Abstracts
Macro- and microscopic ovarian features of Hemiodus microlepis, H. ternetzi and H. unimaculatus were analyzed. Based on these features we proposed an ovarian maturation scale. The nine stages of the preliminary macroscopic fieldwork scale were reduced to five after microscopic analysis of ovaries. The microscopic analysis indicated a group-synchronous oocyte development common to the three species that were characterized as iteroparous synchronous spawners with a total spawning type. The remarkable thickness of the zona radiata layer and the large size of vitellogenic oocytes of Hemiodus ternetzi distinguished this species from the others.
Hemiodontid; oocyte development; zona radiata; fish reproduction; Tocantins River
Características macro- e microscópicas dos ovários de Hemiodus microlepis, H. ternetzi e H. unimaculatus foram analisadas. Com base nestas características foi proposta uma escala de maturação. Após a análise microscópica dos ovários, a escala macroscópica preliminar de nove estádios, utilizada em campo, foi reduzida a cinco. A análise microscópica indicou que o desenvolvimento grupo-sincrônico dos ovócitos foi comum às três espécies, caracterizadas como iteróparas de desova total. A notável espessura da zona pelúcida e o maior tamanho dos ovócitos vitelogênicos de Hemiodus ternetzi diferenciaram esta espécie das demais.
BIOLOGICAL AND APPLIED SCIENCES
Ovary maturation stages and oocyte features in three species of the neotropical fish Hemiodus (Müller, 1842)
Claudia Angelica da Silva Brandão (in memoriam); Maria de Fátima Moraes Valentim; Érica Pellegrini-Caramaschi* * Author for correspondence
Departamento de Ecologia; Instituto de Biologia; Universidade Federal do Rio de Janeiro; C. P. 68020; Ilha do Fundão; 21941-590; mfmvalentim@ig.com.br; erica@biologia.ufrj.br; Rio de Janeiro - RJ - Brazil
ABSTRACT
Macro- and microscopic ovarian features of Hemiodus microlepis, H. ternetzi and H. unimaculatus were analyzed. Based on these features we proposed an ovarian maturation scale. The nine stages of the preliminary macroscopic fieldwork scale were reduced to five after microscopic analysis of ovaries. The microscopic analysis indicated a group-synchronous oocyte development common to the three species that were characterized as iteroparous synchronous spawners with a total spawning type. The remarkable thickness of the zona radiata layer and the large size of vitellogenic oocytes of Hemiodus ternetzi distinguished this species from the others.
Keywords: Hemiodontid, oocyte development, zona radiata, fish reproduction, Tocantins River
RESUMO
Características macro- e microscópicas dos ovários de Hemiodus microlepis, H. ternetzi e H. unimaculatus foram analisadas. Com base nestas características foi proposta uma escala de maturação. Após a análise microscópica dos ovários, a escala macroscópica preliminar de nove estádios, utilizada em campo, foi reduzida a cinco. A análise microscópica indicou que o desenvolvimento grupo-sincrônico dos ovócitos foi comum às três espécies, caracterizadas como iteróparas de desova total. A notável espessura da zona pelúcida e o maior tamanho dos ovócitos vitelogênicos de Hemiodus ternetzi diferenciaram esta espécie das demais.
INTRODUCTION
The characiform family Hemiodontidae is composed of 5 genera and 33 species living in the Amazon, Orinoco, and Paraná-Paraguay rivers, and in other rivers in Guyana, Suriname, and French Guiana (Langeani, 1998). During a survey of fishes in the upper Tocantins Basin, six hemiodontid species were found: Hemiodus microlepis Kner, 1858, Hemiodus unimaculatus (Bloch, 1794), Hemiodus ternetzi Myers, 1927, Argonectes cf. scapularis, Bivibranchia velox and Bivibranchia cf. protractila (UFRJ/BIORIO/ NACIONAL-FURNAS, 1997). The three species of Hemiodus are the most abundant of the family. Hemiodus ternetzi, the shortest species (105 mm, Standard Length SL average) was common in fast-flowing rocky streams. The larger species H. microlepis and H. unimaculatus (153 mm and 167 mm, SL averages respectively), predominates in large rivers of the Tocantins River drainage.
Little is known about reproduction in the family Hemiodontidae. Aspects of reproduction in fish such as size at first maturity, duration of the spawning season, and fecundity require knowledge of the gonad developmental stages in the individual fish (West, 1990). Many investigators have criticized the subjectivity of macroscopic scales of ovarian maturity (e.g., Godinho et al., 1974; Narahara, 1983; Dias et al., 1998). Scales based on microscopic and macroscopic analyses are preferable.
