Abstracts
This study aimed to evaluate the antibacterial activity of Origanum vulgare L. and O. majorana L. essential oils on Staphylococcus aureus, S. coagulase negative, Enterobacter spp., Proteus spp., Acinetobacter spp., Klebsiella spp. isolated from the patients with conjunctivitis. The results showed a prominent inhibitory effect of both the essential oils on all the bacterial strains, noted by the large bacterial growth inhibition zones (15-32mm). The Minimum Inhibitory Concentrations (MIC) values were between 5-20µL/mL and 2.5-10 µL/mL for O. vulgare and O. majorana essential oil, respectively. The MIC were able to cause significant (P<0.05) inhibitory effect on the cell viability of Klebsiella spp., Proteus spp. and S. aureus providing a total elimination of the bacterial inoculum in a maximum time of 24 h of exposure. These results showed the antibacterial effectiveness of O. vulgare and O. marjorana essential oils and supported the possibility of their use as the sources of alternative antimicrobial compounds.
Origanum vulgare L.; Origanum marjorana L.; Essential oil; Antibacterial activity; Conjunctivitis
Este estudo objetivou analisar a atividade antibacteriana do óleo essencial de O. vulgare L. and O. majorana L. sobre cepas bacterianas (Staphylococcus aureus, S. coagulase negative, Enterobacter spp., Proteus spp., Acinetobacter spp., Klebsiella spp.) isoladas de pacientes com conjuntivite. Os resultados mostraram um destacável efeito inibitório de ambos os óleos essenciais ensaiadossobre todas as cepas bacterianas, notado pela formação de amplas zonas de inibição do crescimento bacteriano (15-32 mm). Os valores de Concentração Inibitória Mínima - CIM encontradas estiveram entre 5-20µL/mL e 2.5-10 µL/mL para o óleo essencial de O. vulgare e O. majorana, respectivamente. Os valores de CIM foram capazes de causar significante efeito inibitório sobre a viabilidade celular de Klebisiella spp., Proteus spp. e S. aureus causando uma total eliminação do inóculo microbiano em um tempo máximo de 24 h de exposição. Estes resultados mostraram a efetividade antibacteriana dos óleos essenciais de O. vulgare e O. majorana, bem como suporta a possibilidade do uso de tais produtos como fontes de compostos antimicrobianos.
HUMAN AND ANIMAL HEALTH
Effectiveness of Origanum vulgare L. and Origanum majorana L. essential oils in inhibiting the growth of bacterial strains isolated from the patients with conjunctivitis
Jana Luíza Toscano Mendes de OliveiraI; Margareth de Fátima Melo DinizI; Edeltrudes de Oliveira LimaII; Evandro Leite de SouzaIII, * * Author for correspondence: evandroleitesouza@ccs.ufpb.br ; Vinícius Nogueira TrajanoII; Bernadete Helena Cavalcante SantosIV
ILaboratório de Tecnologia Farmacêutica; Departamento de Ciências Farmacêuticas; Centro de Ciências da Saúde; Universidade Federal da Paraíba; João Pessoa - Paraíba - Brasil
IILaboratório de Micologia; Departamento de Ciências Farmacêuticas; Centro de Ciências da Saúde; Universidade Federal da Paraíba; João Pessoa - Paraíba - Brasil
IIIDepartamento de Nutrição; Departamento de Ciências Farmacêuticas; Centro de Ciências da Saúde; Universidade Federal da Paraíba; João Pessoa - Paraíba - Brasil
IVLaboratório de Bacteriologia Clínica; Departamento de Ciências Farmacêuticas; Centro de Ciências da Saúde; Universidade Federal da Paraíba; João Pessoa - Paraíba - Brasil
ABSTRACT
This study aimed to evaluate the antibacterial activity of Origanum vulgare L. and O. majorana L. essential oils on Staphylococcus aureus, S. coagulase negative, Enterobacter spp., Proteus spp., Acinetobacter spp., Klebsiella spp. isolated from the patients with conjunctivitis. The results showed a prominent inhibitory effect of both the essential oils on all the bacterial strains, noted by the large bacterial growth inhibition zones (15-32mm). The Minimum Inhibitory Concentrations (MIC) values were between 5-20µL/mL and 2.5-10 µL/mL for O. vulgare and O. majorana essential oil, respectively. The MIC were able to cause significant (P<0.05) inhibitory effect on the cell viability of Klebsiella spp., Proteus spp. and S. aureus providing a total elimination of the bacterial inoculum in a maximum time of 24 h of exposure. These results showed the antibacterial effectiveness of O. vulgare and O. marjorana essential oils and supported the possibility of their use as the sources of alternative antimicrobial compounds.
