Abstracts
Functional food presents specific physiological properties, supplies nutrients and can be naturally found in formulated food or added to it. Seafood plays an important role in human diet, representing the largest stock available of w-3 polyunsaturated fatty acids, especially eicosapentaenoic (EPA) and docosahexaenoic (DHA). Scientific researchers have intensified their studies on fatty acids due to their importance in preventing and/or curing diseases, especially cardiovascular and inflammatory ones. This review describes the most important aspects of w-3 fatty acids found in fish, as well as their roles in the mechanism involved in the prevention and control of diseases.
Fish; seafood; polyunsaturated fatty acids; w-3 fatty acids; functional foods
O pescado é um importante constituinte da dieta humana e possui a maior reserva de ácidos graxos polinsaturados, especialmente o eicosapentanóico (EPA) e o docosahexanóico (DHA) da série w-3. Têm-se intensificado as pesquisas científicas a respeito desses ácidos graxos por estes estarem envolvidos na prevenção e/ou cura de doenças, principalmente cardiovasculares e inflamatórias. Esta revisão teve como objetivo discutir os aspectos mais importantes dos ácidos graxos w-3 presentes no pescado, bem como, os seus mecanismos de ação, envolvidos na prevenção e controle de doenças.
ENGINEERING AND TECHNOLOGY
Seafood as functional food
Marcilene C. Heidmann SoccolI, * * Author for correspondence ; Marília OettererII
IMestre em Ciência e Tecnologia de Alimentos
IIDepartamento de Agroindústria, Alimentos e Nutrição; ESALQ/USP; C. P. 9; 13418-900; Piracicaba - SP - Brazil
ABSTRACT
Functional food presents specific physiological properties, supplies nutrients and can be naturally found in formulated food or added to it. Seafood plays an important role in human diet, representing the largest stock available of w-3 polyunsaturated fatty acids, especially eicosapentaenoic (EPA) and docosahexaenoic (DHA). Scientific researchers have intensified their studies on fatty acids due to their importance in preventing and/or curing diseases, especially cardiovascular and inflammatory ones. This review describes the most important aspects of w-3 fatty acids found in fish, as well as their roles in the mechanism involved in the prevention and control of diseases.
Key words: Fish, seafood, polyunsaturated fatty acids, w-3 fatty acids, functional foods
RESUMO
O pescado é um importante constituinte da dieta humana e possui a maior reserva de ácidos graxos polinsaturados, especialmente o eicosapentanóico (EPA) e o docosahexanóico (DHA) da série w-3. Têm-se intensificado as pesquisas científicas a respeito desses ácidos graxos por estes estarem envolvidos na prevenção e/ou cura de doenças, principalmente cardiovasculares e inflamatórias.
Esta revisão teve como objetivo discutir os aspectos mais importantes dos ácidos graxos w-3 presentes no pescado, bem como, os seus mecanismos de ação, envolvidos na prevenção e controle de doenças.
INTRODUCTION
Due to the appearance of several human health disorders, such as obesity, hypertension and heart problems, there has been increasing interest in the study of foods presenting functional components or substances, that is, those that act on the physiological system, not only improving human health but also preventing diseases (Byrne, 1994; Tuley, 1995; Arai, 1996). Such foods are called Functional Foods. Functional components or substances can be naturally found or be added to commercialized food products.
Human beings, as well as other mammals, can synthesize certain saturated and unsaturated fatty acids, but are unable to synthesize polyunsaturated fatty acids (PUFAs), whose absence may lead to malfunctioning of the human body. Due to this fact, these fatty acids are called "essential" and must be included in the diet.
Fish is an important dietary constituent of several population groups, once it is a source of components of significant nutritional value, such as high quality proteins, vitamins, minerals and lipids, besides being the largest source of w-3 series polyunsaturated fatty acids, especially the eicosapentaenoic (EPA) and docosahexaenoic (DHA), which bring several benefits to human organism (Belda and Pourchet-Campos, 1991).
The composition of the eatable portion of fish varies as a function of many factors, such as specie, sex, sexual maturity degree, size, place of capture, water temperature, type of feeding and season (Botta and Siquires, 1986; Castro, 1988; Armstrong et al., 1991). The variation in the fatty acids composition is explained by fluctuations in the quality and amount of food, especially phytoplankton, available to the fish. These foods are the greatest source of several fatty acids, especially the w-3 series ones (Stansby, 1969). Fish oil has been studied and used in food preparation, especially in margarine, for a long time (Labomar, 1989) due to its considerable biochemical, metabolic, nutritional and pharmaceutical importance (Stansby, 1969). Numerous benefits have been attributed to fish oil, particularly to its EPA and DHA fatty acids (Ackman and McLeod, 1988; Wang et al., 1990).
w-6 and w-3 polyunsaturated fatty acids
The organic synthesis of saturated fatty acids takes place in the extra-mitochondrial cell compartment, by means of a complex enzymatic system triggered by the acetyl-coenzyme-A. The monounsaturated fatty acids are formed from saturated ones in the liver through a reaction catalyzed by microsomal desaturases. Polyunsaturated fatty acids are produced from monounsaturated ones by the action of specific desaturases to the double-bound position in the chain (Belda and Pourchet-Campos, 1991).
The w-6 family is represented by the linoleic acid (C18:2 w-6), the precursor of arachidonic acid (C20:4 w-6), which is transformed through the metabolic process of young organisms, allowing the elongation of its carbon chains, as well as proper desaturation. "Older" organisms do not guarantee such transformation process; thus, the ingestion of arachidonic acid becomes necessary from the middle age onwards (Belda and Pourchet-Campos, 1991). The w-3 family includes the a-linolenic acid (C18:3 w-3), which forms the eicosapentaenoic (EPA) (20C:5 w -3) and docosahexaenoic acids (DHA) (22C:6 w -3) through prolongation and desaturation (Belda and Pourchet-Campos, 1991).
