ABSTRACT
Quorum sensing is considered one of the most important discoveries in cell-to-cell communication. Although revealed in Bacteria, it has been identified as well as a mechanism present in the other two domains, Eukaryota and Archaea. This phenomenon consists mainly of an exchange and sensing of "words" produced by each cell: chemical signals known as autoinducers. The process takes places at high cell densities and confined environments, triggering the expression of specific genes that manifest in a determined phenotype. Quorum sensing has a fundamental importance in the organisms' fitness in natural ecosystems since it activates many of the traits needed by cells to survive under specific conditions, and thus a wide variety of chemical signals, which are detailed throughout the review, have evolved in response to the needs of an organism in the ecosystem it inhabits. As a counterpart, derived from the natural occurrence of quorum sensing, comes it's antagonistic process named quorum quenching. Acting in the exact opposite way, quorum quenching interferes or degrades the autoinducers confusing and stopping communication, hence affecting transcriptional regulation and expression of a specific phenotype. The main reasons for stopping this mechanism go from fading their own signals when perceiving scarce nutrients conditions, to degrading competitors' signals to take advantage in the ecosystem. Some of the most studied purposes and means known up to date to be used by cells for making quorum quenching in their ecosystems is what will be discussed along this review, offering information for future works on quorum quencher molecules bioprospection.
Key words:
Quorum Sensing; Autoinducers; Interference; Degradation; Microbial Ecology; Quorum Quenching
INTRODUCTION
Quorum Sensing (QS) discovery has been one the greatest highlights in microbiology on the last 50 years. Since the publication of the earliest studies on the subject, back in the 70's by Nealson et al. (19701 - Nealson KH, Platt T, Hastings JW. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970; 104: 313-322.) [11 - Nealson KH, Platt T, Hastings JW. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970; 104: 313-322.], who dedicated their research to bioluminescence production by the oceanic bacteria Vibrio fischeri and its relative Vibrio harveyi, it became clear that it would be a milestone for unveiling plenty of biological processes. The term QS itself, includes both the sensing process of small diffusible molecules and the quorum or population threshold needed for the whole phenomenon of signals detection and genes regulation to happen. Since its discovery, different QS mechanisms have been described within the three domains of life: Bacteria, Archaea and Eukaryota [22 - Tommonaro G, Abbamondi GR, Toksoy OE, Nicolaus, B. Investigating the quorum sensing system in halophilic bacteria. In: Maheshwari DK, Saraf M, editors Halophiles, Sustainable Development and Biodiversity. Switzerland: Springer; 2015. p. 189-207.], more exhaustedly among bacteria, with more than 70 genera, of both gram negative and gram positive bacteria being nowadays well characterized in terms of their QS systems, with their respective autoinducers and the processes they trigger in cells [33 - Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994; 176: 269-275.,44 - Zhang LH, Dong YH. Quorum sensing and signal interference: diverse implications. Mol Microbiol. 2004; 53: 1563-1571,55 - Garg N, Manchanda G, Kumar A. Bacterial quorum sensing: circuits and applications. A Van Leeuw J Microb. 2014; 105: 289-305. ].
As QS related discoveries and studies have become abundant throughout the years, it is now clear that it differs from one species to another, essentially in two aspects: the kind of autoinducer used by the cell to communicate and the biological processes regulated, these last ranging from bioluminescence, antibiotics synthesis, expression of virulence factors, motility, biofilm formation, among several others [66 - Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. Quorum-sensing in gram-negative bacteria. FEMS Microbiol Rev. 2001; 25: 365-404.] Thus, the biological importance of QS for cellular populations is quite significant because it commonly represents a competitive advantage over the rest of the organisms in the ecosystem, including humans. Therefore, an interest arises in understanding how can this process be downregulated, or interrupted, giving it the term quorum quenching (QQ), since a large number of microbial species regulate through QS, harmful behaviors with negative implications on other species equilibrium or development [77 - Bassler BL. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol. 1999; 2: 582-587.]. The essence of QQ is that autoinducers molecules should be affected in some way or another so that distortion of microbial communication can indeed take place.