Key words:Origanum vulgare L.; Origanum marjorana L.; Essential oil; Antibacterial activity; Conjunctivitis
RESUMO
Este estudo objetivou analisar a atividade antibacteriana do óleo essencial de O. vulgare L. and O. majorana L. sobre cepas bacterianas (Staphylococcus aureus, S. coagulase negative, Enterobacter spp., Proteus spp., Acinetobacter spp., Klebsiella spp.) isoladas de pacientes com conjuntivite. Os resultados mostraram um destacável efeito inibitório de ambos os óleos essenciais ensaiadossobre todas as cepas bacterianas, notado pela formação de amplas zonas de inibição do crescimento bacteriano (15-32 mm). Os valores de Concentração Inibitória Mínima CIM encontradas estiveram entre 5-20µL/mL e 2.5-10 µL/mL para o óleo essencial de O. vulgare e O. majorana, respectivamente. Os valores de CIM foram capazes de causar significante efeito inibitório sobre a viabilidade celular de Klebisiella spp., Proteus spp. e S. aureus causando uma total eliminação do inóculo microbiano em um tempo máximo de 24 h de exposição. Estes resultados mostraram a efetividade antibacteriana dos óleos essenciais de O. vulgare e O. majorana, bem como suporta a possibilidade do uso de tais produtos como fontes de compostos antimicrobianos.
INTRODUCTION
The conjunctiva is a mucous, thin and clear membrane covering the posterior eyelid surface (eyelid conjunctiva) and the eyeball ante-surface (bulb conjunctiva) (Vaughan and Asbury, 1993). Because of its location, the conjunctiva is exposed to several harmful agents, hence some pathogenic microorganisms (bacteria, viruses, rickettsias, fungi) can attack this mucous membrane causing infectious diseases (Nakano, 2002). Conjunctivitis is the ocular disease more common in the western hemisphere with the clinical severity ranging from a light hyperemia to a severe ocular necrotic wound (Solari, 2004). The simple bacterial conjunctivitis is a highly contagious infectious disease caused by the Gram positive cocci (e.g. Staphylococcus epidermidis, S. aureus, Streptococcus pneumoniae) and Gram negative rods (e.g. Haemophillus influenza, Moraxella lacunata) (Kansky, 2000; Nakano, 2002). Anti-bacterial conjunctivitis is made by using the wide spectrum antibiotics such as norfloxacin, ofloxacin, chloramphenicol, gentamicin and tobramicin (Durkin et al., 2006).
Currently, there has been an increasing interest in studying the biological properties of the plant and derivatives in order to discover the alternative biologically active compounds (Araújo et al., 2004; Lima et al., 2005). The plant products have surprised the current scientific skepticism, especially because the discovery of the phytochemicals with prominent pharmacological properties which were not previously identified in a scientific approach (Seidil, 2000).