The essentiality of w -6 and w -3 families fatty acids in humans can no longer be defied (Anderson and Connor, 1989). The quantitative ratio between both families must be taken into consideration for an appropriate physiological balance. In humans, a w -3 fatty acid can be converted to another w -3 type, but a w -3 type cannot be converted to a w -6 one and vice-versa. However, the presence of w -3 in the organism influences the w -6 concentration and vice-versa. For example, arachidonic acid, which is a derivative of w -6 linoleic acid, found in the membranes lipids, has its concentration decreased with the consumption of EPA, a derivative of w -3 a -linolenic acid. Similarly, the human capacity to synthesize the DHA can be inhibited by a high ingestion of w -6 linoleic acid. Thus, the w-3/w -6 intake proportion must be balanced (Tuley, 1995).
Dietary need of w -3 and w -6 polyunsaturated fatty acids
The addition of long-chained w -3 fatty acids (DHA and EPA) in the diet has been discussed and recommended (Robertson, 1993; Byrne, 1994). Epidemiological studies have shown that a regular consumption of fish has favorable effects on the triacylglycerol levels, blood pressure, coagulation mechanism and heart rhythm (Burr, 1991). Glucoronyltransferases are a family of enzymes responsible for detoxifying the body from a series of molecules. The type and amount of lipids associated with a certain diet can affect enzymatic activities. The w -3 fatty acids activate the enzymes, enhancing their detoxicating power and providing protection against the action of oxidizers. Thus, the production of these enzymes can be partially responsible for the protective effect of antioxidants (Health, 1995).
Numerous functions related to membranes lipids are important for the immunological system and the blood platelets, as well as for their association with specific glycoproteins of the cell wall (Kinsella, 1990). The amounts of arachidonic acids and/or their competitors (PUFAs) released after membrane stimulation, via conversion to eicosanoids, have wide reflexes or effects on physiologic functions (Kinsella et al., 1990). The human body has a mechanism capable of regulating the lipidic metabolism and maintaining a homeostatic state, so that several physiologic systems can work with a wide range of alimentary lipids without showing clear clinical symptoms or metabolic aberrations. However, numerous functions may be disturbed if the supply of a certain fatty acid is excessive or inexistent (Belda and Pourchet-Campos, 1991).
The Food and Agriculture Organization and the World Health Organization (FAO/WHO, 1994) have recommended a consumption of 3% of essential oils, mainly w -3 and w -6 ones, based on a total energy consumption of around 3000 calories/day. Yet, it is most important that the w -3 and w -6 consumption is based on a 0.2 w -3/w -6 ratio. This proportion was defined based on the Okinawa's population's diet, in Japan. WHO has recommended the addition of DHA to all infant formulations based on fixed amounts of DHA in human milk. The amounts are based on a study regarding the w -3 fatty acids, particularly the DHA, as being fundamental for the neurological and visual pre and postnatal development (FAO/WHO, 1994). Table 1 shows the appropriate polyunsaturated fatty acids amount for pregnant or lactating women, according to the National Institute of Health (Benisek et al., 2000).
The essentiality of fatty acids
Infant development
The arachidonic (AA) and DHA fatty acids are accumulated in the tissues during the fetal stage and within the first weeks after birth. In the last 3 months of pregnancy, there is an increase (3 to 5 times) in these fatty acids amount in the fetus' brain and cerebellum. In the first stages of prenatal development, the fetus preferably receives long-chained w -3 and w -6 fatty acids, which can be seen by the increase in AA and DHA rates in the fetus' blood, with the opposite being verified for the linoleic and linolenic acids. The concentration of DHA progressively increases from the mother to the child, getting to the child's brain through a selective process, which provides the developing organism with the necessary amounts of such substance (Kuhn and Crawford, 1968). The DHA amount in the mother's milk is variable, and depends on the mother's diet. The American and European women's milk contains about 0.15 to 0.3% of DHA (Dontson et al., 1992; Birch et al., 1998; François et al., 1998), while, in other parts of the world, the DHA amount in the mother's milk can reach 0.1 to 1.4% of the total fatty acids (Innis, 1992).
The w -3 fatty acids are essential for the development of nerve cells, neurons and glial cells. The lack of w -3 fatty acids during the fetal stage can bring tragic consequences for the child's future extra-uterine life. The amount of polyunsaturated fatty acids (PUFAs) in the diet, particularly the docosahexaenoic acid, as well as the w -3/w -6 ratio during pregnancy and lactating periods, influence the mother's and infant's condition, and may play an important role in the infant's neural development (Benisek et al., 2000). Japanese studies showed that schoolchildren who consumed bread and dairy products with added supplements of DHA had their concentration levels improved and their stress levels decreased during exams (Hi - DHATM, 2000).
Adulthood
The adult's health condition as a whole is related to the w -3/w -6 fatty acids ratio, as well as to the eicosanoids genesis (Belda and Pourchet-Campos, 1991). Prostaglandins (PG), which are hormones responsible for biological events in distant cells, derive from the AA, EPA and the g-linolenic acids through the following mechanism: the aforementioned fatty acids, which are found in phospholipids of cellular membranes, are released by means of the A2 phospholipase action. Once these fatty acids have been released by means of the cyclooxygenase's activity, they form endoperoxides, which, in turn, form the prostaglandin (Ribeiro, 1990). PG is produced in virtually all tissues through hormonal, neuronal or other stimuli. Yet, the amounts of PG produced vary as a function of the tissue type and with the availability of phospholipids (20:4n-6) in the tissues. The PG production is sensitive to dietary alterations, especially to 18:2n-6 contents and factors reducing the delta-6-desaturase activity, limiting the conversion rates from 18:2 to 20:4n-6 (Hornstra, 1982). The PGs modulate numerous secretory functions of the digestive, reproductive, circulatory and immune systems (Hornstra, 1982). The prostaglandins may constitute three families, depending on the fatty acid they are derived from. These families are characterized by the number of double-bounds in the lateral chains to the cyclopentane ring: PG1, PG2 and PG3, which come from the linolenic, arachidonic and eicosapentaenoic acids, respectively. Variations in the chemical groups linked to the rings originate several PG types designated by letters (A, B...). The PCs originate prostacyclins (PGI) and thromboxanes (TX) by means of the prostacyclin-synthetase and thromboxane-synthetase actions, respectively. Such compounds are also named with numbers representing the fatty acids they come from (Belda and Pourchet-Campos, 1991).
