Abstract
Ocotea porosa (Nees & Mart.) Barroso, commonly known as “imbuia”, “canela-imbuia” or “imbuia-amarela” in Brazil, is a tree of the Southern Atlantic Forest. The present study investigates the anatomy of leaf and stem, volatile oil chemistry, as well as cytotoxicity and insecticidal activities of the essential oil of O. porosa. Species identification was achieved by anatomy features, mainly due to paracytic and anomocytic stomata; non-glandular trichomes; biconvex midrib and petiole with a collateral open arc vascular bundle; presence of a sclerenchymatous layer, starch grains and crystal sand in the stem; and the presence of phenolic compounds in the epidermis, phloem and xylem of the midrib, petiole and stem. The main volatile components of the essential oil were α-pinene (19.71%), β-pinene (13.86%) and bicyclogermacrene (24.62%). Cytotoxicity against human cancer cell (MCF-7), mouse cancer cell (B16F10) and mouse non-tumoral cell (McCoy) was observed as well as insecticidal activity of the essential oil against susceptible ‘Ft. Dix’ bed bugs (Cimex lectularius L.) by topical application.
Keywords:
anticancer; bed bugs; Cimex lectularius; cytotoxic effect; imbuia; light and scanning electron microscopy
INTRODUCTION
Ocotea Aubl. is one of the most representative genus of Lauraceae comprising 428 species [11 The Plant List. A working list of all plant species [Internet]. [place unknown: publisher: unknown]; [updated 2020 Mar 03; cited 2020 Mar 03] Available from: www.theplantlist.org/1.1/browse/A/Lauraceae/Ocotea/.
www.theplantlist.org/1.1/browse/A/Laurac...
], with 168 of these species occurring in Brazil [22 Ocotea in Flora do Brasil 2020 em construção. [Jardim Botânico do Rio de Janeiro]; [updated 2020 Mar 03; cited 2020 Mar 03]. Available from: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB8440.
http://floradobrasil.jbrj.gov.br/reflora...
]. Ocotea porosa (Nees & Mart.) Barroso and O. odorifera (Vell.) Rohwer are the most important Brazilian representatives of this genus [33 Vattimo-Gil I. Contribuição ao conhecimento da distribuição geográfica das Lauraceae VII. Rodriguésia. 1980 Jul;32(54):351-367. doi:10.1590/2175-78601980325419.
https://doi.org/10.1590/2175-78601980325...
]. The taxonomy and delimitation of Ocotea are problematic due to similar features with Cinnamomum Schaeff. and Nectandra Rol. ex Rottb. [44 Rohwer JG. Prodromus einer Monographie der Gattung Ocotea Aubl. (Lauraceae), sensu lato. Mitt Inst Allg Bot Hamburg. 1986 Apr;20:3-278.].
Several species of Ocotea have been reported to have important biological activities, such as antiherpetic [55 Garrett R, Romanos MTV, Borges RM, Santos MG, Rocha L, Silva AJR. Antiherpetic activity of a flavonoid fraction from Ocotea notate leaves. Rev Bras Farmacogn. 2012 Mar;22(2):306-313. doi:10.1590/S0102-695X2012005000003.
https://doi.org/10.1590/S0102-695X201200...
], antimycobacterial [66 Costa IFB, Calixto SD, Araujo MH, Konno TUP, Tinoco LW, Guimarães DO, Lasunskaia EB, Leal IRC, Muzitano MF. Antimycobacterial and nitric oxide production inhibitory activities of Ocotea notata from Brazilian restinga. Sci World J. 2015;947248:1-9. doi:10.1155/2015/947248.
https://doi.org/10.1155/2015/947248...
], antibacterial [77 Farago PV, Budel JM, Duarte MR, Nakashima T. Análise morfoanatômica de folhas de Ocotea puberula (Rich.) Nees, Lauraceae. Rev Bras Farmacogn. 2005 Jul;15(3):250-255. doi:10.1590/S0102-695X2005000300016.
https://doi.org/10.1590/S0102-695X200500...
], antinociceptive [88 Montrucchio DP, Miguel OG, Zanin SMW, Silva GA, Cardozo AM, Santos ARS. Antinociceptive effects of a chloroform extract and the alkaloid dicentrine isolated from fruits of Ocotea puberula. Planta Med. 2012;78(14):1543-1548. doi:10.1055/s-0032-1315026.
https://doi.org/10.1055/s-0032-1315026...
], antiplatelet and antithrombotic [99 Silva JK, Trindade R, Moreira EC, Maia JGS, Dosoky NS, Miller RS, Cseke LJ, Setzer WN. Chemical diversity, biological activity, and genetic aspects of three Ocotea species from the Amazon. Int J Mol Sci. 2017 May;18(5):1081-1095. doi:10.3390/ijms18051081.
https://doi.org/10.3390/ijms18051081...
], acaricidal [1010 Barbosa LCA, Filomeno CA, Teixeira RR. Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules. 2016 Dec;21(12):1671-1686. doi:10.3390/molecules21121671.
https://doi.org/10.3390/molecules2112167...
,1111 Conceição RS, Carneiro MMAA, Reis IMA, Branco A, Vieira IJC, Braz-Filho R, Botura MB. In vitro acaricide activity of Ocotea aciphylla (Nees) Mez. (Lauraceae) extracts and identification of the compounds from the active fractions. Ticks and Tick-borne Diseases. 2017 Feb;8(2):275-282. doi:10.1016/j.ttbdis.2016.11.013.
https://doi.org/10.1016/j.ttbdis.2016.11...
], anti-inflammatory [1212 Ballabeni V, Tognolini M, Giorgio C, Bertoni S, Bruni R, Barocelli E. Ocotea quixos Lam. essential oil: In vitro and in vivo investigation on its anti-inflammatory properties. Fitoterapia. 2010 Jun;81(4)289-295. doi:10.1016/j.fitote.2009.10.002.
https://doi.org/10.1016/j.fitote.2009.10...
], antiprotozoal activity against Trypanosoma cruzi and Leishmania [1313 Fournet A, Ferreira ME, Arias AR, Guy I, Guinaudeau H, Heinzen H. Phytochemical and antiprotozoal activity of Ocotea lancifolia. Fitoterapia. 2007 Jul;78(5)382-384. doi:10.1016/j.fitote.2007.03.003.
https://doi.org/10.1016/j.fitote.2007.03...
], cytotoxic [1414 Garcez FR, Silva AGR, Garcez WS, Linck G, Matos MCF, Santos ECS, Queiroz LMM. Cytotoxic aporphine alkaloids from Ocotea acutifolia. Planta Med. 2011 Oct;77(4)383-387. doi:10.1055/s-0030-1250401.
https://doi.org/10.1055/s-0030-1250401...
], antioxidant and antimutagenic activities [1515 Gontijo DC, Brandão GC, Gontijo PC, Oliveira AB, Diaz MAN, Fietto LG, Leite JPV. Identification of phenolic compounds and biologically related activities from Ocotea odorifera aqueous extract leaves. Food Chem. 2017 Sep;230:618-626. doi:10.1016/j.foodchem.2017.03.087.
https://doi.org/10.1016/j.foodchem.2017....
]. The essential oils of Ocotea species have also exhibited promising antimicrobial activities against Escherichia coli and showed cytotoxicity against MCF-7 cells [99 Silva JK, Trindade R, Moreira EC, Maia JGS, Dosoky NS, Miller RS, Cseke LJ, Setzer WN. Chemical diversity, biological activity, and genetic aspects of three Ocotea species from the Amazon. Int J Mol Sci. 2017 May;18(5):1081-1095. doi:10.3390/ijms18051081.
https://doi.org/10.3390/ijms18051081...
].
Monoterpenoids, sesquiterpenoids and phenylpropanoids were found in their essential oils [1616 Chaverri C, Cicció JF. Essential oil of trees of the genus Ocotea (Lauraceae) in Costa Rica. I. Ocotea brenesii. Rev Biol Trop. 2005 Sep;53:431-436. doi:10.15517/rbt.v53i3-4.14611.
https://doi.org/10.15517/rbt.v53i3-4.146...
,1717 Takaku S, Haber WA, Setzer WN. Leaf essential oil composition of 10 species of Ocotea (Lauraceae) from Monteverde, Costa Rica. Biochem Syst Ecol. 2007 Aug;35(8):525-532. doi:10.1016/j.bse.2007.02.003.
https://doi.org/10.1016/j.bse.2007.02.00...
]. Other metabolites such as benzylisoquinolinic and aporphinic alkaloids [1818 Vilegas JHY, Gottlieb OR, Kaplan MA, Gottlieb HE. Aporphine alkaloids from Ocotea caesia. Phytochemistry. 1989;28(12):3577-3578. doi:10.1016/0031-9422(89)80403-0.
https://doi.org/10.1016/0031-9422(89)804...
,1919 Dias CS, Silva IG, Cunha EVL, Silva MS, Braz-Filho R, Barbosa-Filho JM. Isolamento e identificação de novos alcalóides de Ocotea duckei Vattimo (Lauraceae). Rev Bras Farmacogn. 2003;13(1 Suppl):62-63. doi:10.1590/S0102-695X2003000300023.
https://doi.org/10.1590/S0102-695X200300...
], lignans [2020 Morais LCSL, Almeida RN, Cunha EVL, Silva MS, Barbosa-Filho JM, Gray AI. Further lignans from Ocotea duckei. Pharm Biol. 1999;37(2):144-147. doi:10.1076/phbi.37.2.144.6084.
https://doi.org/10.1076/phbi.37.2.144.60...
] were also described from the genus.