In this investigation we proposed an ovary maturation scale describing oocyte morphology and development of the three Hemiodus species. The possible relationship among oocyte, egg and habitat features has also been discussed.
MATERIALS AND METHODS
Hemiodus microlepis, H. ternetzi, and H. unimaculatus were caught bimonthly with gill nets (15 to 150 mm knot-to-knot) from December 1995 to August 1999. The fish were collected from the upper Tocantins River and its tributaries near the Serra da Mesa Hydroelectric Dam, Central Brazilian region. The specimens were measured (SL), weighed, dissected, and sexed. Gonads were classified by maturation stage using a fieldwork scale composed by nine stages: Immature, Maturation I (Initial), Maturation II (Intermediary), Maturation III (Final), Mature, Spawning, Spawned, Recuperation and Recovered (UFRJ/BIORIO/NACIONAL-FURNAS, 1997). This classification is based on position and volume of the ovaries in the coelomic cavity and width, length and cross section shape of the gonads, vascularization and size and color of the oocytes.
Ovarian samples from 68 Hemiodus microlepis, 38 H. ternetzi, and 66 H. unimaculatus were preserved in Lille's formalin fluid for microscopic analysis. They were treated using standard histological procedures (e.g., Vazzoler, 1996). The samples were dehydrated and embedded in paraffin wax and sectioned transversely at 5 m m thickness and stained with haematoxylin and eosin (HE). Oocyte structures were measured using a graduated ocular. Oocyte development was characterized as previtellogenic and vitellogenic. The latter was divided into the cortical alveoli phase and true vitellogenesis phase (Wallace and Selman, 1981). In the previtellogenic phase, we called type I oocytes - with a scant cytoplasm and a centrally located nucleus containing a single, large basophilic nucleus; and type II oocytes - with multiple peripheral nucleoli and enlarged cytoplasm. In the vitellogenic phase we called type III oocytes - with predominant cortical alveoli - and type IV oocytes - with yolk granules.
RESULTS
1. Oocyte morphology and development
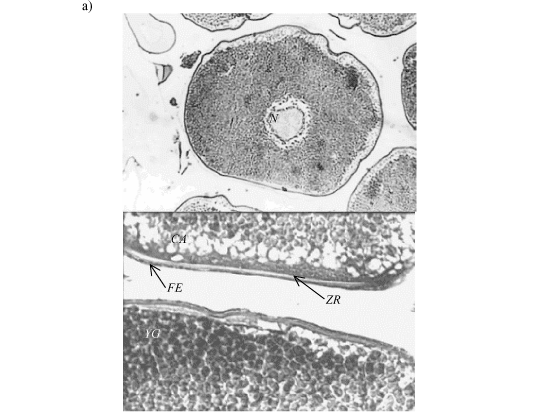
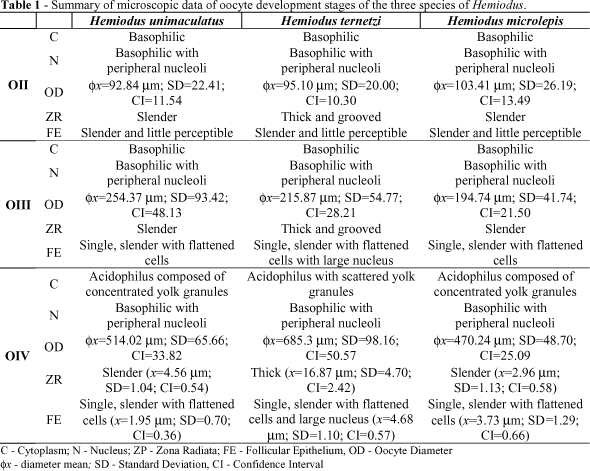
The oocytes found in the samples of the three species were type I (OI), type II (OII), type III (OIII), and type IV (OIV). Type I oocytes were recorded only on recovered ovaries. Features of the cytoplasm, nucleus, zona radiata, and follicular epithelium of OII, OIII and OIV are compared in Table 1. OI were very small to be measured by optical microscope. The main difference among the oocytes of the Hemiodus species was in the size of the vitellogenic oocyte and the thickness of the upper coat (Fig. 1a, b and c ).