Metabolic functions, alterations and effects on diseases
The lipoxygenases action leads fatty acids to form leukotrienes (LTs). The arachidonic acid forms the 4-series leukotrienes, which are similar to thromboxanes, showing vasoconstrictive action, possibly leading to arrhythmias. The EPA forms the 5-series leukotrienes through the lipoxygenase action. The 5-series leukotrienes, although not completely inactive, are far less active than the 4-series or 4-group ones (Belda and Pourchet-Campos, 1991). Leukotrienes are also bioactive modulators when involved in pulmonary functions (asthmas and allergies), chemotaxis, inflammatory responses and immunological functions (Stjernschantz, 1984).
Platelets are blood cells that play an important role in maintaining vascular integrity, controlling the permeability of arterioles (Levin et al., 1984). Platelets show a natural tendency to clump and adhere to blood vessels walls. This phenomenon is controlled by the production of thromboxanes (TXA2 from 20:4n-6) by the platelets enzymes. Excessive TXA2 synthesis causes vasoconstriction and the platelets tend to form aggregates or thrombi, which may block arteries, causing coronary or cerebral thrombosis. This is a major event in death associated with ischemic heart disease (IHD) (Hornstra, 1982).
The tendency of platelets to aggregate in response to TXA2 is continually counteracted by prostacyclin (PGI2), which is also synthesized from 20:4n-6 by enzymes of the endothelial cells which line the vascular blood vessels walls. The PGI2 causes vasodilatation, which minimizes the platelets adhesion to the clumps, causing antithrombotic disaggregation (Levin et al., 1984). Such homeostatic mechanism is extremely important, as it maintains the integrity and functioning of the vascular system. The most important of the w -3 polyunsaturated fatty acids seems to be the EPA, which presents anti-aggregative potential resulting either in a competitive inhibition of the thrombotic synthesis (eicosanoid thromboxane) (Budowski, 1981) or in an increase in the production of prostacyclins, which are anti-aggregative agents (Goodnight et al., 1981).
It has been found that these essential fatty acids, especially the eicosapentanoic acid (20:5 w -3), interfere in the production of thrombotic prostaglandin, thromboxane (Budowski, 1981), or are, alternatively, readily converted into antithrombotic prostaglandin when in vitro (Needleman et al., 1979; Dyerberg et al., 1978). In vivo studies have demonstrated changes in the platelets functions in individuals fed on fish or fish products (Dyerberg and Bang, 1979). Such metabolic peculiarities can account for many supporting arguments of the favorable effects of EPA and DHA on the prevention and cure of cardiovascular diseases. It is believed that EPA, when substituting AA, can promote the formation of TXA3, showing an antithrombotic effect, while PGI2 is substituted by PGI3 in the vascular walls, reinforcing the TXA3 action (Belda and Pourchet-Campos, 1991).
Aging is a multifactorial biological process that accompanies degenerative changes, such as functional decreases of organs and systems, which lead to an increase in the organism's vulnerability and a decrease in its surviving ability. Several biological and biochemical changes related to the aging process have been studied. Metabolic deficiencies, conversion of amino acids, synthesis of collagen and changes in the DNA, proteins and enzymes or in the enzymatic system may be used as biological signals of aging (Campisi et al., 1996). Peluffo et al. (1970) demonstrated that all the processes involved in the synthesis of highly unsaturated fatty acids (HUFAs) and, particularly, the limitation in the delta-6-desaturate proportion are related with the slow aging process. The authors confirmed this observation by demonstrating that the loss of affinity of delta-6-desaturate (D6D) to enzymes from both substrates, linoleic (LA) and a-linolenic acids (ALA), is one of the causes of aging (Hrelia et al. 1989 and Hrelia et al., 1990). Previous studies have shown that low amounts of DHA in the blood increase the risk of insanity, also demonstrating that the supplementation of this substance benefits people suffering from Alzheimer's disease (Hi - DHATM, 2000).
Sources of w -3 and w -6 polyunsaturated fatty acids
Once the availability of w -3 and w -6 fatty acids for human beings depends on the diet, that is, they come from exogenous sources, it is important to know which foods are capable of supplying human needs. Some examples worthy mentioning are fish, fish oil, as well as oil from many sea animals, vegetable oil, margarine, human milk and others of little significance (Belda and Pourchet-Campos, 1991).
Fish and fish oil
The greatest source of w -3 long-chained fatty acids is most frequently found in fish, more frequently used as food than other animals, such as seals and whales. Oysters are also a good w -3 source. The amounts of fatty acids most frequently found in seafood range between 8 and 12% EPA and between 10 to 20% DHA (Badolato et al., 1994). Freshwater fish usually contains lower proportions of w -3 polyunsaturated fatty acids than sea fish (Monsen, 1985). There are reports showing that cold-water fish presents relatively higher amounts of w -3 PUFA (Agren et al., 1988). Water temperature seems to be an important factor influencing such amounts. However, Bimbo (1987) contested the theory of cold-water fish, such as English fish, being richer in PUFAs than fish from tropical areas, such as Brazil (Wang et al., 1990).
Andrade and Lima (1979) studied the influence of sexual and seasonal changes in the fatty acids composition for mandi (Pimelodus clarias), a freshwater Brazilian fish. Comparing the results of tinned and in-brine products, the authors observed significant monthly variations in the total fatty acids amounts, ranging from 7.80 to 17.05 g/100g. As for the sexual changes, similar contents of fatty acids were verified. Tinned and in-brine products showed a decrease in the fatty acids content during the storage period. Bodwell and Anderson (1986) observed a decrease in fatty acids amounts during storage due to water loss and an increase in the lipidic fraction.
Badolato et al. (1994) studied the influence of seasonal variations on the fatty acids composition of 20 fillet samples and 20 mechanically separated fish pulp samples of sea fish commercialized in Sao Paulo State (whitemouth croaker, weakfish, pigfish, sardine and mullet). No significant variation was observed in the fatty acids composition between the fillet and pulp samples. Seasonal variations in the fatty acids percentages were observed for each analyzed species. The pigfish showed the largest combined amounts of two fatty acids (from 27.6 to 37.0 and from 33.9 to 36.5 g/100 g for fillet and pulp, respectively), followed by sardine (from 23.7 to 33.3 and from 24.1 to 34.1 g/100g for filet and pulp, respectively). Considerable amounts of arachidonic acid were observed in all studied species, especially in pigfish and mullet, corroborating the results of other studies showing that tropical waters fish contains larger amounts of w -6-series unsaturated fatty acids, especially eicosatetraenoic (C 20:4), than temperate waters fish (Gibson, 1983).