Ocotea porosa, usually called “imbuia”, “imbuia-amarela”, “imbuia-zebrina” in Brazil, is a typical tree species from Southern Atlantic Forest that reaches up to 15 m high. In spite of legal instruments that prevent the species exploitation, its wood is still considered as one of the most valuable for furniture and construction industry due to its moderately density and resistance to fungal infection [2121 Amato CM. Ecologia de populações de Ocotea porosa (Nees) Barroso em áreas submetidas a diferentes graus de perturbação [dissertation]. Curitiba: Universidade Federal do Paraná Curitiba; 2008. 49 p.]. This species is in danger of extinction due to overexploitation for wood extraction [22 Ocotea in Flora do Brasil 2020 em construção. [Jardim Botânico do Rio de Janeiro]; [updated 2020 Mar 03; cited 2020 Mar 03]. Available from: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB8440.
http://floradobrasil.jbrj.gov.br/reflora...
].
Considering the wide number of biological activities for essential oils from Ocotea, and the fact that no previous work was devoted to investigate the chemical composition of leaf essential oil and the anatomy of O. porosa, the present study aims to investigate the chemical profile of the volatile oil and its cytotoxic and insecticidal activities, as well as the anatomy of the leaf and stem of this species in order to provide accurate information to support the identification of plant materials.
MATERIAL AND METHODS
Plant Material
Fresh samples of leaves and stems of Ocotea porosa were collected from plants growing in open and sunny areas of União da Vitória, Paraná, Brazil (26º13’48’’ S and 51º05’11” W) in July 2017. The voucher specimen was identified and deposited in the herbarium of State University of Ponta Grossa under number HUPG 22243. The access to botanical materials was authorized and licensed by the Genetic Heritage Administration Council (CGEN/SISGEN) according to code AD3F256.
At least six samples of mature leaves (cut from median, intercostal and margin regions) were obtained from the sixth node and below, as well as stem fragments 5 to 10 cm from the shoot were collected for anatomical analysis.
For the extraction of essential oil, the plant material was selected and standardized in order to acquire leaves and stems in the same pattern. The plant material was then dried in shade and cut into small pieces (~1 cm).
Microscopic procedure
Freshly collected leaves and stems of O. porosa were fixed in formalin-acetic acid-alcohol (FAA) solution [2222 Johansen DA. Plant microtechnique. New York: McGraw Hill Book; 1940. 523 p.], for three days, washed in distilled water and then stored in 70% ethanol (v/v) [2323 Berlyn GP, Miksche JP. Botanical microtechnique and cytochemistry. Iowa: The Iowa State University Press; 1976.] until use. Cross and longitudinal sections were freehand prepared using razor blades, placed on glass slides, hydrated, and stained with toluidine blue [2424 O'Brien TP, Feder N, McCully ME. Polychromatic staining of plant cell walls by Toluidine Blue O. Protoplasma. 1964 Jul;59:368-373. doi:10.1007/BF01248568.
https://doi.org/10.1007/BF01248568...
] or Astra blue and basic fuchsine combination [2525 Roeser KR. Die nadel der schwarzkiefer-massenprodukt und kunstwerk der natur. Mikrokosmos. 1962;61:33-36.]. Kraus and Arduin [2626 Kraus JE, Arduin M. Manual básico de métodos em morfologia vegetal. Rio de Janeiro: EDUR; 1997. p. 159-165.] methods were used to analyze epidermal features.
Quantitative studies of stomata were performed by taking ten measurements from multiple leaf specimens. The length and width of stomata were measured from ten stomata at different places on the leaf blade for each species to determine the average stomatal size.
For the analysis by field emission scanning electron microscopy (FEG-SEM), the samples fixed in FAA were passed through a series of ethanol solutions of increasing concentrations and then dried in a critical point dryer (Balzers CPD-030) using liquid CO2. The dried samples were mounted on aluminum stubs using glued carbon tapes and then coated with gold using a Quorum SC7620 sputter coater [2727 Justus B, Almeida VP, Gonçalves MM, Assunção DPSF, Borsato DM, Arana AFM, Maia BHLNS, Paula JFP, Budel JM, Farago PV. Chemical composition and biological activities of the essential oil and anatomical markers of Lavandula dentata L. cultivated in Brazil. Braz Arch Biol Technol. 2018 Nov;61:1-12. doi:10.1590/1678-4324-2018180111.
https://doi.org/10.1590/1678-4324-201818...
]. Photomicrographs were prepared and examined using a Mira 3 Tescan FEG-SEM equipment located at the State University of Ponta Grossa (UEPG).
For histochemical tests, the following standard solutions were used: potassium dichromate (10%) [2828 Gabe M. Techniques Histologiques. Paris: Masson & Cie; 1968. 704 p.] and ferric chloride (2%) [2222 Johansen DA. Plant microtechnique. New York: McGraw Hill Book; 1940. 523 p.] for phenolic compounds, phloroglucinol/HCl for lignin [2929 Sass JE. Botanical microtechnique. 3rd ed. Ames: The Iowa State College Press; 1958. 228 p.], Sudan III for lipophilic components [3030 Foster AS. Practical plant anatomy. 2nd ed. Princeton: D. Van Nostrand; 1949. 228 p.], and iodine (1%) for starch [2323 Berlyn GP, Miksche JP. Botanical microtechnique and cytochemistry. Iowa: The Iowa State University Press; 1976.]. Controls were prepared in parallel with the tests and were carried out as described above. Photomicrographs were prepared using digital camera (C7070) attached to the light microscope (Olympus CX 31) at UEPG.
Extraction of essential oil (EO) and GC-MS analysis
Dried plant material (300 g) was subjected to hydrodistillation for 4 h in triplicate using a modified Clevenger-type apparatus for EO extraction. The obtained oil was dried using anhydrous Na2SO4 and kept in glass vials with Teflon-sealed caps at 4 ± 0.5ºC in absence of light.
The EO was analyzed using a Shimadzu GC-2010 Plus GC-MS/MS apparatus coupled to a TQ8040 triple quadrupole type tandem mass detector and AOC-5000 Plus automatic injector for analysis of liquid samples (headspace) and solid phase microextraction (SPME). The samples were diluted at 1% (v/v) in methylene chloride and characterized using the following analytical conditions: Rtx-5MS fused silica capillary column (5% diphenyl + 95% dimethylpolysiloxane (30 m x 0.25 mm x 0.25 μm). The carrier gas used was helium at a flow rate of 1.02 mL/min, in 1:90 split mode. The injector was set at 250°C and the ionization system at 70 eV. 1 μL of sample was injected into the following heating ramp: initial temperature 60 °C to 250 °C with heating rate at 3 °C/min.
A homologous series of linear saturated hydrocarbons, C8 to C19 was used to calculate the retention index. The experimental retention index was obtained using the following Van den Dool and Kratz equation:
In the above equation, C n is the number of carbon atoms of the n-alkane whose retention time is immediately greater than the retention time of the analyte; C n-1 is the number of carbon atoms of the n-alkane whose retention time is immediately less than the retention time of the analyte; T x is the retention time of the analyte; Tn is the retention time of the C n alkane;T n-1 is the retention time of the C n-1 alkane.
The EO volatile components were identified by comparing retention indices and mass spectra with literature [3131 Adams RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Illinois: Carol Stream; 2007. 804 p.] and mass spectra were also compared with the NIST 02 mass library (NIST, Gaithersburg, MD, USA). The relative quantification was determined from the normalization of each peak area with the total chromatogram, with no use of any correction factor. This experimental was carried out at the Federal University of Paraná.
Cell culture
The human cancer cell (MCF-7, breast cancer, Rio de Janeiro cell bank n. 0162), mouse cancer cell (B16F10, melanoma, Rio de Janeiro cell bank n. 0342) and mouse non-tumoral cell (McCoy, fibroblast, Rio de Janeiro cell bank n. 0160) were maintained in RPMI 1640 medium (pH 7.4) supplemented with 10% fetal bovine serum (FBS), 24 mmol/L of sodium bicarbonate and 1% penicillin and streptomycin under controlled temperature (37 °C) and humidified atmosphere (5% CO2). To cell and subcultures expansion, the same conditions were used.
Cytotoxic assay
This assay relies on the ability of viable cells to metabolically reduce a yellow tetrazolium salt (MTT) to a purple formazan product. This reaction takes place when mitochondrial reductases are active [3232 Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec;65(1-2):55-63. doi:10.1016/0022-1759(83)90303-4.
https://doi.org/10.1016/0022-1759(83)903...
]. Cells were placed in 96 wells plates (4 ×103 cell/well). After 24 h, aliquots of O. porosa EO ranging from 0.77 µg/mL to 77 mg/mL dissolved in RPMI were added upon each cell and incubated for 72 h. The medium was removed, replaced with 100 µL of MTT solution (0.5 mg/mL) and incubated at 37 °C for 2 h. After this period of incubation, the resulting formazan crystals were solubilized in isopropyl alcohol acid and the optical density was red at 550 nm using an Elisa plate reader. The negative control was prepared as described above, but each cell was only incubated using RPMI medium. Tests were performed in quadruplicate and repeated three times.
IC 50 and selectivity index
IC50 were determined using logarithmic dose-response sigmoidal curves based on nonlinear regression (using GraphPad Prism software). The selectivity index (SI) was calculated as the ratio IC50 (control cells - McCoy)/IC50 (tumor cell lines - B6F10 or MCF-7). A selectivity index > 1 indicates that the cytotoxicity on cancer cells surpassed the one on the healthy non-tumor cells.
Morphological study
Cells (3 ×103 cell/well) were seeded in 24-well plates containing a coverslip on the bottom, and incubated at 37 °C and 5% CO2 for 24 h. After this time, the cells were treated with O. porosa EO (77 µg/mL), and incubated for 24 h. After that the culture medium was removed, washed with PBS, fixed with 2% formaldehyde for 2 min and stained with May-Grünwald stain. Coverslips were mounted and observed under a microscope coupled to a digital camera.
