquaculture, v. 119, n. 2, p. 287-298, 1994.
- GYLFASON, G. A.; KNÚTSDÓTTIR, E.; ÁSGEIRSSON, B. Isolation and biochemical characterisation of lipid rafts from Atlantic cod (Gadus morhua) intestinal enterocytes. Comparative Biochemistry and Physiology, v. 155, n. 1, p. 86-95, 2010.
- HARTMAN, L.; LAGO, R. C. A. Rapid preparation of fatty acid methyl esters from lipids. Laboratory Practice, v. 22, n. 3, p. 475-476, 1973.
- HONG, S.; TJONAHEN, E.; MORGAN, E. L.; YU, L.; SERHAN, C. N.; ROWLEY, A. F. Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins mediator lipidomic analysis. Prostaglandins and Other Lipid Mediators, v. 78, n. 1-4, p. 107-116, 2005.
- KIRON, V.; FUKUDA, H.; TAKEUCHI, T; WATANABE, T. Essential fatty acid nutrition and defence mechanisms in rainbow trout O. mykiss Comparative Biochemistry and Physiology, v. 111A, n. 3, p. 361-367, 1995.
- KREUTZ, L. C.; BARCELLOS, L. J. G.; MARTENINGHE, A.; SANTOS, E. D.; ZANATA, R. Exposure to sublethal concentration of glyphosate or atrazine-based herbicides alters the phagocytic function and increases the susceptibility of silver catfish fingerlings (Aeromonas hydrophila hydrophila) to Aeromonas hydrophila challenge. Fish and Shellfish Immunology, v. 29, n. 4, p. 694-697, 2010.
- LENNARTZ, M. Phospholipases and phagocytosis. In: ROSALES, C. (Org.). Molecular mechanisms of phagocytosis. New York: Springer Science, 2005. p. 97-116.
- LIN, Y. H.; SHIAU, S. Y. Dietary lipid requirement of grouper, Epinephelus malabaricus, and effects on immune responses. Aquaculture, v. 225, n. 1-4, p. 243-250, 2003.
- LU, M. W.; CHAO, Y. M.; GUO, T. C.; SANTI, N.; EVENSEN, O.; KASANI, S. K.; HONG, J. R.; WU, J. L. The interferon response is involved in nervous necrosis virus acute and persistent infection in zebra-fish infection model. Molecular Immunology, v. 45, n. 4, p. 1146-1152, 2008.
- MARTINS, M. L.; MOURIÑO, J. L. P.; AMARAL, G. V.; VIEIRA, F. N.; DOTTA, G.; JATOBÁ, A. M. B.; PEDROTTI, F. S.; JERONIMO, G. T.; BUGLIONE NETO, C. C.; PEREIRA, G. Haematological changes in Nile tilapia experimentally infected with Enterococcus sp. Brazilian Journal of Biology, v. 68, n. 3, p. 631-637, 2008.
- MONTERO, D.; GRASSO, V.; IZQUIERDO, M. S.; GANGA, R.; REAL, F.; TORT, L.; CABALLERO, M. J.; ACOSTA, F. Total substitution of fish oil by vegetable oils in gilthead sea bream (Sparus aurata) diets: effects on hepatic Mx expression and some immune parameters. Fish and Shellfish Immunology, v. 24, n. 2, p. 147-155, 2008.
- ROSENFELD, G. Corante pancrômico para hematologia e citologia clínica: nova combinação dos componentes do May-Grünwald e do Giemsa num só corante de emprego rápido. Memórias do Instituto Butantan, v. 20, n. 1, p. 329-334, 1947.
- ROWLEY, A. F.; KNIGHT, J.; LLOYD-EVANS, P.; HOLLAND, J. W.; VICKER, P. J. Eicosanoids and their role in immune modulation in fish-a brief overview. Fish and Shellfish Immunology, v. 5, n. 8, p. 549-567, 1995.
- SARGENT, J. R.; TOCHER, D. R.; BELL, J. G. The lipids. In: HALVER, J. E.; HARDY, R. (Ed.). Fish nutrition. San Diego: Academic Press, 2002. p. 182-246.
- SECOMBES, C. J. The nonspecific immune system: cellular defense. In: IWAMA, G.; NAKANISHI T. (Ed.). The fish immune system. San Diego: Academic Press, 1996. p. 63-105.
- SHELDON JR., W. M.; BLAZER, V. S. Influence of dietary lipid and temperature on bactericidal activity of channel catfish macrophages. Journal of Aquatic Animal Health, v. 3, n. 1, .p. 87-93, 1991.
- SHERAN, C. N. Novel lipid mediators in resolution and their aspirin triggered epimers: lipoxins, resolvins, and protectins. In: ROSSI, A. G.; SAWATZKY, D. A. (Ed.). The resolution of inflammation. New York: Birkhäuser Verlag Basel, 2008. p. 93-117.
- TOCHER, D. R. Glycerophospholipid metabolism. In: HOCHACHKA, P. W; MOMMSEN, T. P. (Org.). Biochemistry and molecular biology of fishes. Amsterdam: Elsevier Press, 1995. p. 119-157.
- TOCHER, D. R. Metabolism and functions of lipids and fatty acids in teleost fish. Reviews in Fisheries Science, v. 11, n. 2, p. 107-184, 2003.
- VARGAS, R. J.; GUIMARÃES DE SOUZA, S. M.; MABILIA, R.; CARLET, F.; BAGGIO, S. Resposta fisiológica à infestação experimental com Ichthyophthirius multifiliis (Fouquet, 1876) em alevinos de jundiá Rhamdia quelen (Quoy and Gaimard, 1824) previamente alimentados com diferentes fontes lipídicas. Revista Brasileira de Parasitologia Veterinária, v. 17, n. 2, p. 81-86, 2008.
- WHYTE, S. The innate immune response of finfish - a review of current knowledge. Fish and Shellfish Immunology, v. 23, n. 6, p. 1127-1151, 2007.
- XU, H.; AI, Q.; MAI, K.; XU, W.; WANG, J.; MA, H.; ZHANG, W.; WANG, X.; LIUFU, Z. Effects of dietary arachidonic acid on growth performance, survival, immune response and tissue fatty acid composition of juvenile Japanese seabass, Lateolabrax japonicas. Aquaculture, v. 307, n. 1, p. 75-82, 2010.
Publication Dates
-
Publication in this collection
12 Nov 2013 -
Date of issue
Dec 2013
History
-
Received
24 Jan 2013 -
Accepted
27 May 2013