Silva (1992) observed combined amounts of both fatty acids (EPA and DHA) of 37.73% in the oil of Sardinella aurita, a Brazilian sardine. The Japanese sardine oil contained no more than 29% (16.8% EPA and 11.3% DHA) (Kinsella, 1986), while the American sardine oil contained 37.1% (11.3% EPA and 25.8% DHA) (Hearn et al., 1987).
Oil extracted from some species of fish is presently used as alimentary supplement to modify the plasma's lipidic profile and to balance the aggregating activity of platelets (Dyerberg et al., 1978; Harris et al., 1983). In the same way, it is recommended that the consumption of fish is increased, preferably fatty species. Some studies show a strong correlation between probable fish consumers and lower mortality rates due to cardiovascular diseases (Kromhout et al., 1985).
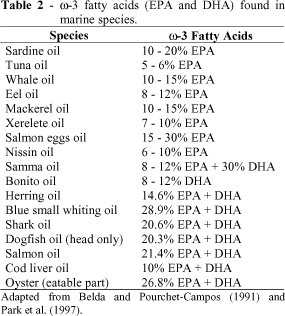
Other relevant data refer to cardiovascular diseases, arteriosclerosis, the awareness of the habitual total cholesterol intake and diets recommending maximum cholesterol intakes of 300 mg. As cholesterol is synthesized by animals, it is found in fish and fish oils (Labuza and Sloam, 1979). Table 2 shows the w -3 fatty acids (EPA and DHA) amounts found in some marine species.
Beneficial effects of PUFAs
The beneficial effects of polyunsaturated fatty acids (PUFAs) in fish oils have been widely discussed (Holub, 1988; Pourchet-Campos, 1989). The PUFAS beneficial effects relate to their role in the integrity maintenance of biological membranes, their capability to reduce the amount of serum lipids and their conversion to compounds called eicosanoids, which show direct influence on the vascular physiology and vascular system (Murphy, 1990). These effects have been more noticeable in populations presenting low fat intake diets, such as Japanese communities of fishermen that show low incidence of heart problems, as well as Eskimos (Kinsella, 1986). Cases of multiple sclerosis are rare in coastal areas, where there is high consumption of seafood (Pourchet-Campos, 1989). It can be said that there are strong evidences that diets based on PUFAs can reduce the development of coronary diseases (Nestel, 1987). Most researches on w -3 fatty acids during the latest years have been aimed at determining their immunological and anti-inflammatory effects, especially in cases of asthma, rheumatic arthritis and autoimmune diseases (Pigott and Tucker, 1987; Horrocks and Yeo, 1999; Simopoulos, 2002; Shapiro, 2003). Evidences for the selective effect of w -3 PUFAs come from epidemiological data indicating a very low incidence of ischemic heart diseases (IHD) among Eskimos living in Greenland, who had a diet rich in seafood (average of 400 g of fish/day) compared to Eskimos living in Denmark, who consumed limited amounts of fish (Dyerberg, 1981; Jorgensen and Dyerberg, 1983). Subsequent analyses revealed that the Greenland Eskimos had significantly lower concentration of serum cholesterol, triglycerides, very-low density lipoproteins (VLDLs), low-density lipoproteins (LDLs) and higher levels of high-density lipoproteins (HDLs), compared to the Denmark Eskimos, who consumed a typical European diet (Dyerberg, 1981; Jorgensen and Dyerberg, 1983). Lower death rates from IHD in Japan have been attributed to the higher consumption of fish, with the lowest rate being observed in Okinawa, where fish consumption exceeds 200 g/day (Kagawa et al., 1982).
Kromhout et al. (1985) suggested fish consumption of 30 g/day to reduce the incidence of IHD-related mortality in a population of Dutch males. These observations stimulated many dietary studies involving the use of fish oil in the diets of humans and animals. Nestel et al. (1984) reported that fish oils inhibit the synthesis of proteins, as well as TG and VLDLs components. The w -3 PUFAs from fish oils may also enhance the removal of VLDL in peripheral tissues and increase the excretion of cholesterol (Harris et al., 1983). They may also reduce the fatty acids synthesis in living cells (Yang and Williams, 1978). Singer et al. (1985) showed that EPA and/or DHA from the mackerel specie are effective in reducing blood pressure in certain groups of patients from East Germany who include fish in their usual diets.
A study carried out by Kromhout et al. (1985) yielded information on foods that provided an EPA supply of 140 mg/day, in a group regularly fed on seafood. This discovery is based on a twenty-year study with male adults in Holland who consumed fish twice or three times a week, amounting to a consumption of 30 g/day. The mortality due to coronary diseases was reduced by 50%. This fact has stimulated the consumption of fish and mollusks.
Whelan et al. (1991) reported experiments with mice with the intent to compare the effects of diets containing a-linolenic acids to those containing fish oils on the metabolism of eicosanoids. The fish oil diet promoted significant modifications in the eicosapentaenoate levels in the tissues, while the a-linolenic acid was mostly metabolized as docosahexanoate.
The w -3 PUFAs components of fatty acids, present in a diet containing fish oil, are readily absorbed and assimilated in various organs and adipose tissues of lab animals, with the phospholipids (PLs) being preferably assimilated, especially the phosphatidylethanolamine and the phosphatidylcholine (Hornstra, 1982). The w -3 fatty acids appear in adipose tissues within 1-2 days, and their PLs concentration increases modestly with continued feeding. However, there seems to be a limit to the amount incorporated, which varies according to the tissue. The w -3 PUFAs are rapidly depleted from most tissues following cessation of feeding (Swanson et al., 1986).
