ABSTRACT
Phytoene synthase (PSY) is the rate-limiting enzyme for carotenoid biosynthesis. To date, several studies focused on PSY genes in the context of abiotic stress responses. In this study, two phytoene synthase encoding genes, IbPSY1 and IbPSY2, were identified from a published transcriptome and bioinformatic analysis showed that they shared conserved domains with phytoene synthases from other plants. The IbPSY1 gene was cloned and carefully characterized. Digital gene expression profiling (DGE) showed that the highest transcription level of IbPSY1 was in young leaves, and the lowest level was in stems. In vivo expression levels of IbPSY1 under abiotic stress were observed to be highest in stems at day 11. Over-expression of IbPSY1 in Escherichia coli and yeast cells endowed the cells with better growth under salt and drought stress than the control cells. This study demonstrated that IbPSY1 not only played an important role in vivo, but also in E. coli and yeast to improve tolerance to salinity and drought stress. Thus, IbPSY1 may be aid in the development of transgenic plants with enhanced stress tolerance.
Keywords:
Ipomoea batatas; phytoene synthase; abiotic stress
INTRODUCTION
Sweet potato (Ipomoea batatas Lam.) is a major root crop that is grown for human consumption, particularly in Sub-Saharan Africa, parts of Asia and the Pacific Islands. Many parts of the plant are edible including its leaves, roots and vines, and varieties exist with a wide range of skin and flesh colors, ranging from white to yellow-orange and deep purple 11 Bovell-Benjamin AC. Sweet potato: a review of its past, present, and future role in human nutrition. Adv Food Nutr Res. 2007; 52:1-59.
2 Woolfe JA. Sweet potato: An untapped food resource. New York: Cambridge University Press; 1992.-33 Zhang L, Zhao H, Gan M, Jin Y, Gao X, Chen Q, et al. Application of simultaneous saccharification and fermentation (SSF) from viscosity reducing of raw sweet potato for bioethanol production at laboratory, pilot and industrial scales. Bioresour Technol. 2011; 102:4573-4579.. Its rich nutritional content and high adaptability to marginal land allow it to support human nutrition and food security in the developing world 44 Fan W, Zhang M, Zhang H, Zhang P. Improved tolerance to various abiotic stresses in transgenic sweet potato (Ipomoea batatas) expressing spinach betaine aldehyde dehydrogenase. PLoS One. 2012; 7:e37344.. However, environmental stresses, such as salinity and drought, have adverse effects on the growth and productivity of sweet potato crops 55 Mitsuya S, Takeoka Y, Miyake H. Effects of sodium chloride on foliar ultrastructure of sweet potato (Ipomoea batatas Lam.) plantlets grown under light and dark conditions in vitro. J plant physiol. 2000; 157:661-667.
6 Dasgupta M, Sahoo M, Kole P, Mukherjee A. Evaluation of orange-fleshed sweet potato (Ipomoea batatas L.) genotypes for salt tolerance through shoot apex culture under in vitro NaCl mediated salinity stress conditions. Plant Cell, Tissue and Organ Culture. 2008; 94:161-170.-77 Kasukabe Y, He L, Watakabe Y, Otani M, Shimada T, Tachibana S. Improvement of environmental stress tolerance of sweet potato by introduction of genes for spermidine synthase. Plant Biotechnol. 2006; 23:75-83..
Carotenoids are the most common group of pigments found in nature, featuring rich conjugated double bond systems 88 Cazzonelli CI. Goldacre review: carotenoids in nature: insights from plants and beyond. Funct Plant Biol. 2011; 38:833-847.. In plants, carotenoids are essential components involved in photoprotection 99 Demmig-Adams B, Adams WW. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends in Plant Sci. 1996; 1:21-26., photosynthesis 1010 Cogdell R. Carotenoids in photosynthesis. Philosophical Transactions of the Royal Society B: Biol Sci. 1978; 284:569-579. and the production of carotenoid-derived phytohormones 1111 Parry AD, Horgan R. Carotenoids and abscisic acid (ABA) biosynthesis in higher plants. Physiol Plantarum. 1991; 82:320-326.. Phytoene synthase (PSY) catalyzes the conversion of two molecules of geranylgeranyl diphosphate (GGPP) to phytoene, playing a pivotal role in the carotenoid pathway as the first committed biosynthetic step and controlling flux through the pathway 1212 Welsch R, Beyer P, Hugueney P, Kleinig H, von Lintig J. Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta. 2000; 211:846-854.. Multiple PSY genes have been well characterized in many plants and exhibit tissue-specific expression that might affect the ability to control carotenogenesis independently of photosynthesis or occur in response to certain stresses 1313 Shumskaya M, Wurtzel ET. The carotenoid biosynthetic pathway: thinking in all dimensions. Plant Sci. 2013; 208:58-63.. For instance, PSY1 is expressed in fruits and flowers of tomatoes, while PSY2 is primarily expressed in leaves 1414 Bartley GE, Scolnik P. cDNA cloning, expression during development, and genome mapping of PSY2, a second tomato gene encoding phytoene synthase. J Biol Chem. 1993; 268:25718-25721..