Origanum species grow abundantly on the stony slopes and rocky mountain areas at a wide range of altitudes (0-400m) (Sahin et al., 2004). Because of the variability in the chemical and aroma characteristics, different species and Origanum ecotypes (biotypes) are widely used in the agricultural, pharmaceutical and cosmetic industries (Nostro et al., 2004; Chun et al., 2005). In addition, they have been used in the folk medicine to treat several illnesses as spasmodic, antimicrobial, digestive, expectorant and aromatic for the whooping and convulsive coughs (Dorman and Deans, 2000; Novak et al., 2003). Some studies have found interesting antimicrobial activity in Origanum species, so that O. vulgare L. (oregano) and O. majorana L. (marjoram) have shown prominent results in inhibiting the growth of the bacteria, fungi and the synthesis of the microbial metabolites (Marino et al., 20001; Baydar et al., 2004). O. vulgare and O. majorana are rich in the essential oil characterized for high amount of phenolic compounds which are believed to be responsible for their antimicrobial property (Sakandamis et al., 2002; Ferrara et al., 2003).
Although some researchers had found antimicrobial property in O. vulgare and O. majorana essential oils against some clinically important microorganisms (standard cultures) there has been a lack of studies focusing their antimicrobial effect against microbial the strains isolated from human infections. Moreover, no scientific report has been found regarding the inhibitory effect of the essential oils against the bacterial strains isolated from conjunctivitis. This study aimed to evaluate the effectiveness of O. vulgare and O. majorana essential oils to inhibit the growth/survival of the bacteria strains isolated from the patients with conjunctivitis.
MATERIAL AND METHODS
Essential oils
O. vulgare L. and O. majorana L. essential oils were obtained from Ferquima Ind. e Com. Ltda. (Vargem Grande Paulista, São Paulo, Brazil) and their quality parameters (appearance, color, purity, odor, density - 20°C, refraction index - 20°C) were as described elsewhere. The essential oil was assayed at absolute concentration and at concentrations of 160, 80, 40, 20, 10, 5 and 2.5µL/mL being the solutions prepared according to Souza et al. (2005).
Bacteria strains
Staphylococcus aureus BH01, S. aureus BH02, S. aureus BH03, S. coagulase negative BH08, S. coagulase negative BH09, Enterobacter BH15, Proteus spp. BH16, Acinetobacter spp. BH17 and Klebsiella spp. BH1 strains isolated from the patients with conjunctivitis were used as the test microorganisms. These strains were isolated and identified according to the standard procedures (McFaddin, 1980; Murray, 1999). The stock cultures were maintained on the nutrient agar slants at 4°C. Inocula were obtained from overnight cultures on nutrient agar slants at 37°C and diluted in the sterile saline solution (0.85% w/v) to a final concentration of 106 colony forming units (cfu)/mL (adjusted according to the turbidity of 0.5 McFarland scale tube).
Screening
The solid medium diffusion technique using the filter paper discs was used for screening the antibacterial activity of O. vulgare and O. majorana essential oils. For this, 1mL of the bacterial suspension (approximately 106cfu/mL) was uniformly spread on the sterile nutrient agar Petri dishes. Filter paper discs (Whatman n. 1, diameter 6mm) were soaked with 20µL of the essential oil and placed on the inoculated agar (Sahin et al., 2004; Nair et al., 2005). The system was incubated at 35-37°C/24 hours. At the end of the incubation period, the bacterial growth inhibition zones diameters were measured using the calipers and expressed in millimeters. When observed growth inhibition zones with diameter equal to or more than 10mm diameter, then it was considered as positive antibacterial activity. The controls included in this assay were essential oil replaced by sterile water.