Several researches have shown that diets rich in fat may be associated with an increase in the incidence, accelerated growth and development of certain tumors (Cave, 1991; Kinsella, 1990). In view of this fact, studies have been carried out to determine the capability of numerous families of fatty acids to promote tumors growth. The effects of a diet rich in w -3 series polyunsaturated fatty acids on the development of certain tumors in a group of animals were examined. At the same time, another group of animals was feed on w -6 series polyunsaturated fatty acids. The animals fed on w -3 fatty acids showed a decrease in tumors growth (mammary, colon, pancreas and prostate), while those fed on w -6 fatty acids presented an increase in the growth of tumor cells (Cave, 1991).
Experimental studies with mice have shown that the linoleic acid is a factor of tumors growth, while fatty acids derived from seafood, mainly the EPA and DHA fatty acids, inhibit tumors growth, as it is the possible case of antioxidants, vitamin C and E, carotenoids and selenium. High levels of prostaglandin (PGE2) have been detected in the tissues and blood of patients with tumors (Kritchevsky, 1996), which evidences that PGE2 is associated with tumors growth. Consequently, high amounts of w -6 fatty acids in tissues facilitate this reaction. There are also evidences that these factors inhibit the PG synthesis, which may inhibit breast cancer. The Eskimos and the Japanese show low incidence of breast cancer, suggesting that fish oil may play a role in this fact (Kinsella, 1986).
The PGE2 and PGF2 prostaglandins, metabolized from w -6 polyunsaturated fatty acids, are found in high amounts in blood and urine of dysmenorrheic women during the menstrual period (Benassi et al., 1993). A balanced diet, that is, showing low amounts of w -6 fatty acids, may indirectly reduce the prostaglandin amounts, as well as the menstrual discomfort. This phenomenon may not occur due to the increase in w -3 fatty acids or monounsaturated fatty acids (w -9 series) amounts. This hypothesis was based on a previous study carried out with Spanish women, whose menstrual colics and other menstrual discomforts were statistically correlated with low amounts of fatty acids in their diets, that is, low w -3/w -6 fatty acids ratios and low amounts of vitamin B12 (Deutch, 1995). Based on these results, an American study indicated that fish oil supplementation could be used to control dysmenorrhea (Hazel Z Biro et al., 1996).
Recent researches have shown that people who consume large quantities of fish tend to show lower depression indexes and/or suicide (Colin et al., 2003). The increase in depression indexes in the United States has been associated to diets containing low amounts of DHA (Hi - DHATM, 2000). Study showed that hospitalized manic-depressives and individuals with other mood disorders showed significant improvement in their depressive state when DHA was part of their diets (Hi - DHATM, 2000; Mischoulon and Fava, 2000; Puri et al., 2001; Peet and Horrobin, 2002). Hibben et al., (1998) and Brunner et al. (2002) suggested the increasing the dietary intake of omega-3 fatty acids might increase central serotonergic activity and reduce impulsive and aggressive behaviors.
The excessive consumption of unsaturated fatty acids might pose a potential threat due to the peroxidation process. High amounts of w -6 fatty acids may trigger physiopathologic events associated with the production of eicosanoids (arthritis, inflammations). Thus, besides meeting the functional requirements of nerve tissues, retina and gonads, w -3 PUFAs from diets may act as effective modifiers in the eicosanoids production.
As for patients under treatment, the supplementation should supply the needs for EPA and DHA in palatable form, which may increase the oxidative stress, favor the lipidic peroxidation and naturally reduce the activity of "killer" cells, decreasing their direct action over extraneous cells. It is necessary to have a balanced diet of polyunsaturated fatty acids, as well as of antioxidants, to avoid the lipidic peroxidation (Kritchevsky, 1996).
Vitamin E is the most important in vivo antioxidant for controlling the peroxidation of polyunsaturated fatty acids. This vitamin is composed mainly of tocopherols, with a -tocopherol being the most effective antioxidant. Although the current American diet provides the average dietary needs of 0.4mg a-tocopherol/g linoleic acid, it is still not clear if sufficient amounts of a-tocopherol are provided in low fat diets containing only polyunsaturated oils, which is the case of fish oil. Therefore, it is important to ensure that an adequate intake of a-tocopherol is provided in order to minimize peroxidation, cellular aging and accumulation of lipofuscin and ceroid pigments, typical of tissue degeneration (Kinsella, 1988).
It was found that in case there was a deficiency of vitamin E in people consuming large amounts of polyunsaturated fatty acids, the production of prostacyclin-synthetase was reduced. Thus, it did not act on the endoperoxide and, consequently, not forming prostacyclin. The reduction in the prostacyclin production increased the thromboxane-prostacyclin rate, which was deleterious for the organism (Ribeiro, 1990).
Other foods containing w-3 and w-6
Human milk is a very rich source of w-3 and w-6 fatty acids for preterm infants. However, polyunsaturated acids amounts in the human milk depend on their ingestion by mothers, so that they may be liberated by the mammary glands (Martinez, 1991; Duchen and Bjorksten, 2001). Jensen (1978) reported that colostrum is richer than milk in unsaturated fatty acids, which are also found in higher amounts in preterm children's mother's colostrums (Bitman et al., 1983). Milk from strictly vegetarian women shows around 1/3 of the DHA amount found in milk from non-vegetarian women and lower seafood consumption also was associated with higher rates of postpartum depression. (Carlson et al., 1985; Hibbeln, 2002. The total long-chained polyunsaturated fatty acids amounts in human milk vary after the childbirth, with amounts dropping quickly three months afterwards (Crawford, 1976). The supply of w -3 and w -6 by vegetable oils varies according to the oil origin, as it can be observed in Table 3.
In Brazil, an African palm oil is broadly used in a typical dish from Bahia, which can supply up to 50% of fatty acids from the three families (10% of w -6 and 1% of w -3) (Tavares and Barbério, 1989). It has been found that purslane, Portulaca oleracea, contributed more to health than most cultivated vegetables. It particularly showed high percentage of a-linolenic acid (Simopoulos et al., 1991; Ezekwe et al., 1999), being the richest source of this fatty acid among all vegetables (Simopoulos et al., 1991).
The algae Fucus vesiculosus and Chondrus crispus provide 38.5% and 9.4%, and 30.5% and 3.3% of w -3 and w -6, respectively. Algae are generally little used as food around the world, except for Japan (Hunter, 1990). Broccoli, lettuce, spinach, certain types of beans, butter and especially margarines may be, depending on the vegetable oils they are made from, sources of fatty acids (Hunter, 1990). Nowadays, nutritious products enriched with DHA, such as milk and soft drinks, not to mention supplements containing EPA and DHA, have been marketed by the food industry (Park et al., 1997).