In previous studies, over-expression of the endogenous Arabidopsis phytoene synthase gene results in increased levels of carotenoids 1515 Lindgren LO, Stålberg KG, Höglund AS. Seed-specific overexpression of an endogenous Arabidopsis phytoene, synthase gene results in delayed germination and increased levels of, carotenoids, chlorophyll, and abscisic, acid. Plant Physiol. 2003; 132:779-85.. PSY from Salicornia europaea was found to alter responses to reactive oxygen species under salt stress in transgenic Arabidopsis1616 Han H, Li Y, Zhou S. Overexpression of phytoene synthase gene from Salicornia europaea alters response to reactive oxygen species under salt stress in transgenic Arabidopsis. Biotechnol Lett. 2008; 30:1501-1507.. Hence, studies have increasingly focused on the anti-stress functions of PSY. To date, the function of PSY from sweet potato has not been studied in the context of abiotic stress. Here, we cloned the PSY gene (IbPSY1) from sweet potato and characterized its molecular function. We determined that the gene responded significantly to both salinity and drought stress. The functionality of the IbPSY1 gene was also determined in prokaryotic (E. coli) and eukaryotic (Saccharomyces cerevisiae) cells.
MATERIAL AND METHODS
Plant materials and growth condition
Sweet potato (Ipomoea batatas Lam., cv. Xushu 18) was used in this study, planted at 26 ± 1 ° C air temperature and grown under cool white fluorescent lamps providing a 16-h photoperiod. The salt and drought stress experiments were performed by supplementing the media with 300 mM NaCl and 10% (w/v) PEG 6000 for 20 days, respectively 1717 Wang HY, Huang YC, Chen SF, Yeh KW. Molecular cloning, characterization and gene expression of a water deficiency and chilling induced proteinase inhibitor I gene family from sweet potato (Ipomoea batatas Lam.) leaves. Plant Sci. 2003; 165:191-203.. Young leaves, stems and expanding tuberous roots were collected from various samples, immediately washed and frozen in liquid nitrogen. At least three independent replicates of each experiment were performed.
Amplification and sequence analysis of Ib PSY1
Total RNA was extracted using Trizol (Invitrogen, USA). The quality and the concentration of the RNA was determined by 1.0% agarose gel electrophoresis and a NanoDrop 2000 spectrophotometer (Thermo, USA). One microgram of total RNA was used for first-strand cDNA synthesis by a PrimeScript First Strand cDNA Synthesis Kit (Invitrogen, USA). The full-length coding sequence of IbPSY1 was amplified from cDNA using the gene-specific primers IbPSY1(SLIC)-F and IbPSY1 (SLIC)-R (Table 1). The vector DNA fragment was cloned from the pET32a(+) using primers PET(SLIC)-F and PET(SLIC)-R. Next, the PCR products were subcloned into pET32a(+) vector using sequence and ligation-independent cloning (SLIC) 1818 Li MZ, Elledge SJ. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat Meth. 2007; 4:251-256..
Genomic analysis was based on our high-throughput sequencing data (GenBank accession no. JP104589 to JP160056) 1919 Tao X, Gu YH, Wang HY, Zheng W, Li X, Zhao CW, et al. Digital gene expression analysis based on integrated de novo transcriptome assembly of sweet potato (Ipomoea batatas (L.) Lam.). PLoS One. 2012; 7:e36234.. Protein modification sites were predicted with Proscan (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_pro scan.html). An analysis of protein physical properties was carried out by the On-line Analysis System (http://web.expasy.org/compute_pi/). Sequence similarities were examined using the BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and searching against the GenBank database. The amino acid sequences of the cloned cDNA fragments were deduced, and protein sequences were aligned using the program DNAMAN 6.0 (Lynnon Biosoft, Quebec, Canada). Phylogenetic relationships were determined using Clustal X2 with the Neighbor-Joining method and 1000 bootstrap replicates 2020 Larkin MA, Blackshields G, Brown N, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007; 23:2947-2948..
Digital gene expression profiling
Using the Illumina pipeline, DGE tags were generated from three different tissues: young leaves, stems and expanding tuberous roots. Next, the tags were mapped to the complete CDS of IbPSY1 by Bowtie with only one base mismatch using Galaxy's web platform 2121 Shao H, Cao Q, Tao X, Gu Y, Chang M, Huang C, et al. Cloning and characterization of ATP synthase CF1 a gene from sweet potato. Afri J Biotechnol. 2013; 10:19035-19042.,2222 Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010; 26(1):139-140.. All clean tags corresponding to the IbPSY1 gene provided a raw expression level in the different tissues, and the expression levels were normalized using the TPM algorithm. This approach generated an accurate and reasonable measurement of gene expression.