The main aspects of QS and some autoinducer molecules are described in a general way in this review, but the connection of this information on cellular communication, population sensing and function regulation with the ecological evidences of QQ it's the main discussion. Through inquiring and revising the literature on different compounds and mechanisms that interfere with QS, this review aims to group information available on the subject and to assess the evidence of QQ as a natural occurring phenomenon with an important ecological role in the equilibrium, especially of microbial populations. Finally, a way of structuring QQ microorganisms bioprospection is proposed here, taking the ecological interactions discussed throughout the review as a basis for determining the QQ potential of a microbial population colonizing a specific habitat.
A variety of signal molecules
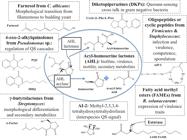
Properties, structures, biochemical nature, biological origin and roles of microbial communication signals, or autoinducers must be taken into account when trying to understand how to degrade them and turn them off in natural environments. With diverse molecular structures from one organism to the other, autoinducers can be produced both in natural and artificial ecosystems [88 - Dessaux Y, Chapelle E, Faure D. Quorum sensing and quorum quenching in soil ecosystems. In: Witzany G, editor. Biocommunications in Soil Microorganisms. Berlin Heidelberg: Spriger-Verlag; 2011. p. 339-367], in concentrations from pM to nM in the first one, scaling up to μM levels in vitro [99 - Leadbetter JR, Greenberg EP. Metabolism of acylhomoserine lactone quorum-sensing signals by Variovorax paradoxus. J Bacteriol. 2000; 182: 6921-6926.,1010 - Schell M. Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network. Annu Rev Phytopathol. 2000; 38:263-292.,1111 - Uroz S, Chhabra SR, Camara M, Williams P, Oger P, Dessaux Y. N-acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology. 2005; 151: 3313-3322.]. The widely known Acyl-Homoserine Lactones (AHL) are produced by various microorganisms belonging to Proteobacteria phylum, most of them containing the luxI and luxR type family of genes that encode different kinds of AHL synthases and proteins that bind to the autoinducer and act as transcriptional regulators, respectively [77 - Bassler BL. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol. 1999; 2: 582-587.,1212 - Boyer M, Wisniewski-Dye F. Cell-cell signalling in bacteria: not simply a matter of quorum. FEMS Microbiol Ecol. 2009; 70:1-19.,1313 - Von Bodman SB, Bauer WD, Coplin DL. Quorum sensing in plant-pathogenic bacteria. Annu Rev Phytopathol. 2003; 41: 455-482.]. More specialized structures are diketopiperazines (cyclic dipeptides), known to activate processes in some cells which are also normally activated by AHL [1414 - Holden MT, Ram Chhabra S, De Nys R, Stead P, Bainton NJ, Hill PJ, Manefield M, Kumar N, Labatte M, England D, Rice S, Givskov M, Salmond GP, Stewart GS, Bycroft BW, Kjelleberg S, Williams P. Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram-negative bacteria. Mol Microbiol. 1999; 33: 1254-1266.], quinolones or 4-hydroxy-2-alkylquinolones, specific for pseudomonads [1515 - Dubern JF, Diggle SP. Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species. Mol Biosyst. 2008; 4: 882-888.], the p-coumaroyl-HSL of Rhodopseudomonas palustris, Bradyrhizobium sp. and other bacteria [1616 - Schaefer AL, Greenberg EP, Oliver CM, Oda Y, Huang JJ, Bittan-Banin G, Peres CM, Schmidt S, Juhaszova K, Sufrin JR, Harwood CS. A new class of homoserine lactone quorum-sensing signals. Nature. 2008; 454: 595-599.], the autoinducer-2 or AI-2 which is vastly produced by both gram-negative and gram-positive bacteria when culture media is nearly spent [1717 - Bassler BL, Wright M, Silverman MR. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994; 13: 273-286], γ-butyrolactones in Actinobacteria [1818 - Yamada Y, Nihira T. Microbial hormones and microbial chemical ecology. In: Barton D, Nakanishi K, editors. Comprehensive natural products chemistry volume 8. Oxford, UK: Elsevier; 1998. p. 377-413.], oligopeptides in various Firmicutes and other gram positive bacteria [1919 - Dunny GM, Winans SC. Bacterial life: neither lonely nor boring. In: Dunny GM, Winans SC, editors. Cell-Cell signaling in bacteria. Washington DC: ASM Press; 1999. p. 1-5], bradyoxetin in Bradyrhizobium japonicum [2020 - Loh J, Carlson RW, York WS, Stacey G. Bradyoxetin, a unique chemical signal involved in symbiotic gene regulation. P Natl Acad Sci USA. 2002; 99: 14446-14451.], the signals of plant pathogenic bacteria Ralstonia solanacearum and Xanthomonas campestris, respectively 3-hydroxypalmitic acid methyl ester (3-OH PAME) and the diffusible signal factor (DSF) or chemically cis-11-methyl- 2-dodecenoic acid, both fatty acid derivatives [2121 - Chun W, Cui J, Poplawsky A. Purification, characterization and biological role of a pheromone produced by Xanthomonas campestris pv. campestris. Physiol Mol Plant P. 1997; 51: 1-14.,2222 - Flavier AB, Clough SJ, Schell MA, Denny TP. Identification of 3-hidroxypalmitic acid methyl ester as a novel autoregulator controlling virulence in Ralstonia solanacearum. Mol Microbiol. 1997; 26: 251-259.,2323 - Wang LH, He YW, Gao YF, Wu JE, Dong YH, He C, Wang SX, Weng LX, Xu JL, Tay L, Fang RX, Zhang LH. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol. 2004; 51: 903-912.] and farnesoic acid from the fungal microorganism Candida albicans [2424 - Oh KB, Miyazawa H, Naito T, Matsuoka H. Purification and characterization of an autoregulatory substance capable of regulating the morphological transition in Candida albicans. P Natl Acad Sci USA. 2001; 98: 4664-4668.].
But what is the reason for such a chemical variety of QS signals? Can the biotic and abiotic factors in the environment in which the QS microorganisms thrive be disturbing, adjusting or influencing the chemical nature of QS signals and creating this diversity? For instance, the case of autoinducer p-coumaroyl-HSL, which is derived from a plant metabolite, can be taken into account. In effect, it comes from p-coumaric acid, a key constituent of lignin, one of plants mayor components itself. This compound is believed to be synthetized by plants as a signaling molecule when wounded [88 - Dessaux Y, Chapelle E, Faure D. Quorum sensing and quorum quenching in soil ecosystems. In: Witzany G, editor. Biocommunications in Soil Microorganisms. Berlin Heidelberg: Spriger-Verlag; 2011. p. 339-367]. As Schaefer et al. (2008) [1616 - Schaefer AL, Greenberg EP, Oliver CM, Oda Y, Huang JJ, Bittan-Banin G, Peres CM, Schmidt S, Juhaszova K, Sufrin JR, Harwood CS. A new class of homoserine lactone quorum-sensing signals. Nature. 2008; 454: 595-599.] discuss it, this fact differs from the traditional way in which AHL-type signal are synthetized, through AHL-synthases expressed by the microorganism which create a bond between an acyl-carrier protein and a derivate of the amino acid metabolism, such as S-adenosylmethionine [2525 - Williams P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology. 2007; 153: 3923-3938.], bonding instead p-coumaroyl acid to HSL, to yield p-coumaroyl-HSL by using environmental p-coumaric acid rather than fatty acids from cellular pools. There represents an evidence of interaction between plant signals and bacterial metabolism, suggesting the presence of evolved generations of these microorganisms as a result of a better adaptation to their environment. Dessaux et al. (2011) [88 - Dessaux Y, Chapelle E, Faure D. Quorum sensing and quorum quenching in soil ecosystems. In: Witzany G, editor. Biocommunications in Soil Microorganisms. Berlin Heidelberg: Spriger-Verlag; 2011. p. 339-367] propose that this abundance of communication molecules could have also evolved to such a considerable number due to physico-chemical limitations that only allowed the biosynthesis or stability of a kind of molecule, illustrating their hypothesis with the case of the phytopathogenic bacterium R. solanacearum that causes bacterial wilt in nearly a hundred plant species [1313 - Von Bodman SB, Bauer WD, Coplin DL. Quorum sensing in plant-pathogenic bacteria. Annu Rev Phytopathol. 2003; 41: 455-482.] and is more abundant in tropical, moist and warm regions of earth, where soil temperatures are higher. The main autoinducer molecule for its QS system through which it regulates virulence is 3-OH PAME, although (R)-methyl 3-hydroxymyristate ((R)-3-OH MAME) has been identified in other strains [2626 - Kai K, Ohnishi H, Shimatani M, Ishikawa S, Mori Y, Kiba A, Ohnishi K, Tabuchi M, Hikichi Y. Methyl 3-hydroxymyristate, a diffusible signal mediating phc quorum sensing in Ralstonia solanacearum. Chembiochem. 