The zona radiata of the vitellogenic oocytes of Hemiodus ternetzi was thicker than in the other two species, and appeared grooved under the light microscope (Fig. 2).
2. Maturation stages of the ovary
Microscopic analysis of the ovaries allowed the preliminary 9-stage scale used in the field to be reduced to 5 stages for the three species. Since juveniles of the three Hemiodus species were not collected, the Immature stage was not included. The final proposed scale, based on both macroscopic and microscopic analyses, was as follows:
Initial Maturation - Ovaries of Hemiodus microlepis and H. unimaculatus occupied less than 15% of the body cavity; they were ribbon-shaped, long and narrow, rectangular in cross-section, cream-colored, translucent, with no evident blood vascularization, and the oocytes were not visible by the naked eye (similar to the Recovered stage). The ovary of H. ternetzi had similar morphology, but occupied nearly 25% of the body cavity. Microscopically the oocytes were organized in wide lamellae. Oocytes II were predominant. H. ternetzi presented a greater quantity of oocytes III when compared to the two other species.
Advanced Maturation - Ovaries of all species occupied nearly 70% of the body cavity; they were leaf shaped, long and wide, oval in cross-section, yellow (Hemiodus ternetzi) or cream-colored (H. microlepis and H. unimaculatus), opaque, with discrete blood vascularization; the oocytes were visible macroscopically. Microscopically, the predominance of OIV and the presence of some OIII were observed. The later were more abundant in H. ternetzi than in the two other species.
Mature - Ovaries reached their maximum size and occupied nearly 80% of the body cavity of all three species. They were leaf-shaped, wider and longer than in the Advanced maturation stage, oval in cross-section, yellow (Hemiodus ternetzi) or cream-colored (H. microlepis and H. unimaculatus), with discrete vascularization. Their oocytes were opaque and visible macroscopically. The vascularization was very weak in mature ovaries of H. ternetzi. Microscopically, OIV predominated and OIII were few in number. Some OII were also noted. OIII were more abundant in H. ternetzi than in the other two species. OIV were larger in this species when compared to the other two.
Spawned - Ovaries occupied 30-40% of the body cavity. They were leaf-shaped, long and wide, oval in cross-section, yellow (Hemiodus ternetzi) or cream-colored (H. microlepis and H. unimaculatus), opaque, hemorrhaged, and with oocytes macroscopically visible. The depleted appearance of the ovarian tissue characterized this stage. Microscopically, the ovarian lamellae became undefined because of tissue disorganization; abundant remains of follicular epithelium were observed, as well as the predominance of OII and scarcity of OIV. Absorption of the oocytes was not observed in any of the ovaries analyzed.
Recovered - Ovaries occupied nearly 10% of the body cavity; they were ribbon shaped, long and narrow, rectangular in cross-section, cream-colored, translucent, vascularization non evident and oocytes were not visible macroscopically. Microscopically, only OII arranged on lamellae strings and few OI nests were observed.
DISCUSSION
Many studies have been carried out on the development of germ cells in fish (e.g., Wallace and Sellman, 1981; Guraya, 1986; Vazzoler, 1996). Bowers and Holliday (1961) classified the germ cells according to their morphological characteristics and different developmental stages. Three of these stages were observed in the species of Hemiodus. The oocyte development of these three species was very similar, although H. ternetzi differed in the extent of occupation of the ovaries in the body cavity, vasculatization intensity of mature ovaries, and in OIV in size, color and arrangement of the yolk globules. These dissimilarities indicated the existence of interspecies differences in the type of spawning and reproductive investment.
Holanda (1982) proposed a macroscopic five-stage scale of ovary maturation for two species of Hemiodontidae (Hemiodopsis sp. and Hemiodus unimaculatus): Immature, Recovered, Maturation, Mature, and Spawning. We also defined five stages, but we did not collect specimens at an Immature stage throughout the period of this study. The division in Initial and Advanced for the Maturation stage better characterized the maturation process. Due to the difficulty in detecting macroscopically the early oocytes, many individuals of the three species were recognized as in the Initial Maturation only by microscopic analysis.