Minimum Inhibitory Concentration MIC
The microplate bioassay was used to determine the MIC of O. vulgare and O. majorana essential oil. The 96-well plates were prepared by dispensing l 100 µL of double strength nutrient broth inoculated with the bacterium inoculum into each well prior to the assay. An aliquot (100 µL) of the essential oil solutions at their respective concentrations was transferred into seven consecutive wells. The final volume in each well was 200 µL. The solution having the highest essential oil concentration (160 µL/mL) was added into the first well and the smallest concentration (2.5 µL/mL) was added into the penultimate well. The last well, containing 200 µL of the nutrient broth inoculated with the bacteria suspension, was used as the positive control. The microplate was aseptically sealed, followed by mixing on a plate shaker (300 rpm) for 30 s and incubated at 35-37/24-48 h (Viljoen et al., 2003; Sahin et al., 2004). The antibacterial activity was detected using a colorimetric method by adding 200 µL of resauzurin staining (0.1 % w/w) aqueous solution in each well at the end of the incubation period. The MIC was defined as the lowest essential oil concentration able to inhibit the bacterial growth as indicated by resauzurin staining (dead cells were not able to change the staining color by visual observation blue to red) (Palomino et al., 2002; Burt and Reinders, 2003).
Kill time study
The kill time study was carried out with the MIC values previously found and for this the viable cells count method was used. 5 mL of double strength nutrient broth was inoculated with 1 mL of the bacterial suspension. After that, 4 mL of the essential oil solution, with concentration adjusted to provide an essential oil final concentration similar to the MIC previously determined, was
added to the system and followed by shaking for 30 s using vortex. The system was incubated at 37 °C. At different time intervals (1, 2, 4, 8, 12 and 24hs) of the exposure, 1 mL of the suspension was serially diluted (10-1 10-5) in the sterile peptone water (0.1% w/v) and inoculated on the nutrient agar Petri dishes for 24hs at 35-37°C (Viljoen et al., 2003). In the control assay the essential oil solution was replaced by the sterile distilled water. At the end of the incubation period, the mean number of the colonies (cfu/mL) was counted and compared with that found in the control assay. The results were expressed in log of cfu/mL.
Statistical analysis
Statistical analysis was performed to determine the significant differences (P<0.05) by the Tukey test in the bacteria kill time assays. For this, Sigma stat 2.03 computer program was used.
RESULTS AND DISCUSSION
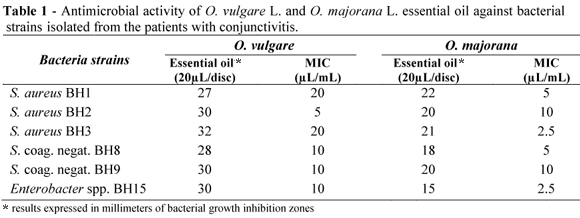
Table 1 shows the antimicrobial activity of O. vulgare and O. majorana essential oils on the bacterial strains isolated from the patients with the conjunctivitis. The results showed that both the tested essential oils provided a significant inhibitory effect on the growth of all the assayed bacterial strains. O. vulgare and O. majorana essential oils developed bacterial large growth inhibition zones with the diameter between 27-32 mm and 15-24 mm, respectively. The MIC value for the O. vulgare and O. majorana essential oils was 10 µL/mL mL for the most strains. O. majorana essential oil showed the smallest MIC (2.5 µL/mL for S. aureus BH2 and Enterobacter spp. BH15) while the highest one (20µL/mL S. aureus) was found for the O. vulgare essential oil.
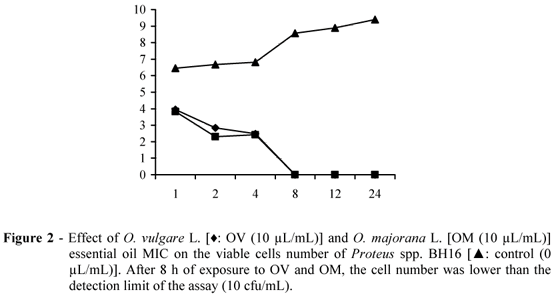
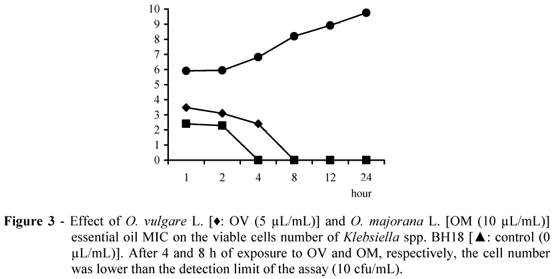
The inhibitory effect of O. vulgare and O. majorana essential oil MIC on the cell viability of S. aureus BH2, Proteus spp. BH16 and Klebsiella spp. BH18 are shown in Figs. 1, 2 and 3, respectively. The kill time study is a dynamic analysis about the microbial population mortality when exposed to some antimicrobial compound, showing the rapidity of a bactericidal effect or the length of a bacteriostatic effect noted by the viable cell number (Burt, 2004).