Insecticidal activity studies
Strains of Cimex lectularius, Bayonne (insecticide resistant) and Ft. Dix (susceptible) were provided by Dr. Changlu Wang, Department of Entomology, Rutgers University, New Brunswick, NJ, and their colony was raised using blood feeders (CG-1836-75 ChemGlass). The insecticidal activity of O. porosa EO against bed bugs was assessed by fumigation, topical application, and residual studies.
For fumigation study, bed bugs were exposed to vapor toxicity in 125 mL clear glass jars. A small piece of paper was placed in the jar's bottom to deliver a substrate for the bed bugs to rest during the tests. Bed bugs were introduced in the jars 2-4 h before treatment to acclimatize. A treatment solution or acetone aliquot of 2 μL was located directly onto the internal surface of the bottle side wall ∼4 cm from bottle bottom using a 50 μL gas-tight syringe (Hamilton Company, Reno, NV) attached to a PB600 (Hamilton Company, Reno, NV) repeating dispense. Five concentrations viz., 15.6, 31.25, 62.5, 125 and 250 μg EO/125 sq.cm were tested against the bed bugs. The jars were placed in the growth chamber and data for mortality were verified 24 h after the treatment. The 2,2-diclorovinil-dimetylphosphate (DDVP) was used as the standard.
Studies using O. porosa EO in topical application were carried out with adult insects, which were separated in the Petri dishes and anesthetized with CO2. 1 µL of treatment solution (50 µg/bug) in acetone was carried onto the dorsal surface of the abdomen, using a hand-held repeating dispenser. Control bugs received 1 μL of acetone alone. Data for the mortality of the bed bugs were verified for seven days after treatment. There were three replicates with ten bugs (mixed sex)/replicate. The standard was deltamethrin (2.4 ng/bug).
For residual studies, an aliquot of 100 µL of treatment (diluted in acetone) was applied on 20 cm2 Whatman #1 filter paper achieving 100 μg/cm2 of residues. The treated filter papers were then placed in the Petri dish. Only acetone was used in control tests. Ten adult bed bugs were released on the filter paper and mortality was recorded as mentioned in topical application. Deltamethrin was used as standard insecticide.
Statistical analysis
Cytotoxicity assays were analyzed by the difference of experimental statistical significance using analysis of variance (ANOVA) followed by Tukey's test. The experimental values were expressed as the mean ± standard error of the mean. The data were analyzed using Graph Pad Prism 7.0 software. The level of p<0.05 was used to determine statistical significance.
Treatment means of vapor toxicity were compared using two factor analysis of variance (ANOVA) and separated by Tukey’s honestly significant difference (HSD). Analysis were performed in SAS® 9.3 and JMP®10.0.
RESULTS AND DISCUSSION
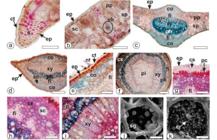
Microscopical analysis
Ocotea porosa (Figure 1 A, B) leaves have domatia in the nerve axils beneath (Figure 1 D). This feature has also been observed in other species, such as O. urbaniana Mez, O. pulchella(Nees & Mart.) Mez, O. tristis (Nees & Mart.) Mez and O. catharinensis Mez [3333 Santos M, Oliveira PL. Aspectos anatômicos do pecíolo de quatro espécies do gênero Ocotea Aubl. (Lauraceae) ocorrentes no Rio Grande do Sul. INSULA Revista de Botânica. 1995;24:3-14.].
From the surface view, the leaf possesses straight anticlinal cell walls on adaxial side and wavy on abaxial epidermis (Figure 1 C, E). This feature is also found in O. puberula (Rich.) Nees [3434 Farago PV, Paula JP, Nakashima T, Döll-Boscardin P, Budel JM, Maia BHLNS. Chemical composition and antibacterial activity of the essential oil from bark of Ocotea puberula (Rich.) Ness. Lat Am J Pharm. 2010 Mar;29(7):1242-1245.]. However, O. indecora (Schott) Mez had sinuous anticlinal walls on both sides [3535 Gonçalves RA, Pinheiro AB, Oliveira MA, Nascimento RT, Rosalem PF, Garcia VL, Martins AR. Anatomical characters and chemical profile of leaves of three species in Lauraceae family. Rev Bras Farmacogn. 2018 Jan;28(1):1-8. doi:10.1016/j.bjp.2017.11.008.
https://doi.org/10.1016/j.bjp.2017.11.00...
] whereas O. gardneri (Meisn.) Mez had both epidermises with straight anticlinal cell walls [3636 Coutinho DF, Agra MF, Barbosa-Filho JM, Basílio IJLD. Morfo-anatomia foliar de Ocotea gardneri (Meisn.) Mez (Lauraceae-Lauroideae). Rev Bras Farmacogn. 2006 Apr;16(2):178-184. doi:10.1590/S0102-695X2006000200008.
https://doi.org/10.1590/S0102-695X200600...
]. This is a significant feature in distinguishing various species of Ocotea.
Epicuticular waxes are found on the epidermis, especially on the stomata (Figure 1 G, H). This characteristic was not mentioned for other species of Ocotea. The stomata are of paracytic or anomocytic types (Figure 1 E) and the leaves are hipostomatic as also reported for several other Ocotea species [77 Farago PV, Budel JM, Duarte MR, Nakashima T. Análise morfoanatômica de folhas de Ocotea puberula (Rich.) Nees, Lauraceae. Rev Bras Farmacogn. 2005 Jul;15(3):250-255. doi:10.1590/S0102-695X2005000300016.
https://doi.org/10.1590/S0102-695X200500...
,3535 Gonçalves RA, Pinheiro AB, Oliveira MA, Nascimento RT, Rosalem PF, Garcia VL, Martins AR. Anatomical characters and chemical profile of leaves of three species in Lauraceae family. Rev Bras Farmacogn. 2018 Jan;28(1):1-8. doi:10.1016/j.bjp.2017.11.008.
https://doi.org/10.1016/j.bjp.2017.11.00...
36 Coutinho DF, Agra MF, Barbosa-Filho JM, Basílio IJLD. Morfo-anatomia foliar de Ocotea gardneri (Meisn.) Mez (Lauraceae-Lauroideae). Rev Bras Farmacogn. 2006 Apr;16(2):178-184. doi:10.1590/S0102-695X2006000200008.
https://doi.org/10.1590/S0102-695X200600...
37 Toledo MGT. Estudo botânico e fitoquímico de Ocotea odorifera (Vell.) Rohwer. (Lauraceae) da região metropolitana de Curitiba [dissertation]. Curitiba: Universidade Federal do Paraná; 2000. 62 p.
38 Coutinho DF, Agra MF, Basílio IJLD, Barbosa-Filho JM. Estudo morfoanatômico das folhas de Ocotea duckei Vattimo (Lauraceae-Lauroideae). Rev Bras Farmacogn. 2006 Oct;16(4):537-544. doi:10.1590/S0102-695X2006000400017.
https://doi.org/10.1590/S0102-695X200600...
-3939 Santos M, Oliveira PL. Aspectos anatômicos da lâmina foliar de Ocotea porosa (Nees et Mart. ex Nees) J. Angely (Lauraceae). INSULA Revista de Botânica. 1988;18:3-22.]. The average size of stomata is 29 × 23 µm. The stomatal index calculated for O. porosa is 16.43 per unit area (1 sq. mm) on the abaxial side.
Ocotea porosa evidences simple unicellular non-glandular trichomes on both surfaces although rarely on the adaxial side (Figure 1 F, G). Similar trichomes have also been reported for O. puberula [3434 Farago PV, Paula JP, Nakashima T, Döll-Boscardin P, Budel JM, Maia BHLNS. Chemical composition and antibacterial activity of the essential oil from bark of Ocotea puberula (Rich.) Ness. Lat Am J Pharm. 2010 Mar;29(7):1242-1245.] and O. indecora [3535 Gonçalves RA, Pinheiro AB, Oliveira MA, Nascimento RT, Rosalem PF, Garcia VL, Martins AR. Anatomical characters and chemical profile of leaves of three species in Lauraceae family. Rev Bras Farmacogn. 2018 Jan;28(1):1-8. doi:10.1016/j.bjp.2017.11.008.
https://doi.org/10.1016/j.bjp.2017.11.00...
]. Even though this feature is common in the taxa of Lauraceae [3535 Gonçalves RA, Pinheiro AB, Oliveira MA, Nascimento RT, Rosalem PF, Garcia VL, Martins AR. Anatomical characters and chemical profile of leaves of three species in Lauraceae family. Rev Bras Farmacogn. 2018 Jan;28(1):1-8. doi:10.1016/j.bjp.2017.11.008.
https://doi.org/10.1016/j.bjp.2017.11.00...
], O. gardneri had glabrous leaves [3636 Coutinho DF, Agra MF, Barbosa-Filho JM, Basílio IJLD. Morfo-anatomia foliar de Ocotea gardneri (Meisn.) Mez (Lauraceae-Lauroideae). Rev Bras Farmacogn. 2006 Apr;16(2):178-184. doi:10.1590/S0102-695X2006000200008.
https://doi.org/10.1590/S0102-695X200600...
].
Morpho-anatomy ofOcotea porosa [c, e: Light microscopy; d, f, g, h: FEG-SEM]. a- Plant in habit. b- Leaves. c-h- Leaf in surface view (c- adaxial, e-h- abaxial). [gl-domatia of glands, st- stomata, nt- non-glandular trichome, wa- waxes]. Scale bar: a = 70 cm; b = 4 cm; d = 1 mm; f = 100 µm; c, e = 50 µm; g = 20 µm; h= 5µm.