The ovaries initially classified macroscopically as in the Spawning stage, were actually in the Advanced maturation stage. The depleted appearance, typical of the Spawning stage, resulted from the fragility of the mesovarium, which provides little support for the ovaries of Hemiodus. The macroscopic appearance of the Advanced maturation stage probably also induced Holanda (1982) to report a spawning condition to some hemiodontid fishes. The Spawning stage is characterized by the presence of remains of the follicular epithelium among maturing and mature oocytes (e.g. Caramaschi et al., 1982; Narahara, 1983). For the three Hemiodus species, we only had follicular epithelium remains and few mature oocytes. We named this stage as Spawned (sensu Cunningham, 1897), but we did not observe the adipose degeneration of the non-expelled oocytes (Cunningham, 1897; Vizziano and Berois, 1990). This is probably due to the rapidity of the absorption process (Agostinho et al., 1984). The absence of intermediate ovary maturation stages, the predominance of OIV in the Mature stage, and the rapidity of oocyte absorption, in all three species of Hemiodus, are characteristic of a group-synchronous oocyte development (sensu Wallace and Selman, 1981). Fishes with those characteristics are iteroparous synchronous spawners (sensu Blaber, 2000) with a total type of spawning (Vazzoler, 1996). Hemiodus ternetzi has shown mature ovaries with a greater relative abundance of OIII than the other two species. However, this probably means a slower development process in H. ternetzi and not a multiple spawning type. The oocyte diameter frequency of mature ovaries of this species showed that the abundance of OIII in mature ovaries did not interfere in the single spawning oocyte distribution characteristic of H. ternetzi (non published data).
The main microscopic difference observed among the three species of Hemiodus was the thickness of the zona radiata layer of the oocyte. The grooves of the zona radiata observed in Hemiodus ternetzi, were pores or channels filled by filaments of the oocyte and of the follicular epithelium that could also be related to adhesiveness (Guraya, 1996). Mills (1981) and Guraya (1986) related this trait to substratum adhesiveness; Suzuki (1993), otherwise, related the granulosa layer to the adhesiveness and the zona radiata layer to resistance to substratum abrasion. These grooves are radials striations observed by optical microscope and are easily observed in some teleosts (like H. ternetzi). Theses pores are present in adhesive and non-adhesive oocytes and also seem to be related to metabolic changes (oxygenation) of the egg after spawning. These pores must also be present in H. microlepis and H. unimaculatus, despite not observed in our analysis.
The main difference among the zona radiata of the oocytes regarding the Hemiodus species seemed to be related to the resistance of their oocytes. H. ternetzi had the largest oocytes, which could become heavy eggs that sink after fertilization. This species is more abundant in fast flowing, shallow streams with a rocky and sandy bottom. The thicker zona radiata can provide protection against the abrasion of the bottom (Guraya, 1986; Suzuki et al., 2000). Demersal eggs, which are often subjected to abrasive forces, generally develop thick envelopes with complex lamellae (Guraya, 1986; Suzuki et al., 2000). Hemiodus unimaculatus and H. microlepis, have thinner zona radiata and smaller lighter eggs. Besides they are more abundant in the large tributaries and in the main channel of the Tocantins River, environments where the egg flotation would be more propitious for guarantee egg integrity. Field or experimental evidences are necessary to confirm these hypotheses. In different species of loricariid, the oocyte size and the thickness of the egg upper coat were different at genus level (Suzuki et al., 2000). Here we observed that species pertaining to a single genus have differences concerning the oocyte size and thickness of the zona radiata layer. These characteristics suggested additional resources for the reproductive success of this species in the particular conditions of the tributaries of the upper Tocantins Basin.
ACKNOWLEDGEMENTS
This study was supported by Serra da Mesa Energia - FURNAS/ BIORIO/ UFRJ program as part of the "Studies on the Ichthyofauna of the Serra da Mesa Hydroelectric Plant in the Upper Tocantins River, Goiás" project. We thank the field and laboratory team of the project. Nelsy Fenerich-Verani made important comments and Ricardo Campos-da-Paz and Miriam Pilz Albrecht revised the manuscript.
Received: June 07, 2001
Revised: April 24, 2002
Accepted: January 15, 2003
- Agostinho, C. A.; Molina, S. L.; Agostinho, A. A. and Verani, J. R. (1984), Ciclo reprodutivo e primeira maturação sexual de fêmeas do lambari, Astyanax bimaculatus (L.) (Osteichthyes, Characidae) do rio Ivaí. Estado do Paraná. Rev. Brasil. Biol, 44 : (1), 31-36.
- Blaber, S. J. M. (2000), Tropical Estuarine Fishes. Ecology, Exploitation and Conservation Blackwell Science. London.
- Bowers, A. B. and Holliday, F. G. T. (1961), Histological changes in the gonad associated with the reproductive cycle of the herring (Clupea harengus L.). Mar. Res., 5, 3-14.