The essential oils presented significant inhibitory effect (P<0.05) on the cell viability of the assayed bacterial strains, showing a bactericidal effect. The essential oils provided the killing of the initial inoculum on exposure time ranging from 4 to 24 h, depending on the essential oil concentration and assayed strain. The inhibitory effect on the bacterial cell viability was noted on 1 h of exposure and this effect was enhanced on enhanced exposure. Most intense inhibitory effect was showed by O. majorana essential oil on Proteus spp. BH16, killing the initial inoculum after 4 h of exposure. O. majorana essential oil presented a more intense inhibitory effect on the viability of all the assayed bacterial strains when compared to O. vulgare essential oil.
Myrcene, γ-terpinene, α-terpinene, p-cimene, borneol, thymol, carvacrol, β-cariophyllene, limonene, α-pinene, β-pinene, linalool and sabinene are some compounds found in O. vulgare and O. majorana essential oils (Novack et al., 2003; Burt, 2004; Chun et al., 2005). Thymol and carvacrol, which have been known as major compounds of these essential oils (Lambert et al., 2001; Marino et al., 2001), are able to increase the microbial cytoplasm membrane permeability, probably because their capability of dissolving into the phospholipid bilayer aligning between the fatty acid chains and causing a distortion of the membrane physical structure (Ultee et al., 2000; Ultee et al., 2001). Presented results showed the intense antimicrobial potential of O. vulgare and O. majorana essential oils which were able to inhibit significantly the growth and cell viability of the assayed bacterial strains. These results supported the recognizing of these essential oils as the possible source of the antimicrobial compounds to be used in pharmaceutical formulations used to treat the bacterial infections, particularly, conjunctivitis. Still, further studies would be needed to verify their antimicrobial effectiveness against the pathogenic microorganisms able to act as etiological agents of different infections diseases, as well studies regarding their phytochemical, toxicological and pharmacological aspects.
Received: July 03, 2006;
Revised: November 13, 2007;
Accepted: May 13, 2008.
- Araújo, J.C.L.V.; Lima, E.O.; Ceballos, B.S.O.; Freire, K.R.L.; Souza, E.L. and Santos Filho, L. Ação antimicrobiana de óleos essenciais sobre microrganismos potencialmente causadores de infecções oportunistas. Revista de Patologia Tropical, 33, 55-64, 2004.
- Baydar, H.; Sagdiç, O.; Ozkan, G. and Karadogan, T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Sartureja species with commercial importance in Turkey. Food Control, 15, 169-172, 2004.
- Burt, S. Essential oils: their antibacterial properties and potential applications in foods a review. International Journal of Food Microbiology, 94, 223-253, 2004.
- Burt, S.A. and Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Letters in Applied Microbiology, 26, 162-167, 2003.
- Chun, S.S.; Vattem, A.V.; Lin, Y.T. and Shetty, K Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Procces Biochemistry, 40, 809-816, 2005.
- Dorman, H.J.D. and Deans, S.G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. Journal of Applied Bacteriology, 88, 308-316, 2000.
- Ferrara, L.K.; Montesanto, D. and Chiantese, C. Origanum marjoran L. in medicine and foods. Ingredientia Alimentaria, 2, 23-25, 2003.
- Kansky, J.J. Oftalmologia Clínica uma abordagem sistemática Rio de Janeiro, Rio Med, 2000.