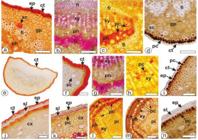
In transverse section (TS) of the leaf, the epidermis is uniseriate (Figure 2 A, B) and the cells contain phenolic compounds that reacted positively for histochemical tests. Epidermis is covered by a thin cuticle that reacted for Sudan III. The leaf is dorsiventral and is formed by two layers of palisade and 5-6 layers of spongy parenchyma (Figure 2 B, C). The veinlets traversing the mesophyll region are represented by small collateral vascular bundles surrounded by sclerenchymatous sheath with extensions that reach both faces of the epidermis (Figure 2 B). Dorsiventral mesophyll is frequent in the family Lauraceae [4040 Metcalfe CR, Chalk L. Anatomy of the Dicotyledones: leaves, stem and wood in relation to taxonomy with notes on economic uses. Oxford: Clarendon Press; 1972. 1500 p.]. However, O. gardneri evidenced isobilateral mesophyll [3636 Coutinho DF, Agra MF, Barbosa-Filho JM, Basílio IJLD. Morfo-anatomia foliar de Ocotea gardneri (Meisn.) Mez (Lauraceae-Lauroideae). Rev Bras Farmacogn. 2006 Apr;16(2):178-184. doi:10.1590/S0102-695X2006000200008.
https://doi.org/10.1590/S0102-695X200600...
].
The edge of the lamina is slightly curved downwards. The epidermis possesses cells with irregular shape and covered by thick cuticle. Underneath the epidermis many layers of sclerenchyma cells are found (Figure 2 A). These characteristics have also been observed in O. gardneri [3636 Coutinho DF, Agra MF, Barbosa-Filho JM, Basílio IJLD. Morfo-anatomia foliar de Ocotea gardneri (Meisn.) Mez (Lauraceae-Lauroideae). Rev Bras Farmacogn. 2006 Apr;16(2):178-184. doi:10.1590/S0102-695X2006000200008.
https://doi.org/10.1590/S0102-695X200600...
].
Secretory cells with spherical to oblong shape (Figure 2 B) and with light yellow lipophilic contents reacted with Sudan III in the histochemical test (Figure 3 A) are found in the lamina, especially in the adaxial side as well as in the midrib regions. Secretory cells are widely reported in the species of Ocotea [3434 Farago PV, Paula JP, Nakashima T, Döll-Boscardin P, Budel JM, Maia BHLNS. Chemical composition and antibacterial activity of the essential oil from bark of Ocotea puberula (Rich.) Ness. Lat Am J Pharm. 2010 Mar;29(7):1242-1245.
35 Gonçalves RA, Pinheiro AB, Oliveira MA, Nascimento RT, Rosalem PF, Garcia VL, Martins AR. Anatomical characters and chemical profile of leaves of three species in Lauraceae family. Rev Bras Farmacogn. 2018 Jan;28(1):1-8. doi:10.1016/j.bjp.2017.11.008.
https://doi.org/10.1016/j.bjp.2017.11.00...
36 Coutinho DF, Agra MF, Barbosa-Filho JM, Basílio IJLD. Morfo-anatomia foliar de Ocotea gardneri (Meisn.) Mez (Lauraceae-Lauroideae). Rev Bras Farmacogn. 2006 Apr;16(2):178-184. doi:10.1590/S0102-695X2006000200008.
https://doi.org/10.1590/S0102-695X200600...
37 Toledo MGT. Estudo botânico e fitoquímico de Ocotea odorifera (Vell.) Rohwer. (Lauraceae) da região metropolitana de Curitiba [dissertation]. Curitiba: Universidade Federal do Paraná; 2000. 62 p.
38 Coutinho DF, Agra MF, Basílio IJLD, Barbosa-Filho JM. Estudo morfoanatômico das folhas de Ocotea duckei Vattimo (Lauraceae-Lauroideae). Rev Bras Farmacogn. 2006 Oct;16(4):537-544. doi:10.1590/S0102-695X2006000400017.
https://doi.org/10.1590/S0102-695X200600...
-3939 Santos M, Oliveira PL. Aspectos anatômicos da lâmina foliar de Ocotea porosa (Nees et Mart. ex Nees) J. Angely (Lauraceae). INSULA Revista de Botânica. 1988;18:3-22.].
Anatomy ofOcotea porosa leaves and stems [a-i: Light microscopy; j, k: FEG-SEM]. a. TS through leaf margin; b. TS of lamina; c. TS of midrib and lamina; d and e. TS of petiole; f-k. TS of stem. [co- collenchyma, cr- crystals, cs- subepidermal layer, ct- cuticle, cx- cortex, ep- epidermis, fi- fibers, nt- non-glandular trichome, pc- phenolic compounds, sc- secretory cell, sg- starch grains, ph- phloem, pp- palisade parenchyma, pi- pith, sp- spongy parenchyma, vb- vascular bundle, xy- xylem]. Scale bar: a, b, e, g, h, i = 50 μm; c, d, f = 200 μm; k =10 μm; j = 5 μm.
In transverse section, the midrib is biconvex in outline (Figure 2 C). This characteristic has also been observed in O. odorifera (Vell.) Rohwer [3737 Toledo MGT. Estudo botânico e fitoquímico de Ocotea odorifera (Vell.) Rohwer. (Lauraceae) da região metropolitana de Curitiba [dissertation]. Curitiba: Universidade Federal do Paraná; 2000. 62 p.], O. puberula [3434 Farago PV, Paula JP, Nakashima T, Döll-Boscardin P, Budel JM, Maia BHLNS. Chemical composition and antibacterial activity of the essential oil from bark of Ocotea puberula (Rich.) Ness. Lat Am J Pharm. 2010 Mar;29(7):1242-1245.], O. indecora [3535 Gonçalves RA, Pinheiro AB, Oliveira MA, Nascimento RT, Rosalem PF, Garcia VL, Martins AR. Anatomical characters and chemical profile of leaves of three species in Lauraceae family. Rev Bras Farmacogn. 2018 Jan;28(1):1-8. doi:10.1016/j.bjp.2017.11.008.
https://doi.org/10.1016/j.bjp.2017.11.00...
] and O. gardneri [3636 Coutinho DF, Agra MF, Barbosa-Filho JM, Basílio IJLD. Morfo-anatomia foliar de Ocotea gardneri (Meisn.) Mez (Lauraceae-Lauroideae). Rev Bras Farmacogn. 2006 Apr;16(2):178-184. doi:10.1590/S0102-695X2006000200008.
https://doi.org/10.1590/S0102-695X200600...
]. The uniseriate epidermis is formed by cells with different shapes and sizes containing phenolic compounds which reacted with ferric chloride (Figure 3 D). The epidermis is covered by a thick cuticle evidenced by Sudan III in the histochemical tests (Figure 3 A). Beneath the epidermis several layers of annular collenchyma are found. Annular collenchyma was also found in O. odorifera [3737 Toledo MGT. Estudo botânico e fitoquímico de Ocotea odorifera (Vell.) Rohwer. (Lauraceae) da região metropolitana de Curitiba [dissertation]. Curitiba: Universidade Federal do Paraná; 2000. 62 p.] whereas the angular type was observed in O. puberula [3434 Farago PV, Paula JP, Nakashima T, Döll-Boscardin P, Budel JM, Maia BHLNS. Chemical composition and antibacterial activity of the essential oil from bark of Ocotea puberula (Rich.) Ness. Lat Am J Pharm. 2010 Mar;29(7):1242-1245.].
The vascular system of the midrib is represented by one collateral vascular bundle in open arc that is surrounded by a continuous sheath of sclerenchymatous fibers (Figures 2 C, 3 A-D) which reacted with phloroglucinol/HCl (Figure 3 B). Phenolic compounds are found in some cells of xylem and in several cells of phloem. These compounds are evidenced in the histochemical tests using potassium dichromate 10% (Figure 3 C). Idioblasts containing phenolic compounds were also found in the midrib of O. odorifera [3737 Toledo MGT. Estudo botânico e fitoquímico de Ocotea odorifera (Vell.) Rohwer. (Lauraceae) da região metropolitana de Curitiba [dissertation]. Curitiba: Universidade Federal do Paraná; 2000. 62 p.] and O. puberula [3434 Farago PV, Paula JP, Nakashima T, Döll-Boscardin P, Budel JM, Maia BHLNS. Chemical composition and antibacterial activity of the essential oil from bark of Ocotea puberula (Rich.) Ness. Lat Am J Pharm. 2010 Mar;29(7):1242-1245.].
The petiole, in cross-section, varies from biconvex shape with two lateral extensions in the distal region (Figure 2 D), flat-convex in the medial region (Figure 3 E) to cylindrical in the proximal region. This pattern has also been observed in O. diospyrifolia (Meisn.) Mez, O. pulchella (Nees & Mart.) Mezand, O. tristis (Ness & Mart.) Mez in the proximal region. However, biconvex shape with two lateral extensions occurred in the median and distal regions of O. pulchella, whereas flat-convex shape was found in the same regions in O. tristis [3333 Santos M, Oliveira PL. Aspectos anatômicos do pecíolo de quatro espécies do gênero Ocotea Aubl. (Lauraceae) ocorrentes no Rio Grande do Sul. INSULA Revista de Botânica. 1995;24:3-14.]. The petiole shape can help the Ocotea species identification as observed in Passiflora L. [4141 Wosch L, Imig DC, Cervi AC, Moura BB, Budel JM, Santos CAM. Comparative study of Passiflora taxa leaves: I. A morpho-anatomic profile. Rev Bras Farmacogn. 2015 Jul;25(4):328-343. doi:10.1016/j.bjp.2015.06.004.
https://doi.org/10.1016/j.bjp.2015.06.00...