CONCLUSIONS
Functional foods are associated with changes in lifestyle and alimentary habits. The w -3 and w -6 fatty acids found mainly in seafood and vegetable oils are indicated for the prevention of cardiovascular diseases, as they decrease cholesterol amounts and blood pressure. They are also correlated with the cerebral and visual development. Therefore, a balanced diet, in which fish is consumed at least 2 or 3 times per week, supplies the daily needs of w -3 polyunsaturated fatty acids, and keeps the integrity of cellular membranes and nervous tissues, as well as, ensures good functionality of the organism as a whole.
Received: April 10, 2001
Revised: June 27, 2002
Accepted: April 08, 2003
- Andrade, M. O. and Lima, U. A. (1980), The effects of season and processing on the lipids of mandi (Pimelodus clarias, Bloch), a Brazilian freshwater fish. In: Connell, J. J. (ed.). Advances in Fish Science and Technology, Fishing News Books, England. pp.387-393.
- Ackman, R. G. and McLeod, C. (1988), Total lipids and nutritionally importanty fatty acids of some Nova Scotia fish and shelfish products. Canadian Institute of Food Science and Technology, 24, 390-398.
- Agren, J.; Hanninen, O.; Laitinen, M.; Seppanen, K.; Bernhardt, I.; Fogelholm, L. and Herranen, J. (1988), Boreal freshwater fish diet modifies the plasma lipids and prostanoids and membrane fatty acids in man. Lipids, 23, 924-929.
- Anderson, G. J. and Connor, W. E. (1989), On the demonstration of w -3 essential fatty acid deficiency in humans. The American Journal of Clinical Nutrition, 49, 585-587.
- Arai, S. (1996), Studies on functional foods in Japan - State of the art. Bioscience Biotechnology Biochemical, 60, 9-15.
- Armstrong, S. G.; Leach, D. N. and Wyllie, S. G. (1991), Nutricional evaluation of lipids in fish from Australian temperate waters. Journal of Food Science, 56, 1111-1112.
- Badolato, E. S. G.; Carvalho, J. B.; Amaral Mello, M. R. P.; Tavares, M.; Campos N. C.; Aued-Pimentel, S. and Morais C. (1994), Composição centesimal de ácidos graxos e valor calórico de cinco espécies de peixes marinhos nas diferentes estações do ano. Revista Instituto Adolfo Lutz, 54, 27-35.
- Belda, M. C. R. and Pourchet-Campos, M. A. (1991), Ácidos graxos essenciais em nutrição: uma visão atualizada. Ciência e Tecnologia de Alimentos, 11, 5-35.
- Benassi, L.; Bertani, D. and Avanzine, A. (1993), An attempt at real prophylaxis of primary dysmenorrhea: comparison between Meclofenamate sodium and Naproxen sodium. Clinical and Experimental Obstetrics Gynecology, 20, 102-107.
- Benisek, D.; Shabert, J. and Skornik, R. (2000), Dietary intake of polyunsaturated fatty acids by pregnant or lactating women in the United States. Obstetrics and Gynecology, 95 : (Suppl. 4), 77-78.
- Bimbo, A. P. (1987), The emerging marine oil industry. The American Journal of the Oil Chemists' Society, 46, 706-715.
- Birch, E. E.; Hoffman, D. R.; Uauy, R.; Birch, D. G. and Prestidge, C. (1998), Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatrics Reasearch, 44, 201-209.
- Bitman, J. et al. (1983), Comparison of the lipid composition of breast milk from mothers of term and preterm infants. The American Journal of Clinical Nutrition, 38, 300-312.
- Bodwell, C. E. and Anderson, B. A. (1986), Muscle as Food Academic Press Inc., London.
- Botta, J. R.; Kennedy, K. and Squires, B. E. (1986), Effect of method of catching and time of season on the composition of Atlantic cod (Gadus morhua). Journal of Food Science, 52, 922-924, 927.
- Brunner, J.; Parhofer, K. G.; Schwandt, P. and Bronisch, T. (2002), Cholesterol, essential fatty acids, and suicide. Pharmacopsychiatry, 35, 1-5.
- Budowski, P. (1981), Nutritional effects of n3-polyunsaturated fatty acids. Israel Journal of Medical Sciences, 17, 223-231.
- Burr, M. L. (1991), Is fish good for the heart? Trends in Food Science and Technology, 2, 17-20.
- Byrne, M. (1994), Nutraceuticals: Food fad or future trend. Chilton's Food Engineering International, 19, 42-43.
- Campisi, J.; Dimri, G. and Hara, E. (1996), Control of replicative senescence. In: Handbook of the biology of ageing Academic Press, San Diego. pp.121-149.
- Carlson, S. E.; Rhodes, P. G. and Fergunson, M. C. (1985), DHA status of preterm infants at birth and following feeding with human milk or formula. The American Journal of Clinical Nutrition, 44, 798-804.
- Castro, L. A. B. (1988), Bioquímica do pescado I - Composição química. Boletim Técnico do Instituto da Pesca, 2, 1-16.
- Cave Jr., W. T. (1991), Dietary w -3 polyunsaturared fatty acid effects on animal tumorigenesis. The FASEB Journal, 5, 2160-2166.
- Colin, A.; Reggers, J.; Castronovo, V. and Ansseau, M. (2003), Lipids, depression and suicide. Encephale, 29, 49-58.
- Crawford, M. A. et al. (1976), Milk lipids and their variability. Current Medical Research and Opinion, 4, 33-39.
- Deutch, B. (1995), Menstrual pain in Danish women correlated with low w -3 polyunsaturated fatty acid intake. European Journal of Clinical Nutrition, 49, 508-516.
- Dontson, K.; Jerrel, J.; Picciano, M. and Perkins, E. (1992), High-performance liquid chromatography of human milk triacyglycerols and gas chromatography of component fatty acids. Lipids, 27, 933-939.
- Duchen, K.; Bjorksten, B. (2001), Polyunsaturated n-3 fatty acids and the development of atopic disease. Lipids, 36, 1033-1042.