Expression analysis of Ib PSY1 by real-time RT-PCR
Total RNA was extracted from young leaves, stems and expanding tuberous roots of sweet potato under three growth conditions: normal, high-salt and drought. After first-strand cDNA synthesis, qRT-PCR was carried out with primers IbPSY1-qF and IbPSY1-qR (Table 1) with the SsoFast™ EvaGreen® Supermix (BIO-RAD, Singapore) on a Bio-Rad CFX96 Real-time PCR machine. The amplification procedure was 95 °C for 10 s initially, followed by 40 cycles, each comprising 95 °C for 10 s, 60 °C for 15 s and 72 °C for 20 s and 3 min at 72 °C thereafter. In addition, the actin gene (GenBank accession no. EU250003.1) from I. batatas was used as the internal reference.
Expression of IbPSY1 in E.coli BL21 and yeast
The recombinant plasmid (pET32-IbPSY1) constructed by the SLIC method was transformed into E. coli BL21. A single colony of E. coli strain BL21 harboring pET32-IbPSY1 or the empty vector pET32a(+) was inoculated at 37 °C in LB liquid medium containing ampicillin (100 mg/L) with shaking (180 rpm) until the OD600 value reached 0.6. Protein expression was induced by addition of isopropyl-β-D-thiogalactoside (IPTG) to a final concentration of 1 mM and the cultures were grown at 18 °C for 16 hours. Expressed proteins were visualized by SDS-PAGE.
To construct the yeast expression vector, pYES2 and pET32-IbPSY1 were digested with EcoRV and EcoRI and later re-ligated to construct the expression vector pYES2-IbPSY1. The recombinant plasmid was extracted from E. coli and validated by sequencing. pYES2-IbPSY1 and pYES2 were transformed into S. cerevisiae INVSc1 by electrop with a capacitance of 25 μF, a resistance of 186 Ω and a voltage of 1500 V. Samples were plated onto SD-URA solid medium at 30 °C and incubated for 48 h before being confirmed by PCR.
Functional analysis of IbPSY1 in E.coli and yeast
For E. coli, the OD600 value of the induced culture was diluted to 0.8. Next, 500 μL of culture from each treatment was inoculated in 50 mL LB medium containing 0.8 M NaCl and 30% PEG. The effects of salt and drought on the growth of transformed E. coli BL21(DE3) cells with pET32a(+) (empty vector), and the pET32-IbPSY1 were examined every 4 h. The growth curve of the recombinant strains was compared to the control cells.
To evaluate the functional significance of IbPSY1 with respect to salinity and drought stress in yeast, transformants containing pYES2-IbPSY1 and pYES2 (empty vector) were grown on SC-URA solid medium with 2% glucose and incubated for 24 h at 30 °C 2323 Wang BF, Wang YC, Zhang DW, Li HY, Yang CP. Verification of the resistance of a LEA gene from Tamarix expression in Saccharomyces cerevisiae to abiotic stresses. J Fores Res. 2008; 19:58-62.. Subsequently, a fixed number of cells from each treatment (approximately 1 × 107 cells) were used for the abiotic stress experiments. For high-salinity stress, 5 M NaCl solution was used. After centrifugation, yeast cells were incubated in the presence of 5 M NaCl and placed at 4 °C for 24 h. Next, cells were diluted 1000-fold and plated on SC-URA solid medium. Cells were cultured at 30 °C until single colonies were grown, and the number of colonies was determined. Similarly, to analyze the tolerance to drought stress in transgenic yeast, 4 M sorbitol solution was used following the above method.
RESULTS
Isolation and bioinformatic analysis of Ib PSY1
We identified two full-length PSY encoding sequences based on the results of transcriptomic database established previously in our laboratory. The two encoded proteins shared 80.2 % amino acid sequence identity (Figure 1A). The IbPSY1 gene was isolated and characterized from the cDNA of sweet potato. The PCR product was approximately 1.3 Kb, as expected. The predicted protein molecular weight was 49 kD, and its theoretical pI was 9.18. An evaluation of the hydrophilicity of IbPSY1 was -0.253 such that it could be a hydrophilic protein.
IbPSY1 protein sequence alignment and phylogenetic analysis. (A) Alignment of IbPSY1 and IbPSY2 based on full-length amino acid sequences; (B) Multiple sequence alignment of IbPSY1 with PSY sequences from other plants. The highly conserved regions are shaded; (C) Phylogenetic analysis of IbPSY1 with homologues from other plants.