] and Mikania Willd. [4242 Almeida VP, Hirt AA, Raeski PA, Mika BE, Justus B, Santos VLP, Franco CRC, Paula JP, Farago PV, Budel JM. Comparative morphoanatomical analysis of Mikania species. Rev Bras Farmacogn. 2017 Jan;27(1):9-19. doi:10.1016/j.bjp.2016.05.002.
https://doi.org/10.1016/j.bjp.2016.05.00...
].
The epidermis is unilayered and covered by thick cuticle (Figure 3 E) and has numerous non-glandular trichomes. Underneath the epidermis, several layers of annular collenchyma are observed on both sides (Figure 3 E). Some secretory cells are distributed in the petiole (Figure 3 F). Ocotea gardneri showed similar non-glandular trichomes only in the petiole [3636 Coutinho DF, Agra MF, Barbosa-Filho JM, Basílio IJLD. Morfo-anatomia foliar de Ocotea gardneri (Meisn.) Mez (Lauraceae-Lauroideae). Rev Bras Farmacogn. 2006 Apr;16(2):178-184. doi:10.1590/S0102-695X2006000200008.
https://doi.org/10.1590/S0102-695X200600...
].
Histochemistry of Ocotea porosa [a, e, f, j: Sudan III; b, g, k: phloroglucinol/HCl; d, i, m, n: ferric chloride (2%); c, h, l: potassium dichromate solution (10%). Transverse sections. a-d: midrib; e-i: petiole; j-n: stem [ct, cuticle; cx, cortex; en, endodermis; ep, epidermis; fi: fibers, gp, ground parenchyma; pc, phenolic compounds; ph, phloem; pi, pith; sc, secretory cavity; sl, sclerenchymatous layer; xy, xylem]. Scale bars: a-d, f-k = 50 μm, e = 200 μm, l, m = 100 μm.
In an incipient secondary structure, the stem is circular in shape (Figure 2 F). The epidermis is uniseriate and covered by a thin cuticle. Beneath the epidermis, a layer of sclerenchymatous cells (Figure 2 G) and a layer of cells containing phenolic compounds are found (Figure 2 G, H). The cortical parenchyma presents 10-12 layers (Figure 2 G, H). Secretory idioblasts are also present in the cortex (Figure 2 H). The vascular system has phloem towards the periphery, and xylem facing the pith, separated by a cambium (Figure 2 I). Perivascular fiber patches are adjoined to the phloem (Figure 2G-I). The fibers and xylem are reacted with phloroglucinol/HCl and stained pink evidencing lignification in the walls (Figure 3 K). The pith is made up of thin-walled parenchymatous cells. Starch grains (Figure 2 J) and sand crystals are found (Figure 2 K) in the pith region.
Yield and chemical composition of essential oil (EO)
The EO of O. porosa presents a light-yellow color and a strong and characteristic aroma. The light-yellow coloration is common to several Ocotea species [1717 Takaku S, Haber WA, Setzer WN. Leaf essential oil composition of 10 species of Ocotea (Lauraceae) from Monteverde, Costa Rica. Biochem Syst Ecol. 2007 Aug;35(8):525-532. doi:10.1016/j.bse.2007.02.003.
https://doi.org/10.1016/j.bse.2007.02.00...
]. The yield of O. porosa EO is 1.03%. The species O. caudata (Nees) Mez, O. cujumary Mart. and O. canaliculata (Rich.) Mez, which were collected in the National Forest of Caxiuanã, Amazonas, Brazil, presented an average yield of 0.8% [77 Farago PV, Budel JM, Duarte MR, Nakashima T. Análise morfoanatômica de folhas de Ocotea puberula (Rich.) Nees, Lauraceae. Rev Bras Farmacogn. 2005 Jul;15(3):250-255. doi:10.1590/S0102-695X2005000300016.
https://doi.org/10.1590/S0102-695X200500...
]. In the present study, leaves were used for EO extraction. The anatomical study evidenced several secretory cavities (Figures. 2 B, 3 A, D, F) that store EO in the leaf blade and petiole.
The chemical composition of the EO extracted from O. porosa leaves was analyzed by GC-MS and is summarized in Table 1. Comparing the groups, O. porosa EO has 38.19% of monoterpenoid hydrocarbons, 1.02% of oxygenated monoterpenoids, 35.21% of sesquiterpenoid hydrocarbons and 18.20% of oxygenated sesquiterpenoids. High concentrations of sesquiterpenoids were also found in other species of Ocotea, such as O. gomezii W.C. Burger and O. morae Gómez-Laur [4343 Chaverri C, Diaz C, Cicció JF. Chemical Analysis of Essential Oils from Ocotea gomezii W.C. Burger and Ocotea morae Gómez-Laur. (Lauraceae) Collected at "Reserva Biológica Alberto M. Brenes" in Costa Rica and their Cytotoxic Activity on Tumor Cell Lines. J Braz Chem Soc. 2011;22(4):741-745. doi:10.1590/S0103-50532011000400018.
https://doi.org/10.1590/S0103-5053201100...
].
Thirty-five volatile compounds (92.54%) of EO of O. porosa were identified. The major compounds were bicyclogermacrene (24.62%), α-pinene (19.71%) and β-pinene (13.86%). Weyerstahl and coworkers [4444 Weyerstahl P, Wahlburg HC, Splittgerber U, Marschall H. Volatile Constituents of Brazilian Phoebe Oil. Flavour and Fragrance Journal. 1994 Jul;9(4):179-186. doi:10.1002/ffj.2730090407.
https://doi.org/10.1002/ffj.2730090407...
] verified a distinct chemical composition for the EO of O. porosa extracted from the wood. These authors found carquejila acetate (2.1%), α-copaene (5.6%), γ-copaene (3.5%), δ-cadinene (3.1%), cremoligenol (8.4%), β-eudesmol (8.4%), valerianol (5%), α-bisabolol (3.6%) and β-bisabolol (2.9%) as the major compounds of EO derived from the wood. Reynolds and coworkers [4545 Reynolds T, Kite G. Volatile constituents of Brazilian Phoebe porosa Mez. J Essent Oil Res. 1995 Jul;7(4):415-418. doi:10.1080/10412905.1995.9698551.
https://doi.org/10.1080/10412905.1995.96...
] analyzed EO extracted from O. porosa stem barks and found as major components α-copaene (6.25%), δ-cadinene (3.28%), β-eudesmol (6.86%), valerianol (7.55%) and α-bisabolol (3.33%).
In the present study, the differences found in the chemical composition of O. porosa in relation to the literature data occurred due to the fact that EO was obtained from leaves and not from wood or stem barks. In addition, not only the composition of EO, but also the concentrations of the compounds vary depending on the age of the plant as well as other factors, such as circadian rhythms, seasonal conditions and environmental influences [4646 Brito AFR. Análise de variação sazonal e das atividades antifúngica e antimicrobiana em óleos essenciais de Ocotea porosa (Nees) Barroso e Nectandra megapotamica (Spreng.) Menz [dissertation]. São Paulo: Instituto de Química; 2009. 121p.]. Takaku and coauthors [1717 Takaku S, Haber WA, Setzer WN. Leaf essential oil composition of 10 species of Ocotea (Lauraceae) from Monteverde, Costa Rica. Biochem Syst Ecol. 2007 Aug;35(8):525-532. doi:10.1016/j.bse.2007.02.003.
https://doi.org/10.1016/j.bse.2007.02.00...
] analyzed the volatile components of leaves of several Ocotea species from Costa Rica, namely O. floribunda (Sw.) Mez, O. holdridgeana W.C.Burger, O. meziana C.K.Allen, O. sinuata (Mez) Rohwer, O. tonduzii Standl, O. valerioana (Standl.) W.C.Burger, O. veraguensis (Meisn.) Mez, and O. whitei Woodson. The most common volatile compounds among these species were α-pinene, β-pinene, β-caryophyllene and germacrene D. In the present study, α-pinene and β-pinene were present in high concentrations.
Considering the biological activities, the chemical composition of EO is extremely important and should be evaluated [1010 Barbosa LCA, Filomeno CA, Teixeira RR. Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules. 2016 Dec;21(12):1671-1686. doi:10.3390/molecules21121671.
https://doi.org/10.3390/molecules2112167...
]. In the present study, bicyclogermacrene, the most abundant compound in EO of O. porosa, showed a larvicidal action on the vectors of malaria, dengue, Japanese encephalitis [4747 Govindarajan M, Benelli G. Eco-friendly larvicides from Indian plants: Effectiveness of lavandulyl acetate and bicyclogermacrene on malaria, dengue and Japanese encephalitis mosquito vectors. Ecotoxicol Environ Saf. 2016 Nov;133:395-402. doi:10.1016/j.ecoenv.2016.07.035.
https://doi.org/10.1016/j.ecoenv.2016.07...
] and fungicide activities [99 Silva JK, Trindade R, Moreira EC, Maia JGS, Dosoky NS, Miller RS, Cseke LJ, Setzer WN. Chemical diversity, biological activity, and genetic aspects of three Ocotea species from the Amazon. Int J Mol Sci. 2017 May;18(5):1081-1095. doi:10.3390/ijms18051081.
https://doi.org/10.3390/ijms18051081...
].
The volatile compounds α-pinene and β-pinene were also found in high concentrations in the present study and have been evaluated for biological activities. Both compounds presented antibacterial, antiviral, antifungal and hypotensive activities [4848 Koziol A, Stryjewska A, Librowski T, Salat K, Gawel M, Moniczewski A, Lochynski S. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Rev Med Chem. 2014 Dec;14(14):1156-1168. doi:10.2174/1389557514666141127145820.
https://doi.org/10.2174/1389557514666141...