- Dyerberg, J.; Bang, H.O.; Stoffersen E.; Moncada S. and Vane J. R. (1978), Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis. The The Lancet, 2, 117-119.
- Dyerberg, J. and Bang, H. O. (1979), Haemostatic function and platelet polyunsaturated fatty acids in Eskimos. Lancet, 2, 433-435.
- Ezekwe, M. O.; Omara-Alwala, T. R. and Membrahtu, T. (1999), Nutrite chracterization of purslane accession as influenced by planting date. Plant Foods for Human Nutrition, 54, 183-192.
- FAO/WHO - EXPERT COMMITTEE. (1994), The role of essential fatty acids in neural development: implications for perinatal nutrition. The American Journal of Clinical Nutrition, 7, 703-710.
- François, C. A.; Connor, S. L.; Wander, R. C. and Connor, W. E. (1998), Acute effects of dietary fatty acids on the fatty of human milk. The American Journal of Clinical Nutrition, 67, 301-308.
- Gibson, R. A. (1983), Australian fish - An excellent source of both arachidonic acid and w -3 polyunsaturated fatty acids. Lipids, 18, 743 752.
- Goodnight, S. H.; Harris S. W.; Connor W. E. and Allingworth, R. D. (1981), The effect of dietary w -3 fatty acids on platelet composition and function in man: a prospective controlled study. Blood, 58, 880-885.
- Harris, W. S.; Connor, W. E. and Mcmurray, M. P. (1983), The comparative reductions of plasma lipids and lipoproteins by dietary polyunsaturated fats: salmon oil versus vegetable oils. Metabolism, 32, 178-184.
- Hazel Z Biro, F. M.; Kottenhahn, R. K. and Rosenthal, S. L. (1996), Suplementation with Omega-3 polyunsaturated fatty acids in management of dysmenorrhea in adolescents. The American Journal of Clinical Nutrition, 174, 1335-1338.
- Health (1995), Care foods'possible under current redulations, professor says. Food Chemical News, 36, 71-73.
- Hearn, T. L.; Sgoutas, S. A.; Hearn, J. A. and Sgoutas, D.S. (1987), Polyunsaturated fatty acids and fat in fish flesh for selecting species for health benefits. Journal of Food Science, 52, 1209-1211.
- Hibbeln, J.; Linnoila, M.; Umhau, J. C.; Rawlings, R.; George, D. T. and Salem Jr., N. (1998), Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early- and late-onset alcoholics. Biol Psychiatry; 44, 235-242.
- Hibbeln, J. (2002), Seafood consumption, the DHA content of mothers' milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. Journal Affect Disord., 69, 15-29.
- Hi - DHA TM (2000), Next generation omega -3 fatty acids for brain and nervous system function, MERCK kgaA, 2-11.
- Holub, B. (1988), Health effects of fish oils. The Journal of the American Oil Chemists' Society, 65, 1722-1726.
- Hornstra, G. (1982), Dietary fats, prostanoids and arterial thrombosis. Martinus Nijhoff Publishers, Boston.
- Horrocks, L. A. and Yeo, Y. K. (1999), Health Benefits of Docosahexaenoic Acid (DHA). Pharmacol. Res., 40, 211-225.
- Hrelia, S.; Bordoni, A.; Celadon, M.; Turchetto, E.; Biagi, P. L. and Rossi, C. A. (1989), Ade-related changes in linoleate and a -linoleate desaturation by rat liver microsomes. Biochemical Biophysiology Research Communications, 163, 348-355.
- Hrelia, S.; Celadon, M.; Rossi, C. A.; Biagi, P. L. and Bordoni, A. (1990), Changes in linoleate and a -linoleate desaturation by rat liver microsomes. Biochemical International, 22, 659-667.
- Hunter, J. E. (1990), N-3 fatty acids from vegetable oils. The American Journal of Clinical Nutrition, 51, 809-814.
- Innis, S. M. (1992), Human milk and formula fatty acids. Journal of Pediatrics, 60, 56-61.
- Jensen, R. G.; Hagerty, M. M. and Mc Mahon, K. E. (1978), Lipids of human milk and infant formulas: a review. The American Journal of Clinical Nutritional, 31, 990-1016.
- Jorgensen, K. A. and Dyerberg, J. (1983), Platelets and atherosclerosis. Advance in Nutrition Research, 5, 57.
- Kagawa, Y.; Niskizawa, M.; Suzuki, M.; Miyataki, T.; Hamamoto, T.; Goto, K.; Motonaya, E.; Izumikawa, H.; Hirata, A. and Edihara, A. (1982), Eicosapolyenoic acids of serum lipids of Japanese islanders with low incidence of cardiovascular diseases. The Journal Nutrition of Science Vitaminology, 28, 441-445.
- Kinsella, J. E. (1986), Food components with potential therapeutic benefits: the n-3 polyunsaturated fatty acids of fish oils. Food Technology, 40, 89-97.
- Kinsella, J. E. (1988), Food lipids and fatty acids: importance in food quality, nutrition and health. Food Technology, 42, 124-145.
- Kinsella, J. E. (1990), Lipids, membrane receptors and enzymes: Effects of dietary fatty acids. Journal of Parenteral and Enteral Nutrition, 14, 200-217.
- Kinsella, J. E.; Broughton, K. S. and Whelan, J. W. (1990), Dietary unsaturated fatty acids: Interaction and possible needs in relation to eicosanoid synthesis. Journal Nutrition Biochemical, 1, 123-141.
- Kritchevsky, D. (1996), An international symposium on cancer cachexia, cytokines, and EPA: Introduction. The Journal of Nutrition, 12, 1.
- Kromhout, D.; Boschieler, E. B. and Coulander, D. C. (1985), The inverse relation between fish oil consumption and mortality from coronary heart disease. New England Journal of Medicine, 312, 1205-1209.
- Kuhn, D. C. and Crawford, M. (1968), Placental essential fatty acid transport and prostaglandin synthesis. Progress in Lipid Research, 25; 345-353.
- Labomar - Mun. Cida Program. (1989), Ciência e tecnologia de produtos pesqueiros St. John's, New foundland, MUN Printing Services. Ciência e tecnologia de organismos aquáticos. 1A, 1162.