- Caramaschi, E. P.; Godinho, H. M. and Foresti, F. (1982), Reprodução de Hoplias malabaricus (Bloch, 1794) (Teleostei, Erythrinidae) na represa do rio Pardo (Botucatu, SP) I. Histologia e escala de maturação do ovário. Rev. Brasil. Biol,, 42 : (3), 635-640.
- Cunningham, J. T. (1897), On the histology of the ovary and ovarian ova in certain marine fishes. Quart. Jl. Microsc. Sci., 40, 101-163.
- Dias, J. R.; Peres-Rios, E.; Chaves, P. T. and Rossi-Wongstchowski, C. L. B. (1998), Análise macroscópica dos ovários de teleósteos: problemas de classificação e recomendação de procedimentos. Rev. Brasil. Biol., 58 : (1), 55-69.
- Godinho, H. M.; Ferris, S.; Medeiros, L. O. and Barker, J. M. B. (1974), Morphological changes in the ovary of Pimelodus maculatus Lacépède, 1803 (Pisces, Siluroidei) related to the reproductive cycle. Rev. Brasil. Biol., 34 : (4), 581-588.
- Guraya, S. S. (1986), The cell and molecular biology of fish oogenesis. Karger, New York.
- Holanda, O. M. D. (1982), Captura, distribuição, alimentação e aspectos reprodutivos de Hemiodus unimaculatus (Bloch, 1794) e Hemiodopsis sp (Osteichthyes, Characoidei, Hemiodidae), na represa hidrelétrica de Curuá-una, Pará Master Thesis. Instituto Nacional de Pesquisas da Amazônia/ Fundação Universidade do Amazonas, Manaus, Brasil.
- Langeani, F. (1998), Phylogeny study of the Hemiodontidae (Ostariophysi: Characiformes). In: Malabarba, L. R.; Reis, R. E.; Vari, R. P.; Lucena, Z. M. and Lucena, C. A. S. (ed.). Phylogeny and Classification of Neotropical Fishes, Porto Alegre : EdiPUCRS. pp.141-159.
- Mills, C. A. (1981), The attachment of dace, Leuciscus leuciscus L., eggs to the spawning substratum and the influence of changes in water current on their survival. J. Fish Biol, 19 : (2), 129-134.
- Narahara, M. Y. (1983), Estrutura da população e reprodução de Rhamdia hilari (Valenciennes, 1840) (Osteichthyes, Siluriformes, Pimelodidae) Ph.D. Thesis. Universidade de São Paulo, São Paulo, Brasil.
- Suzuki, H. I. (1992), Variações na morfologia ovariana e no desenvolvimento do folículo de espécies de peixes teleósteos da bacia do rio Paraná, no trecho entre a foz do rio Paranapanema e a do Rio Iguaçu Master Thesis. Universidade Federal do Paraná. Curitiba, Brasil.
- Suzuki, H. I.; Agostinho, A. A. and Winemiller, K. O. (2000), Relationship between oocyte morphology and reproductive strategy in loricariid catfishes of the Paraná River, Brazil. J. Fish Biol, 57 : (3), 791-807.
- UFRJ / BIORIO / NACIONAL - FURNAS (1997), Relatório da Fase II do projeto "Estudos Básicos Sobre a Ictiofauna do Aproveitamento Hidrelétrico Serra da Mesa, GO". Technical Report. Rio de Janeiro.
- Vazzoler, A. E. A. M. (1996), Biologia da reprodução de peixes teleósteos: teoria e prática. Editora Universidade Estadual de Maringá. Maringá.
- Vizziano, D. and Berois, N. (1990), Histología del ovario de Macrodon ancylodon (Bloch y Schneider, 1801) (Teleostei , Sciaenidae) ovogénesis. Folículos post-ovulatorios. Atresia. Rev. Brasil. Biol, 50 : (2), 523-536.
- Wallace, R. A. and Selman, K. (1981), Cellular and Dynamic Aspects of Oocytes Growth in Teleosts. Amer. Zool., 21, 325-343.
- West, G. (1990), Methods of assessing ovarian development in fishes: a review. Austr. J. Mar. and Freshw. Res., 41 : (2), 199-222.
Publication Dates
-
Publication in this collection
19 Nov 2003 -
Date of issue
June 2003
History
-
Accepted
15 Jan 2003 -
Reviewed
24 Aug 2002 -
Received
07 June 2001