], α-pinene presented anti-inflammatory, hypoglycemic [4949 Özbek H, Ylmaz BS. Anti-inflammatory and hypoglycemic activities of alpha-pinene. Acta Pharm Sci. 2017;55(04):7-14. doi:10.23893/1307-2080.APS.05522.
https://doi.org/10.23893/1307-2080.APS.0...
], and gastroprotective activities [5050 Pinheiro MA, Magalhães RM, Torres DM, Cavalcante RC, Mota FSX, Coelho EMAO, Moreira HP, Lima GC, Araújo PCC, Cardoso JHL, Souza ANC, Diniz LRL. Gastroprotective effect of alpha-pinene and its correlation with antiulcerogenic activity of essential oils obtained from Hyptis. Pharmacogn Mag. 2015 Jan;11(41):123-130. doi:10.4103/0973-1296.149725.
https://doi.org/10.4103/0973-1296.149725...
] whereas β-pinene showed antidepressant [5151 Guzmán-Gutiérrez SL, Bonilla-Jaime H, Gómez-Cansino R, Reyes-Chilpa R. Linalool and ß-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sciences. 2015 May;128:24-29. doi:10.1016/j.lfs.2015.02.021.
https://doi.org/10.1016/j.lfs.2015.02.02...
] and antiviral [5252 Astani A, Schnitzler P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran J Microbiol. 2014 Jun;6(3)149-155.] properties.
Insecticidal activities
Ocotea porosa EO was exposed to toxicity test against two strains of Cimex lectularius (Insecticide resistant ‘Bayonne’ and susceptible ‘Ft. Dix’) using three delivery methods i.e topical, residual and fumigation. The EO of O. porosa (100 µg/bug) produced 13.3% mortality in Ft. Dix strain that could reach to 23.3% 7 days after treatment, whereas no mortality was recorded in Bayonne strain. EO of O. porosa was not toxic to bed bugs in fumigation (250 µg/125 mL of air) and residual (100 µg/cm.sq.) assays. Using the same methods, EO of Baccharis sphenophylla Dusén ex Malme produced 66.67 ± 3.33% mortality in the insecticide-resistant strain ‘Bayonne’, while producing 83.33 ± 3.33% mortality in the susceptible strain ‘Ft.Dix’, 24 h after treatment [5353 Budel JM, Wang M, Raman V, Zhao J, Khan SI, Rehman JU, Techen N, Tekwani B, Monteiro LM, Heiden G, Takeda IJM, Farago PV, Khan IA. Essential Oils of Five Baccharis Species: Investigations on the Chemical Composition and Biological Activities. Molecules. 2018 Oct;23(10):2620-2639. doi:10.3390/molecules23102620.
https://doi.org/10.3390/molecules2310262...
]. The EO of Schinus molle L. produced 100.0 ± 0.00% (Ft. Dix) and 90.0 ± 5.77% (Bayonne) of mortality 24 h after the treatment [5454 Machado CD, Raman V, Rehman JU, Maia BHLNS, Meneghetti EK, Almeida VP, Silva RZ, Farago PV, Khan IA, Budel JM. Schinus molle: anatomy of leaves and stems, chemical composition and insecticidal activities of volatile oil against bed bug (Cimex lectularius). Rev Bras Farmacogn. 2018 Jan;29(1):1-10. doi:10.1016/j.bjp.2018.10.005.
https://doi.org/10.1016/j.bjp.2018.10.00...
]. In that sense, O. porosa EO cannot be considered as an effective insecticide against bed bugs.
Cytotoxicity activities
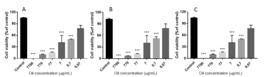
There are no reports of the activities of O. porosa EO against melanoma and breast cancer cell lines or the possible mechanisms related to these activities. Thus, an initial evaluation of the cytotoxicity effect of O. porosa EO against MCF-7 and B16F10 cells lines was performed (Figure 4) by an MTT reduction assay, and the IC50 and SI (selectivity index) values are presented in Table 2. Ocotea porosa EO showed cytotoxic effects against all cell lines tested at different concentrations with the lowest IC50 value achieved after 72 h of treatment.
Statistically significant results were obtained for MCF-7 and B16F10 cells up to the concentration of 7 μg/mL. McCoy cells presented cytotoxicity with statistical difference to the control until 77 μg/mL. Essential oils of O. caudata, O. cujumary and O. caniculata displayed promising cytotoxic activities against MCF-7 cells showing median inhibitory concentration (IC50) ∼= 65.0 μg·mL−1 [99 Silva JK, Trindade R, Moreira EC, Maia JGS, Dosoky NS, Miller RS, Cseke LJ, Setzer WN. Chemical diversity, biological activity, and genetic aspects of three Ocotea species from the Amazon. Int J Mol Sci. 2017 May;18(5):1081-1095. doi:10.3390/ijms18051081.
https://doi.org/10.3390/ijms18051081...
]. The major compounds found in the present study, α-pinene, β-pinene and bicyclogermacrene also showed cytotoxic activities against MCF-7 cells in a study by Grecco and coworkers [5555 Grecco SS, Martins EG, Girola N, Figueiredo CR, Matsuo AL, Soares MG, Bertoldo BC, Sartorelli P, Lago JHG. Chemical composition and in vitro cytotoxic effects of the essential oil from Nectandra leucantha leaves. Pharm Biol. 2015;53(1):133-137. doi:10.3109/13880209.2014.912238.
https://doi.org/10.3109/13880209.2014.91...
]. Taking all these into account, EO of O. porosa can be further investigated regarding both selectivity and cytotoxic mechanisms.
However, an ideal anticancer drug must produce a cytotoxic effect for cancer cells in low concentrations without affecting normal cells. Ashley and coworkers [5656 Ashley EA, Dhorda M, Fairhurst RM, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014 Jul;371:411-423. doi:10.1056/NEJMoa1314981.
https://doi.org/10.1056/NEJMoa1314981...
] suggested that for a compound to be considered of low toxicity and has good chances of became a new anticancer drug, it should present an SI higher than 2. The results showed in Table 2 presented an SI of 1.05 and 0.05 for B16F10 and MCF-7 when compared to fibroblast normal cells (McCoyline), respectively. These data restrict possible use of EO from O. porosa as novel anticancer product. However, these values may be improved after EO fractionation and isolation of more suitable compounds.
Cell cytotoxicity was determined using MTT assay. A - MCF-7, B - B16F10 and C - McCoy cells. **p< 0.01 and *** p < 0.001 compared to control. One-way ANOVA with Tukey’s post hoc test. Three independent experiments were performed.
IC50 values of O. porosa oil for different cell lines and selectivity index (SI). IC50 data were expressed as mean and ± standard error of the mean of three independent experiments.
The morphological features of MCF-7 and B16F10 cell lines were also investigated by studying the effects of O. porosa EO on cells (77 µg/mL, for 24 h). EO of O. porosa induced cell death with apoptotic characteristics as cell rounding, membrane blebbing and chromatin condensation (Figure 5). Apoptosis and necrosis are the two major processes leading to cell death. Apoptosis occurs under normal physiological conditions and the cell is an active participant in its own demise. Due to this efficient mechanism for the removal of apoptotic cells, no inflammatory response is elicited [5757 Pereira CB, Kanunfre CC, Farago PV, Borsato DM, Budel JM, Maia BHLNS, Campesatto EA, Sartoratto A, Miguel MD, Miguel OG. Cytotoxic mechanism Baccharis milleflora (Less.) DC. essential oil. Toxicol in Vitro. 2017 Aug;42:214-21. doi:10.1016/j.tiv.2017.04.031.
https://doi.org/10.1016/j.tiv.2017.04.03...
]. These results suggest that EO of O. porosa provides a more suitable cell death mechanism than other essential oils as EO of Baccharis milleflora (Less.) DC. [5757 Pereira CB, Kanunfre CC, Farago PV, Borsato DM, Budel JM, Maia BHLNS, Campesatto EA, Sartoratto A, Miguel MD, Miguel OG. Cytotoxic mechanism Baccharis milleflora (Less.) DC. essential oil. Toxicol in Vitro. 2017 Aug;42:214-21. doi:10.1016/j.tiv.2017.04.031.
https://doi.org/10.1016/j.tiv.2017.04.03...
] and Lavandula dentata L. [5858 Justus B, Kanunfre CC, Budel JM, Faria MF, Raman V, Paula JP, Farago PV. New insights into the mechanisms of French lavender essential oil on non-small-cell lung cancer cell growth. Ind Crops Prod. 2019 Sep;136:28-36. doi:10.1016/j.indcrop.2019.04.051.
https://doi.org/10.1016/j.indcrop.2019.0...
] which promoted necrotic and apoptotic processes, simultaneously.
Morphology of MCF-7and B16F10 cells after 24 h of treatment with O. porosa EO. A and C - Control cells incubated with RPMI only. B and D - cells treated with 77 µg/mL of O. porosa EO. ↑: cell rounding, ➨: bleb formation, ▲: chromatin condensation. Magnification = 1000x, bar = 20 μm.