Putative post-translational modification sites were also found in IbPSY1, including four N-glycosylation sites, five protein kinase C phosphorylation sites, four casein kinase II phosphorylation sites, one tyrosine kinase phosphorylation site, two N-myristoylation sites and one amidation site. Homology analysis for the PSY protein sequence obtained in this study was carried out using BLAST software at the NCBI server. The results demonstrated that IbPSY1 was homologous to known PSY sequences from other species, including Ipomoea sp. Kenyan (96% sequence identity), Nicotiana tabacum (84%), Nicotiana sylvestris (84%), Gardenia jasminoides (83%), Nicotiana langsdorffii (83%), Coffea canephora (83%), Lycium barbarum (82%), Solanum tuberosum (82%) (Figure 1B). Based on analysis using Clustal X2, the homology tree for the deduced amino acid sequences of IbPSY1 with eight other plants showed that IbPSY1 had the highest sequence identity with the PSY of Ipomoea sp. Kenyan (Figure 1C).
Digital gene expression profiling
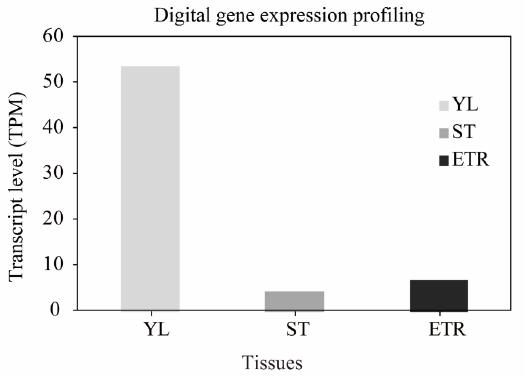
To further characterize the IbPSY1 gene, DGE profiling was used to determine expression levels in various tissues. The transcriptional level of IbPSY1 in different tissues is shown in Figure 2. Young leaves (53 transcripts per million clean tags) had higher levels of IbPSY1 expression than did stems (5 TPM) or expanding tuberous roots (8 TPM).
The expression level of IbPSY1 in different sweet potato tissues by digital gene expression profiling (TPM means transcripts per million). YL, young leaf; ST, stem; ETR, expanding tuberous root.
Expression patterns of Ib PSY1 under abiotic stress in vivo
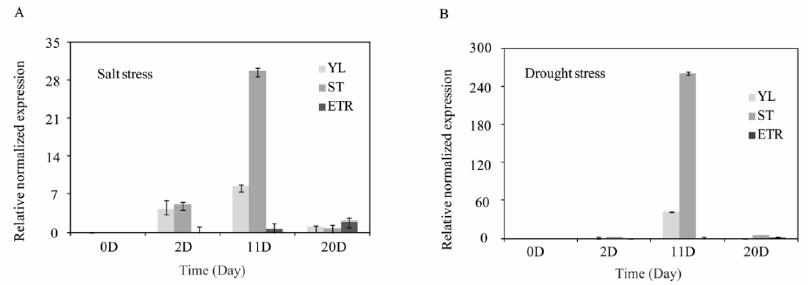
To determine the tissue specific expression patterns of IbPSY1, mRNA isolated from seedlings under different abiotic stresses was analyzed by qRT-PCR at 4 time points (0 D, 2 D, 11 D, 20 D). As shown in Figure 3, IbPSY1 mRNA was induced and reached a maximum at 11 days post treatment with NaCl (300 mM) and PEG6000 (10% (w/v)). Expression of IbPSY1 in both young leaves and stems was strongly induced by 300 mM NaCl during the first two days and continuously increased until day 11. As shown in Figure 3, IbPSY1 mRNA reached a maximum at day 11 in stems, whereas young leaves showed lower levels of induction under the same stress. IbPSY1 expression was reduced from day 11 onwards under salt stress. During PEG6000 treatment, IbPSY1 mRNA also peaked at day 11, but its transcriptional level was low, and only a faint signal could be detected at other time points. All of the results mentioned above showed that IbPSY1 could be induced by NaCl or PEG6000 treatment.
Expression patterns of IbPSY1 under abiotic stress in vivo. (A) Normal growth conditions and after NaCl (300 mM) treatment; (B) Normal growth conditions and after PEG6000 (10% (w/v)) treatment.