- Labuza, T. P. and Sloam, A. E. (1979), Comtemporary Nutrition Controversies, West Publishing Company, New York.
- Levin, R. I.; Weksler, B. B.; Marcus, A. J. and Jaffe, E. A. (1984), Prostacyclin production by endopithelial cells. In - Biology of endothelial cell, ed. JAFFE, E. A. Martinus Nijhoff Publishers, Boston, pp. 288-328.
- Martinez, M. (1991), Developmental profiles of polyunsaturated fatty acids in the brain of normal infants and patientes with peroxisomal disease: severe deficiency of docosahexaenoic acid in Zellweger's and pseudo-Zellweger's syndromes. World Review of Nutrition and Dietetic, 66, 87-120.
- Mischoulon, D. and Fava, M. (2000), Docosahexanoic acid and omega-3 fatty acids in depression. Psychiatr. Clin. North Am. 23, 785-794.
- Monsen, E. R. (1985), NIH lauching major reaserch program on fish oil and health. Food Chemical News, 6, 34-39.
- Murphy, M. G. (1990), Dietary fatty acids and membrane protein function. The Journal of Nutrition Biochemical, 1, 68-79.
- Needleman, P.; Minkes, M. S.; Ferrendelli, J. A. and Sprecher, H. (1979), Triene prostaglandins, prostacyclin and thromboxane biossynthesis and unique biological properties. Proceedings National Academy of Sciences, 76, 944-948.
- Nestel, P. J.; Connor, W. E.; Reardon, M. F.; Connor, S.; Wong, S. and Boston, R. (1984), Suppression by diets rich in fish oil of very low density lipoprotein production in man. Journal of Clinical Investigation, 74, 82-89.
- Nestel, P. J. (1987), Fish oil fatty acids: the answer to heart disease? CSIRO Food Research Quarterly, 47, 9-12.
- Park, Y. K.; Koo, M. H. and Carvalho, P. O. (1997), Recentes progressos dos alimentos funcionais. Boletim SBCTA, 31, 200-206.
- Peet, M. and Horrobin, D. F. (2002), A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch. Gen. Psychiatry, 59, 913-919.
- Peluffo, R. O.; Ayala, S. and Brenner, R. R. (1970), Metabolism of fatty acids of the linoleic acid series in testicles of diabetic rat. American Journal of Physiology, 218, 669-673.
- Pigott, G. M. and Tucker, B. W. (1987), Science opens new horizons for marine lipids in human nutrition. Food Review International, 3, 105-138.
- Pourchet-Campos, M. A. (1989), Os lipídeos na alimentação: Uma benção e um desafio. In: 5th Encontro Nacional de Analistas de Alimentos - ENAAL, Salvador, BA.
- Puri, B. K.; Counsell, S. J.; Hamilton, G.; Richardson, A. J. and Horrobin, D. F. (2001), Eicosapentaenoic acid in treatment-resistant depression associated with symptom remission, structural brain changes and reduced neuronal phospholipid turnover. Int. Journal Clin. Pract., 55, 560-563.
- Ribeiro, L. (1990), Prostaglandinas e ácidos omega-3 na prevenção da aterosclerose. Arquivos Brasileiros de Cardiologia, 54, 279-281.
- Robertson, D. (1993), Increasing Omega-3 fatty acid consumption through food fortification. British Journal of Food, 95, 121-123.
- Shapiro, H. (2003), Could n-3 polyunsaturated fatty acids reduce pathological pain by direct actions on the nervous system? Prostaglandins Leukot. Essent. Fatty Acids, 68, 219-224.
- Silva, S. M. C. S. (1992), Efeito do processamento sobre ácidos graxos polinsaturados da fração lipidica de duas espécies de peixes. Dissertação de Mestrado, Faculdade de Ciências Farmacêuticas da USP, São Paulo.
- Simopoulos, A. P. (1991), Omega-3 fatty acids in health and development. The American Journal of Clinical Nutrition, 54, 438-463.
- Simopoulos, A. P. (2002), Omega-3 fatty acids in inflammation and autoimmune disease. The American Journal of Clinical Nutrition, 21, 495-505.
- Singer, P.; Wirth, M.; Voight, S.; Richtert-Heinrich, E.; Godicke, W.; Berger, I.; Naumann, E.; Listing, J.; Hartrodt, W. and Taube, C. (1985), Blood pressure and lipid lowering effect of mackerel and herring diet in parients with mild essential hypertension. Atherosclerosis, 56, 223-235.
- Stansby, M. E. (1969), Nutritional properties of fish oils. World Review of Nutrition and Dietetics, 11, 46-105.
- Stansby, M. E. (1973), Polyunsaturated and fat in fish flesh. The Journal of the American Dietetic Association, 63, 625-630.
- Stjernschantz, J. (1984), The leukotrienes. Medical Biology, 62, 215-230.
- Swanson, J.; Black, M. and Kinsella, J. E. (1986), Uptake by and depletion of n-3 PUFA from various tissues of mice following administration of fish oil. Cornell University, New York.
- Tavares, M. and Barbério, J. C. (1989), Composição em ácidos graxos do azeite de dendê brasileiro. Revista de Farmácia e Bioquímica da Universidade de São Paulo, 25, 5-15.
- Tuley, L. (1995), Functional foods - The technical issues. Food Manufacture, 70, 30-32.
- Wang, Y. J.; Miller, L. A.; Perren, M. and Addis, P. B. (1990), Omega-3 fatty acids in Lake Superior fish. Journal of Food Science, 55, 71-73, 76.
- Whelan, J.; Broughton, K. S. and Kinsella, J. E. (1991), The comparative effects of dietary a -linolenic acid and fish oil on 4 and 5 series leukotriene formation "in vivo". Lipids, 26, 119-126.
- Yang, W. T. and Williams, M. A. (1978), Comparison of unsaturated fatty acids in reducing fatty acid synthesis in isolated rat hepatocytes. Biochemical and Biophysical Acta, 531, 133-137.
Publication Dates
-
Publication in this collection
18 Nov 2003 -
Date of issue
June 2003
History
-
Received
10 Apr 2001 -
Reviewed
27 June 2002 -
Accepted
08 Apr 2003