CONCLUSION
In the present work, the chemical profiles of Ocotea porosa EO were analyzed. The volatile compositions of EO of the leaves were reported for the first time. The major volatile compounds were α-pinene, β-pinene and bicyclogermacrene. EO of O. porosa demonstrated 13.3% mortality in Ft. Dix strain of bed bugs that could reach to 23.3% 7 days after treatment, while no mortality was recorded in Bayonne strain. EO of O. porosa was not toxic to bed bugs in fumigation and residual assays. The EO of O. porosa was cytotoxic to murine fibroblast cell lines (McCoy), murine melanoma (B16F10) and human breast adenocarcinoma (MCF7) probably by apoptosis. However, there was no evidence of selectivity against the tumor cells under study. The anatomical characteristics that were observed in this study may help in the correct identification of O. porosa. Noteworthy anatomical features include the hypostomatic leaves with paracytic and anomocytic stomata; epicuticular wax, especially on the stomata; non-glandular trichomes; biconvex midrib and petiole with a collateral open arc vascular bundle; presence of a sclerenchymatous layer, starch grains and crystal sand in the stem; and the presence of phenolic compounds in the epidermis, phloem and xylem of the midrib, petiole and stem.
Acknowledgments
The authors would like to thank the Electron Microscopy Center of the c-LABMU at the State University of Ponta Grossa for providing the FEG-SEM images. The bed bug research is supported by USDA-Discovery & Development of Natural Products based insect management for medical, veterinary & Urban (58-6066-6-043).
REFERENCES
-
1The Plant List. A working list of all plant species [Internet]. [place unknown: publisher: unknown]; [updated 2020 Mar 03; cited 2020 Mar 03] Available from: www.theplantlist.org/1.1/browse/A/Lauraceae/Ocotea/
» www.theplantlist.org/1.1/browse/A/Lauraceae/Ocotea/ -
2Ocotea in Flora do Brasil 2020 em construção. [Jardim Botânico do Rio de Janeiro]; [updated 2020 Mar 03; cited 2020 Mar 03]. Available from: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB8440
» http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB8440 -
3Vattimo-Gil I. Contribuição ao conhecimento da distribuição geográfica das Lauraceae VII. Rodriguésia. 1980 Jul;32(54):351-367. doi:10.1590/2175-78601980325419.
» https://doi.org/10.1590/2175-78601980325419 -
4Rohwer JG. Prodromus einer Monographie der Gattung Ocotea Aubl. (Lauraceae), sensu lato. Mitt Inst Allg Bot Hamburg. 1986 Apr;20:3-278.
-
5Garrett R, Romanos MTV, Borges RM, Santos MG, Rocha L, Silva AJR. Antiherpetic activity of a flavonoid fraction from Ocotea notate leaves. Rev Bras Farmacogn. 2012 Mar;22(2):306-313. doi:10.1590/S0102-695X2012005000003.
» https://doi.org/10.1590/S0102-695X2012005000003 -
6Costa IFB, Calixto SD, Araujo MH, Konno TUP, Tinoco LW, Guimarães DO, Lasunskaia EB, Leal IRC, Muzitano MF. Antimycobacterial and nitric oxide production inhibitory activities of Ocotea notata from Brazilian restinga. Sci World J. 2015;947248:1-9. doi:10.1155/2015/947248.
» https://doi.org/10.1155/2015/947248 -
7Farago PV, Budel JM, Duarte MR, Nakashima T. Análise morfoanatômica de folhas de Ocotea puberula (Rich.) Nees, Lauraceae. Rev Bras Farmacogn. 2005 Jul;15(3):250-255. doi:10.1590/S0102-695X2005000300016.
» https://doi.org/10.1590/S0102-695X2005000300016 -
8Montrucchio DP, Miguel OG, Zanin SMW, Silva GA, Cardozo AM, Santos ARS. Antinociceptive effects of a chloroform extract and the alkaloid dicentrine isolated from fruits of Ocotea puberula. Planta Med. 2012;78(14):1543-1548. doi:10.1055/s-0032-1315026.
» https://doi.org/10.1055/s-0032-1315026 -
9Silva JK, Trindade R, Moreira EC, Maia JGS, Dosoky NS, Miller RS, Cseke LJ, Setzer WN. Chemical diversity, biological activity, and genetic aspects of three Ocotea species from the Amazon. Int J Mol Sci. 2017 May;18(5):1081-1095. doi:10.3390/ijms18051081.
» https://doi.org/10.3390/ijms18051081 -
10Barbosa LCA, Filomeno CA, Teixeira RR. Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules. 2016 Dec;21(12):1671-1686. doi:10.3390/molecules21121671.
» https://doi.org/10.3390/molecules21121671 -
11Conceição RS, Carneiro MMAA, Reis IMA, Branco A, Vieira IJC, Braz-Filho R, Botura MB. In vitro acaricide activity of Ocotea aciphylla (Nees) Mez. (Lauraceae) extracts and identification of the compounds from the active fractions. Ticks and Tick-borne Diseases. 2017 Feb;8(2):275-282. doi:10.1016/j.ttbdis.2016.11.013.
» https://doi.org/10.1016/j.ttbdis.2016.11.013 -
12Ballabeni V, Tognolini M, Giorgio C, Bertoni S, Bruni R, Barocelli E. Ocotea quixos Lam. essential oil: In vitro and in vivo investigation on its anti-inflammatory properties. Fitoterapia. 2010 Jun;81(4)289-295. doi:10.1016/j.fitote.2009.10.002.
» https://doi.org/10.1016/j.fitote.2009.10.002 -
13Fournet A, Ferreira ME, Arias AR, Guy I, Guinaudeau H, Heinzen H. Phytochemical and antiprotozoal activity of Ocotea lancifolia. Fitoterapia. 2007 Jul;78(5)382-384. doi:10.1016/j.fitote.2007.03.003.
» https://doi.org/10.1016/j.fitote.2007.03.003 -
14Garcez FR, Silva AGR, Garcez WS, Linck G, Matos MCF, Santos ECS, Queiroz LMM. Cytotoxic aporphine alkaloids from Ocotea acutifolia. Planta Med. 2011 Oct;77(4)383-387. doi:10.1055/s-0030-1250401.
» https://doi.org/10.1055/s-0030-1250401 -
15Gontijo DC, Brandão GC, Gontijo PC, Oliveira AB, Diaz MAN, Fietto LG, Leite JPV. Identification of phenolic compounds and biologically related activities from Ocotea odorifera aqueous extract leaves. Food Chem. 2017 Sep;230:618-626. doi:10.1016/j.foodchem.2017.03.087.
» https://doi.org/10.1016/j.foodchem.2017.03.087 -
16Chaverri C, Cicció JF. Essential oil of trees of the genus Ocotea (Lauraceae) in Costa Rica. I. Ocotea brenesii. Rev Biol Trop. 2005 Sep;53:431-436. doi:10.15517/rbt.v53i3-4.14611.
» https://doi.org/10.15517/rbt.v53i3-4.14611 -
17Takaku S, Haber WA, Setzer WN. Leaf essential oil composition of 10 species of Ocotea (Lauraceae) from Monteverde, Costa Rica. Biochem Syst Ecol. 2007 Aug;35(8):525-532. doi:10.1016/j.bse.2007.02.003.
» https://doi.org/10.1016/j.bse.2007.02.003 -
18Vilegas JHY, Gottlieb OR, Kaplan MA, Gottlieb HE. Aporphine alkaloids from Ocotea caesia. Phytochemistry. 1989;28(12):3577-3578. doi:10.1016/0031-9422(89)80403-0.
» https://doi.org/10.1016/0031-9422(89)80403-0 -
19Dias CS, Silva IG, Cunha EVL, Silva MS, Braz-Filho R, Barbosa-Filho JM. Isolamento e identificação de novos alcalóides de Ocotea duckei Vattimo (Lauraceae). Rev Bras Farmacogn. 2003;13(1 Suppl):62-63. doi:10.1590/S0102-695X2003000300023.
» https://doi.org/10.1590/S0102-695X2003000300023 -
20Morais LCSL, Almeida RN, Cunha EVL, Silva MS, Barbosa-Filho JM, Gray AI. Further lignans from Ocotea duckei. Pharm Biol. 1999;37(2):144-147. doi:10.1076/phbi.37.2.144.6084.
» https://doi.org/10.1076/phbi.37.2.144.6084 -
21Amato CM. Ecologia de populações de Ocotea porosa (Nees) Barroso em áreas submetidas a diferentes graus de perturbação [dissertation]. Curitiba: Universidade Federal do Paraná Curitiba; 2008. 49 p.
-
22Johansen DA. Plant microtechnique. New York: McGraw Hill Book; 1940. 523 p.
-
23Berlyn GP, Miksche JP. Botanical microtechnique and cytochemistry. Iowa: The Iowa State University Press; 1976.
-
24O'Brien TP, Feder N, McCully ME. Polychromatic staining of plant cell walls by Toluidine Blue O. Protoplasma. 1964 Jul;59:368-373. doi:10.1007/BF01248568.
» https://doi.org/10.1007/BF01248568 -
25Roeser KR. Die nadel der schwarzkiefer-massenprodukt und kunstwerk der natur. Mikrokosmos. 1962;61:33-36.
-
26Kraus JE, Arduin M. Manual básico de métodos em morfologia vegetal. Rio de Janeiro: EDUR; 1997. p. 159-165.
-
27Justus B, Almeida VP, Gonçalves MM, Assunção DPSF, Borsato DM, Arana AFM, Maia BHLNS, Paula JFP, Budel JM, Farago PV. Chemical composition and biological activities of the essential oil and anatomical markers of Lavandula dentata L. cultivated in Brazil. Braz Arch Biol Technol. 2018 Nov;61:1-12. doi:10.1590/1678-4324-2018180111.
» https://doi.org/10.1590/1678-4324-2018180111 -
28Gabe M. Techniques Histologiques. Paris: Masson & Cie; 1968. 704 p.
-
29Sass JE. Botanical microtechnique. 3rd ed. Ames: The Iowa State College Press; 1958. 228 p.
-
30Foster AS. Practical plant anatomy. 2nd ed. Princeton: D. Van Nostrand; 1949. 228 p.