Expression of the recombinant IbPSY1
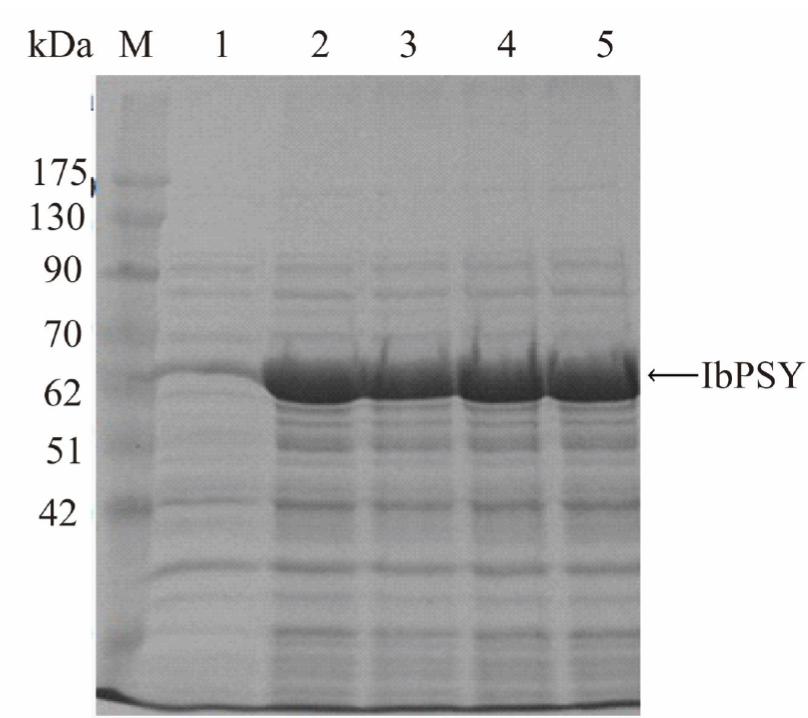
The IbPSY gene was expressed in E. coli BL21 (DE3) as a fusion protein with TrxA (approximately 17.6 kDa) and visualized by SDS-PAGE (Figure 4). The over-expressed recombinant protein was observed at 66.6 kDa, which was in agreement with its predicted size. However, there was no difference in protein expression level when it was induced by different concentrations of IPTG (0.1, 0.5, 1 and 2 mM).
Analysis of recombinant IbPSY1 protein expression. SDS-PAGE analysis of IbPSY1 over-expression in E. coli BL21; Lane M, protein marker; lane 1, crude extracts of E. coli BL21 containing the pET32- IbPSY1 without IPTG induction; lane 2-5, crude extracts of E. coli BL21 containing the pET32-IbPSY1 with different concentrations of IPTG (0.1, 0.5, 1 and 2 mM) respectively.
Expression of IbPSY1 in E. coli enhances growth during stress
Standard growth curves showed that there was no significant difference in growth rate between the control strain and recombinant strain (Figure 5A), with the control strain showing only slightly better growth than the cells harboring pET32-IbPSY1. This phenomenon is probably due to the burden imposed by the over-expression of a foreign protein on the growth of E. coli.
The growth curves of IbPSY1 in E. coli BL21 under salt or drought stress. (A) Growth curves of IbPSY1 in E. coli BL21 harboring pET32-IbPSY1 (circles), pET32a(+) (diamond) under salt stress; (B) Growth curves of IbPSY1 in E. coli BL21 harboring pET32-IbPSY1 (circles), pET32a(+) (diamond) under drought stress.
The growth of the recombinant strain was first arrested, but exponential growth began after a lag phase of 20 h when grown in LB liquid medium containing 800 mM NaCl. For the control strain, the lag phase lasted only 3 h. In general, the recombinant strain displayed improved growth compared with the control strain when exposed to 800 mM NaCl.
To determine if the expression of IbPSY1 altered the drought tolerance of E. coli, the two strains were grown in LB liquid medium containing 30% PEG6000. Similarly, the drought tolerance of E. coli cells harboring pET32-IbPSY1 was improved compared to that of the control strain. The recombinant strain exhibited a shorter lag period (24 h) than the control strain (44 h) (Figure 5B).
Expression of IbPSY1 in yeast enhances growth during stress
To analyze the possible function of IbPSY1, IbPSY1 was over-expressed in S. cerevisiae from the expression vector pYES2. Yeast cells were pre-treated by either salt (5 M NaCl) or drought (4 M sorbitol) stress and were then diluted and spread onto SC-URA solid medium plates, with the number of colonies on the plates being counted thereafter. The result showed that there was an obvious difference in yeast cells harboring IbPSY1 compared to the control. Figure 6 shows that cell viability decreased rapidly in the presence NaCl or PEG6000, but yeast cells expressing IbPSY1 exhibited higher survival rates than control cells. Following a 24-h salt shock, the survival rate of cells without IbPSY1 was approximately 52.4% of the cells expressing IbPSY1. Similarly, after 24 h of drought treatment, the survival rate of cells without IbPSY1 was approximately 44.8 % of the cells expressing IbPSY1. These results indicated that IbPSY1 improved tolerance to salt and drought stresses in both E. coli and S. cerevisiae.
Growth of IbPSY1 in yeast under salt or drought stress. (A) Salt tolerance of yeast cells harboring pYES2-IbPSY1 or pYES2; (B) Drought tolerance of yeast cells harboring pYES2-IbPSY1 or pYES2. (C) Number of yeast cells under different abiotic stress conditions.