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P. and Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol, and carvacrol. Journal of Applied Microbiology, 91, 453-462, 2001.
- Lima, I.O.; Oliveira, R.A.G.; Lima, E.O.; Souza, E.L.; Farias, N.P. and Navarro, D.F. Inhibitory effect of some phytochemicals on the growth of yeasts potentially causing of opportunistic infections. Revista Brasileira de Ciências Farmacêuticas, 41, 188-203, 2005.
- Marino, M.; Bersani, C. and Comi, G. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. International Journal of Food Microbiology, 67, 187-195, 2001.
- Murray, P.R. Manual of Clinical Microbiology Washington, DC: Ellen Jo Baron, 1773p, 1999.
- Nair, M.K.N.; Vasudevan, P. and Venkitanara, K. Antibacterial effect of black seed oil on Listeria monocytogenes. Food Control, 16, 395-398, 2005.
- McFaddin, J.F. Biochemical tests for identification of medical bacteria Baltimore: Ed. William e Wilkins, 1980.
- Nakano, E. Microbiota aeróbia conjuntival nas conjuntivites adenovirais. Arquivos Brasileiros de Oftalmologia, 65, 319-322, 2002.
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Roccaro, A.S. and Alonzo, V. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiology Letters, 230, 191-195, 2004.
- Novak, J.; Zambori-Nemeth, E.; Horvath, H.; Seregely, Z. and Kaffka K Study of essential oil components in different Origanum species by GC and sensory analysis. Acta Alimentaria, 32, 141-150, 2003.
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J. and Portaels, F. Resauzurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrobial Agents and Chemotherapy, 46, 2720-2722, 2002.
- Plause, E.A.; Flores, G.S. and Atacusi, S.G. Composición química y actividad antibacteriana del aceite esencial de Origanum vulgare (orégano). Revista de Medicina, 12, 16-19, 2001.
- Sahin, F.; Gulluce, M.; Daferera, D.; Sokmen, A.; Polissiou, M.; Agar, G. and Ozer, H. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control, 15, 549-557, 2004.
- Seidil, P.R. Pharmaceuticals from natural products: current trends. Anais da Academia Brasileira de Ciências, 74, 145-150, 2000.
- Skandamis, P.; Tsigarida, E. and Nichas, G.J.E. The effect of oregano essential oil on survival/death of Salmonella typhimurium in meat stored at 5°C under aerobic, VP/MAP conditions. Food Microbiology, 19, 97-108, 2002.
- Solari, H.P. Características laboratoriais das ceratites e conjuntivites causadas por Streptococcus sp. Arqivos Brasileiros de Oftalmologia, 67: 781-784, 2004.
- Souza, E.L.; Lima, E.O.; Freire, K.R.L. and Sousa, C.P. Inhibition action of some essential oils and phytochemicals on the growth of moulds isolated from foods. Brazilian Archives of Biology Technology, 2, 245-250, 2005.
- Ultee, A.; Slump, R.A.; Stechini, G. and Smid, E.J. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. Journal of Food Protection, 63, 620-624, 2000.
- Ultee, A. and Smid, E.J. Influence of carvacrol on growth and toxin production by Bacillus cereus. Journal of Food Microbiology, 64, 373-378, 2001.
- Vaughan, D. and Asbury, T. Oftalmologia Geral São Paulo, Editora Atheneu, 1993.
- Viljoen, A.; Van Vuuren, S.; Ernst, E.; Klepser, M.; Demirci, B.; Baser, H. and Van Wyk, B.E. Osmitopsis astericoides (Asteraceae) - the antimicrobial activity and essential oil composition of a Cape-Dutch remedy. Journal of Ethopharmacology, 88, 137-143, 2003.
Publication Dates
-
Publication in this collection
20 Mar 2009 -
Date of issue
Feb 2009
History
-
Received
03 July 2006 -
Reviewed
13 Nov 2007 -
Accepted
13 May 2008