-
31Adams RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Illinois: Carol Stream; 2007. 804 p.
-
32Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec;65(1-2):55-63. doi:10.1016/0022-1759(83)90303-4.
» https://doi.org/10.1016/0022-1759(83)90303-4 -
33Santos M, Oliveira PL. Aspectos anatômicos do pecíolo de quatro espécies do gênero Ocotea Aubl. (Lauraceae) ocorrentes no Rio Grande do Sul. INSULA Revista de Botânica. 1995;24:3-14.
-
34Farago PV, Paula JP, Nakashima T, Döll-Boscardin P, Budel JM, Maia BHLNS. Chemical composition and antibacterial activity of the essential oil from bark of Ocotea puberula (Rich.) Ness. Lat Am J Pharm. 2010 Mar;29(7):1242-1245.
-
35Gonçalves RA, Pinheiro AB, Oliveira MA, Nascimento RT, Rosalem PF, Garcia VL, Martins AR. Anatomical characters and chemical profile of leaves of three species in Lauraceae family. Rev Bras Farmacogn. 2018 Jan;28(1):1-8. doi:10.1016/j.bjp.2017.11.008.
» https://doi.org/10.1016/j.bjp.2017.11.008 -
36Coutinho DF, Agra MF, Barbosa-Filho JM, Basílio IJLD. Morfo-anatomia foliar de Ocotea gardneri (Meisn.) Mez (Lauraceae-Lauroideae). Rev Bras Farmacogn. 2006 Apr;16(2):178-184. doi:10.1590/S0102-695X2006000200008.
» https://doi.org/10.1590/S0102-695X2006000200008 -
37Toledo MGT. Estudo botânico e fitoquímico de Ocotea odorifera (Vell.) Rohwer. (Lauraceae) da região metropolitana de Curitiba [dissertation]. Curitiba: Universidade Federal do Paraná; 2000. 62 p.
-
38Coutinho DF, Agra MF, Basílio IJLD, Barbosa-Filho JM. Estudo morfoanatômico das folhas de Ocotea duckei Vattimo (Lauraceae-Lauroideae). Rev Bras Farmacogn. 2006 Oct;16(4):537-544. doi:10.1590/S0102-695X2006000400017.
» https://doi.org/10.1590/S0102-695X2006000400017 -
39Santos M, Oliveira PL. Aspectos anatômicos da lâmina foliar de Ocotea porosa (Nees et Mart. ex Nees) J. Angely (Lauraceae). INSULA Revista de Botânica. 1988;18:3-22.
-
40Metcalfe CR, Chalk L. Anatomy of the Dicotyledones: leaves, stem and wood in relation to taxonomy with notes on economic uses. Oxford: Clarendon Press; 1972. 1500 p.
-
41Wosch L, Imig DC, Cervi AC, Moura BB, Budel JM, Santos CAM. Comparative study of Passiflora taxa leaves: I. A morpho-anatomic profile. Rev Bras Farmacogn. 2015 Jul;25(4):328-343. doi:10.1016/j.bjp.2015.06.004.
» https://doi.org/10.1016/j.bjp.2015.06.004 -
42Almeida VP, Hirt AA, Raeski PA, Mika BE, Justus B, Santos VLP, Franco CRC, Paula JP, Farago PV, Budel JM. Comparative morphoanatomical analysis of Mikania species. Rev Bras Farmacogn. 2017 Jan;27(1):9-19. doi:10.1016/j.bjp.2016.05.002.
» https://doi.org/10.1016/j.bjp.2016.05.002 -
43Chaverri C, Diaz C, Cicció JF. Chemical Analysis of Essential Oils from Ocotea gomezii W.C. Burger and Ocotea morae Gómez-Laur. (Lauraceae) Collected at "Reserva Biológica Alberto M. Brenes" in Costa Rica and their Cytotoxic Activity on Tumor Cell Lines. J Braz Chem Soc. 2011;22(4):741-745. doi:10.1590/S0103-50532011000400018.
» https://doi.org/10.1590/S0103-50532011000400018 -
44Weyerstahl P, Wahlburg HC, Splittgerber U, Marschall H. Volatile Constituents of Brazilian Phoebe Oil. Flavour and Fragrance Journal. 1994 Jul;9(4):179-186. doi:10.1002/ffj.2730090407.
» https://doi.org/10.1002/ffj.2730090407 -
45Reynolds T, Kite G. Volatile constituents of Brazilian Phoebe porosa Mez. J Essent Oil Res. 1995 Jul;7(4):415-418. doi:10.1080/10412905.1995.9698551.
» https://doi.org/10.1080/10412905.1995.9698551 -
46Brito AFR. Análise de variação sazonal e das atividades antifúngica e antimicrobiana em óleos essenciais de Ocotea porosa (Nees) Barroso e Nectandra megapotamica (Spreng.) Menz [dissertation]. São Paulo: Instituto de Química; 2009. 121p.
-
47Govindarajan M, Benelli G. Eco-friendly larvicides from Indian plants: Effectiveness of lavandulyl acetate and bicyclogermacrene on malaria, dengue and Japanese encephalitis mosquito vectors. Ecotoxicol Environ Saf. 2016 Nov;133:395-402. doi:10.1016/j.ecoenv.2016.07.035.
» https://doi.org/10.1016/j.ecoenv.2016.07.035 -
48Koziol A, Stryjewska A, Librowski T, Salat K, Gawel M, Moniczewski A, Lochynski S. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Rev Med Chem. 2014 Dec;14(14):1156-1168. doi:10.2174/1389557514666141127145820.
» https://doi.org/10.2174/1389557514666141127145820 -
49Özbek H, Ylmaz BS. Anti-inflammatory and hypoglycemic activities of alpha-pinene. Acta Pharm Sci. 2017;55(04):7-14. doi:10.23893/1307-2080.APS.05522.
» https://doi.org/10.23893/1307-2080.APS.05522 -
50Pinheiro MA, Magalhães RM, Torres DM, Cavalcante RC, Mota FSX, Coelho EMAO, Moreira HP, Lima GC, Araújo PCC, Cardoso JHL, Souza ANC, Diniz LRL. Gastroprotective effect of alpha-pinene and its correlation with antiulcerogenic activity of essential oils obtained from Hyptis. Pharmacogn Mag. 2015 Jan;11(41):123-130. doi:10.4103/0973-1296.149725.
» https://doi.org/10.4103/0973-1296.149725 -
51Guzmán-Gutiérrez SL, Bonilla-Jaime H, Gómez-Cansino R, Reyes-Chilpa R. Linalool and ß-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sciences. 2015 May;128:24-29. doi:10.1016/j.lfs.2015.02.021.
» https://doi.org/10.1016/j.lfs.2015.02.021 -
52Astani A, Schnitzler P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran J Microbiol. 2014 Jun;6(3)149-155.
-
53Budel JM, Wang M, Raman V, Zhao J, Khan SI, Rehman JU, Techen N, Tekwani B, Monteiro LM, Heiden G, Takeda IJM, Farago PV, Khan IA. Essential Oils of Five Baccharis Species: Investigations on the Chemical Composition and Biological Activities. Molecules. 2018 Oct;23(10):2620-2639. doi:10.3390/molecules23102620.
» https://doi.org/10.3390/molecules23102620 -
54Machado CD, Raman V, Rehman JU, Maia BHLNS, Meneghetti EK, Almeida VP, Silva RZ, Farago PV, Khan IA, Budel JM. Schinus molle: anatomy of leaves and stems, chemical composition and insecticidal activities of volatile oil against bed bug (Cimex lectularius). Rev Bras Farmacogn. 2018 Jan;29(1):1-10. doi:10.1016/j.bjp.2018.10.005.
» https://doi.org/10.1016/j.bjp.2018.10.005 -
55Grecco SS, Martins EG, Girola N, Figueiredo CR, Matsuo AL, Soares MG, Bertoldo BC, Sartorelli P, Lago JHG. Chemical composition and in vitro cytotoxic effects of the essential oil from Nectandra leucantha leaves. Pharm Biol. 2015;53(1):133-137. doi:10.3109/13880209.2014.912238.
» https://doi.org/10.3109/13880209.2014.912238 -
56Ashley EA, Dhorda M, Fairhurst RM, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014 Jul;371:411-423. doi:10.1056/NEJMoa1314981.
» https://doi.org/10.1056/NEJMoa1314981 -
57Pereira CB, Kanunfre CC, Farago PV, Borsato DM, Budel JM, Maia BHLNS, Campesatto EA, Sartoratto A, Miguel MD, Miguel OG. Cytotoxic mechanism Baccharis milleflora (Less.) DC. essential oil. Toxicol in Vitro. 2017 Aug;42:214-21. doi:10.1016/j.tiv.2017.04.031.
» https://doi.org/10.1016/j.tiv.2017.04.031 -
58Justus B, Kanunfre CC, Budel JM, Faria MF, Raman V, Paula JP, Farago PV. New insights into the mechanisms of French lavender essential oil on non-small-cell lung cancer cell growth. Ind Crops Prod. 2019 Sep;136:28-36. doi:10.1016/j.indcrop.2019.04.051.
» https://doi.org/10.1016/j.indcrop.2019.04.051
HIGHLIGHTS
-
1
The anatomy features were useful for identification of Ocotea porosa.
-
2
The major volatile compounds were α-pinene, β-pinene and bicyclogermacrene.
-
3
Essential oil of O. porosa was cytotoxic against McCoy, B16F10 and MCF7cell lines.
-
4
The cytotoxic mechanism might be related to apoptotic events.
Publication Dates
-
Publication in this collection
10 Aug 2020 -
Date of issue
2020
History
-
Received
11 Sept 2019 -
Accepted
10 Feb 2020