DISCUSSION
At present, the continuous deterioration of the environment means that plants are much more likely to suffer a variety of stresses. High salinity and drought stress are major environmental factors that limit crop productivity in arid and semi-arid regions 2323 Wang BF, Wang YC, Zhang DW, Li HY, Yang CP. Verification of the resistance of a LEA gene from Tamarix expression in Saccharomyces cerevisiae to abiotic stresses. J Fores Res. 2008; 19:58-62.
24 Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, et al. Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol. 2003; 133:1755-1767.
25 Mahajan S, Tuteja N. Cold, salinity and drought stresses: an overview. Arch Biochem Biophys. 2005; 444:139-158.
26 Munns R, James RA, Läuchli A. Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot. 2006; 57:1025-1043.-2727 Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003; 218:1-14.. Carotenoids are essential for photosynthesis and for photoprotection. In addition, carotenoids also serve as precursors to signaling molecules that influence plant development and the biotic/abiotic stress response 2828 Li F, Vallabhaneni R, Yu J, Rocheford T, Wurtzel ET. The maize phytoene synthase gene family: overlapping roles for carotenogenesis in endosperm, photomorphogenesis, and thermal stress tolerance. Plant Physiol. 2008; 147:1334-1346.. PSY is an important rate limiting regulatory enzyme in the carotenoid pathway, constituting the first committed step and a bottleneck in carotenogenesis 88 Cazzonelli CI. Goldacre review: carotenoids in nature: insights from plants and beyond. Funct Plant Biol. 2011; 38:833-847.. Overexpression of the PSY gene in Narcissus pseudonarcissus L 2929 Burkhardt PK, Beyer P, Wünn J, Klöti A, Armstrong GA, Schledz M, et al. Transgenic rice (Oryza sativa) endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin A biosynthesis. The Plant J. 1997; 11:1071-1078., Solanum tuberosum3030 Ducreux LJ, Morris WL, Hedley PE, Shepherd T, Davies HV, Millam S, et al. Metabolic engineering of high carotenoid potato tubers containing enhanced levels of ß-carotene and lutein. J Exp Bot. 2005; 56:81-89., and Zea mays3131 Wong J, Lambert R, Wurtzel E, Rocheford T. QTL and candidate genes phytoene synthase and ?-carotene desaturase associated with the accumulation of carotenoids in maize. Theor Appl Genet. 2004; 108:349-359. resulted in significantly increased carotenoid levels. PSY is also transcriptionally responsive to drought, temperature, photoperiod, ABA, salt and post-transcriptional feedback regulation 3232 Cazzonelli CI, Pogson BJ. Source to sink: regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010; 15:266-274..
Previous work on the genetic manipulation of PSY isolated from Salicornia europaea conferred tolerance to salt and oxidative stress in transgenic Arabidopsis1616 Han H, Li Y, Zhou S. Overexpression of phytoene synthase gene from Salicornia europaea alters response to reactive oxygen species under salt stress in transgenic Arabidopsis. Biotechnol Lett. 2008; 30:1501-1507.. To date, there have been no reports regarding whether PSY from sweet potato (Ipomoea batatas) could improve salt and drought stress tolerance in plants. In this study, we cloned and characterized a full-length cDNA clone encoding IbPSY1 from sweet potato. Bioinformatic analysis showed that IbPSY1 contained an ORF of 1314 bp and encoded a protein of 438 amino acids. Generally, most protein functions are regulated by phosphorylation/ dephosphorylation 3333 Song S, Huo J, Li D, Yuan Y, Yuan F, Miao Y. Molecular cloning, sequence characterization, and gene expression profiling of a novel water buffalo (Bubalus bubalis) gene, AGPAT6. Genet Mol Res. 2013; 12:4116.. Six types of putative phosphorylation sites were found in IbPSY1, including N-glycosylation, protein kinase C phosphorylation, casein kinase II phosphorylation, tyrosine kinase phosphorylation, N-myristoylation and amidation sites. These modification sites are important for the function of phytoene synthase 3434 Klimczak LJ, Schindler U, Cashmore AR. DNA binding activity of the Arabidopsis G-box binding factor GBF1 is stimulated by phosphorylation by casein kinase II from broccoli. The Plant Cell. 1992; 4(1):87-98.
35 Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995; 270(48):28495-28498.-3636 Hollmann M, Maron C, Heinemann S. N-glycosylation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluR1. Neuron. 1994; 13:1331-1343., especially the N-myristoylation site, which is necessary for salt tolerance in plants 3636 Hollmann M, Maron C, Heinemann S. N-glycosylation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluR1. Neuron. 1994; 13:1331-1343.. Therefore, we speculated that this protein may be involved in the regulation of stress responses. We examined the transcript levels of IbPSY1 in seedlings that were treated with salt and drought stress and found that the expression of IbPSY1 in both young leaves and stems was strongly induced by salt or drought stress at day 11 and continuously increased for the next 20 days. Interestingly, IbPSY1 mRNA peaked at day 11 in stems in both stress conditions. However, transcription of IbPSY1 was highest in young leaves of sweet potato grown under normal conditions. According to the expression profiles of carotenoid biosynthetic genes in Brassica rapa, the BrPSY gene exhibited a higher expression level in leaves than in stems 3838 Li P, Zhang S, Zhang S, Li F, Zhang H, Cheng F , et al. Carotenoid biosynthetic genes in Brassica rapa: comparative genomic analysis, phylogenetic analysis, and expression profiling. BMC Genomics. 2015; 16:1.. Similarly, when maize inbred B73 seedlings were subjected to salt stress, PSY transcript levels were barely altered in leaves, but increased in roots within 30 min 3939 Li F, Vallabhaneni R, Wurtzel ET. PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress-induced root carotenogenesis. Plant Physiol. 2008; 146:1333-1345.. These results are consistent with the expression level of IbPSY1, implying that IbPSY1 may play an important role in stems in the response to salt or drought stress. Growth measurements in salt or drought stress media revealed that E. coli cells with pET32-IbPSY1 exhibited a shorter lag period and better growth than the control cells. This finding indicates that IbPSY1 may have different protective functions in E. coli. Our study also shows that the expression of IbPSY1 confers salt and drought tolerance by enhancing the survival of yeast cells. Salt and drought stress affect plant growth and development, resulting in a knock-on effect on crop yields. In summary, analysis of the stress tolerance effect of IbPSY1 suggests that this gene functions not only in sweet potato but also in microbes. This study improves our understanding of how IbPSY1 functions during stress and might help us to enhance the abiotic stress tolerance of crops by genetic engineering methods in the future.
CONCLUSION
In this study, IbPSY1 was shown to increase tolerance to abiotic stresses in E. coli and yeast cells. In addition, the expression level of IbPSY1 was affected by drought or salt stress. Therefore, we conclude that IbPSY1 plays an important role in the defense response of plants to abiotic stresses in vivo and may be beneficial in plant anti-stress genetic engineering.
ACKNOWLEDGMENT:
This research was supported by the National Natural Science Foundation of China (21472132), Research Fund of Sichuan Normal University (341426001), Research Fund for Young Teacher of Sichuan Normal University (14qn08).
REFERENCES
-
1Bovell-Benjamin AC. Sweet potato: a review of its past, present, and future role in human nutrition. Adv Food Nutr Res. 2007; 52:1-59.
-
2Woolfe JA. Sweet potato: An untapped food resource. New York: Cambridge University Press; 1992.
-
3Zhang L, Zhao H, Gan M, Jin Y, Gao X, Chen Q, et al. Application of simultaneous saccharification and fermentation (SSF) from viscosity reducing of raw sweet potato for bioethanol production at laboratory, pilot and industrial scales. Bioresour Technol. 2011; 102:4573-4579.
-
4Fan W, Zhang M, Zhang H, Zhang P. Improved tolerance to various abiotic stresses in transgenic sweet potato (Ipomoea batatas) expressing spinach betaine aldehyde dehydrogenase. PLoS One. 2012; 7:e37344.
-
5Mitsuya S, Takeoka Y, Miyake H. Effects of sodium chloride on foliar ultrastructure of sweet potato (Ipomoea batatas Lam.) plantlets grown under light and dark conditions in vitro. J plant physiol. 2000; 157:661-667.
-
6Dasgupta M, Sahoo M, Kole P, Mukherjee A. Evaluation of orange-fleshed sweet potato (Ipomoea batatas L.) genotypes for salt tolerance through shoot apex culture under in vitro NaCl mediated salinity stress conditions. Plant Cell, Tissue and Organ Culture. 2008; 94:161-170.
-
7Kasukabe Y, He L, Watakabe Y, Otani M, Shimada T, Tachibana S. Improvement of environmental stress tolerance of sweet potato by introduction of genes for spermidine synthase. Plant Biotechnol. 2006; 23:75-83.
-
8Cazzonelli CI. Goldacre review: carotenoids in nature: insights from plants and beyond. Funct Plant Biol. 2011; 38:833-847.
-
9Demmig-Adams B, Adams WW. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends in Plant Sci. 1996; 1:21-26.
-
10Cogdell R. Carotenoids in photosynthesis. Philosophical Transactions of the Royal Society B: Biol Sci. 1978; 284:569-579.
-
11Parry AD, Horgan R. Carotenoids and abscisic acid (ABA) biosynthesis in higher plants. Physiol Plantarum. 1991; 82:320-326.
-
12Welsch R, Beyer P, Hugueney P, Kleinig H, von Lintig J. Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta. 2000; 211:846-854.
-
13Shumskaya M, Wurtzel ET. The carotenoid biosynthetic pathway: thinking in all dimensions. Plant Sci. 2013; 208:58-63.
-
14Bartley GE, Scolnik P. cDNA cloning, expression during development, and genome mapping of PSY2, a second tomato gene encoding phytoene synthase. J Biol Chem. 1993; 268:25718-25721.
-
15Lindgren LO, Stålberg KG, Höglund AS. Seed-specific overexpression of an endogenous Arabidopsis phytoene, synthase gene results in delayed germination and increased levels of, carotenoids, chlorophyll, and abscisic, acid. Plant Physiol. 2003; 132:779-85.
-
16Han H, Li Y, Zhou S. Overexpression of phytoene synthase gene from Salicornia europaea alters response to reactive oxygen species under salt stress in transgenic Arabidopsis. Biotechnol Lett. 2008; 30:1501-1507.
-
17Wang HY, Huang YC, Chen SF, Yeh KW. Molecular cloning, characterization and gene expression of a water deficiency and chilling induced proteinase inhibitor I gene family from sweet potato (Ipomoea batatas Lam.) leaves. Plant Sci. 2003; 165:191-203.
-
18Li MZ, Elledge SJ. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat Meth. 2007; 4:251-256.
-
19Tao X, Gu YH, Wang HY, Zheng W, Li X, Zhao CW, et al. Digital gene expression analysis based on integrated de novo transcriptome assembly of sweet potato (Ipomoea batatas (L.) Lam.). PLoS One. 2012; 7:e36234.
-
20Larkin MA, Blackshields G, Brown N, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007; 23:2947-2948.
-
21Shao H, Cao Q, Tao X, Gu Y, Chang M, Huang C, et al. Cloning and characterization of ATP synthase CF1 a gene from sweet potato. Afri J Biotechnol. 2013; 10:19035-19042.
-
22Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010; 26(1):139-140.
-
23Wang BF, Wang YC, Zhang DW, Li HY, Yang CP. Verification of the resistance of a LEA gene from Tamarix expression in Saccharomyces cerevisiae to abiotic stresses. J Fores Res. 2008; 19:58-62.
-
24Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, et al. Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol. 2003; 133:1755-1767.
-
25Mahajan S, Tuteja N. Cold, salinity and drought stresses: an overview. Arch Biochem Biophys. 2005; 444:139-158.
-
26Munns R, James RA, Läuchli A. Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot. 2006; 57:1025-1043.
-
27Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003; 218:1-14.
-
28Li F, Vallabhaneni R, Yu J, Rocheford T, Wurtzel ET. The maize phytoene synthase gene family: overlapping roles for carotenogenesis in endosperm, photomorphogenesis, and thermal stress tolerance. Plant Physiol. 2008; 147:1334-1346.
-
29Burkhardt PK, Beyer P, Wünn J, Klöti A, Armstrong GA, Schledz M, et al. Transgenic rice (Oryza sativa) endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin A biosynthesis. The Plant J. 1997; 11:1071-1078.
-
30Ducreux LJ, Morris WL, Hedley PE, Shepherd T, Davies HV, Millam S, et al. Metabolic engineering of high carotenoid potato tubers containing enhanced levels of ß-carotene and lutein. J Exp Bot. 2005; 56:81-89.
-
31Wong J, Lambert R, Wurtzel E, Rocheford T. QTL and candidate genes phytoene synthase and ?-carotene desaturase associated with the accumulation of carotenoids in maize. Theor Appl Genet. 2004; 108:349-359.
-
32Cazzonelli CI, Pogson BJ. Source to sink: regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010; 15:266-274.
-
33Song S, Huo J, Li D, Yuan Y, Yuan F, Miao Y. Molecular cloning, sequence characterization, and gene expression profiling of a novel water buffalo (Bubalus bubalis) gene, AGPAT6. Genet Mol Res. 2013; 12:4116.
-
34Klimczak LJ, Schindler U, Cashmore AR. DNA binding activity of the Arabidopsis G-box binding factor GBF1 is stimulated by phosphorylation by casein kinase II from broccoli. The Plant Cell. 1992; 4(1):87-98.
-
35Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995; 270(48):28495-28498.
-
36Hollmann M, Maron C, Heinemann S. N-glycosylation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluR1. Neuron. 1994; 13:1331-1343.
-
37He C, Fong SHT, Yang D, Wang GL. BWMK1, a novel MAP kinase induced by fungal infection and mechanical wounding in rice. Mol Plant Microbe Interact. 1999; 12:1064-1073.
-
38Li P, Zhang S, Zhang S, Li F, Zhang H, Cheng F , et al. Carotenoid biosynthetic genes in Brassica rapa: comparative genomic analysis, phylogenetic analysis, and expression profiling. BMC Genomics. 2015; 16:1.
-
39Li F, Vallabhaneni R, Wurtzel ET. PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress-induced root carotenogenesis. Plant Physiol. 2008; 146:1333-1345.
Publication Dates
-
Publication in this collection
2018
History
-
Received
29 June 2016 -
Accepted
21 Oct 2016