2015; 16: 2309-2318.]. These two compounds, both fatty acids derivates, can be more heat-stable than the sensible AHLs [2727 - Byers JT, Lucas C, Salmond GP, Welch M. Nonenzymatic turnover of an Erwinia carotovora quorum-sensing signaling molecule. J Bacteriol. 2002; 184: 1163-1171.] and were probably generated during evolutionary selection and host coevolution of this pathogenic bacterium, developing some advantages over the rest of the microflora [2828 - Denny TP. Plant Pathogenic Ralstonia Species. In: Gnanamanickam SS, editor. Plant-associated bacteria. The Netherlands: Springer; 2006. p. 573-644.,1010 - Schell M. Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network. Annu Rev Phytopathol. 2000; 38:263-292.]. Similar is the case for the DSF or cis-11-methyl-2-dodecenoic acid of X. campestris that causes black rot of crucifers, which also being a fatty acid derivate [2121 - Chun W, Cui J, Poplawsky A. Purification, characterization and biological role of a pheromone produced by Xanthomonas campestris pv. campestris. Physiol Mol Plant P. 1997; 51: 1-14.,2323 - Wang LH, He YW, Gao YF, Wu JE, Dong YH, He C, Wang SX, Weng LX, Xu JL, Tay L, Fang RX, Zhang LH. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol. 2004; 51: 903-912.] is more stable to adverse conditions in the environment and has probably been selected by evolution to ensure communication under specific climate conditions. In Archaea for instance, QS could be involved in the strategy evolved by these organisms to thrive in hypersaline environments [22 - Tommonaro G, Abbamondi GR, Toksoy OE, Nicolaus, B. Investigating the quorum sensing system in halophilic bacteria. In: Maheshwari DK, Saraf M, editors Halophiles, Sustainable Development and Biodiversity. Switzerland: Springer; 2015. p. 189-207.], with the halophilic archaeon Haloterrigena hispanica producing diketopiperazines that activate AHL biosensors and that, as the authors speculate, could be involved in metabolic pathways controlling key functions to survive under such harsh conditions.
The constant interactions between microbial communities and their environments, have then probably favored the development of new QS systems and more complex communications among different species of microorganisms and even among eukaryotes. For instance, the cases of AI-2, or furanosyl borate diester, which has been detected in spent culture supernatant of several bacteria [1717 - Bassler BL, Wright M, Silverman MR. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994; 13: 273-286] and described as an interspecies signal or putative universal QS signal [2929 - Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev. 2005; 21: 319-346.] and the one of the fungal pathogen Candida albicans, reported firstly by Oh et al (2001) [2424 - Oh KB, Miyazawa H, Naito T, Matsuoka H. Purification and characterization of an autoregulatory substance capable of regulating the morphological transition in Candida albicans. P Natl Acad Sci USA. 2001; 98: 4664-4668.], in which they were able to demonstrate that the yeast produces a compound named farnesoic acid (FA) as a regulator in the yeast-to-mycelium transition, fundamental for the development of fungal virulence. These studies set examples of complex communication processes in prokaryotes and eukaryotes, while the case proposed by Dourado et al. (2013) [3030 - Dourado MN, Bogas AC, Pomini AM, Andreote FD, Quecine MC, Marsaioli AJ, Araújo WL. Methylobacterium-plant interaction genes regulated by plant exudate and quorum sensing molecules. Braz J Microbiol. 2013; 44: 1331-1339.] provides yet another evidence for the interaction between the environment and the QS regulated processes of a determined microorganism suggesting that root and plant exudates affect, though in a minor way that autoinducers, the expression ratios of genes involved in the plant-bacteria interaction of strains from the genus Methylobacterium, which have been reported as plant growth promoter and systemic resistance inducers. Considering this evidence and discussion, it seems rather evident that the expression of QS systems may be manipulated by the activities of other bacteria within complex microbial communities, by higher organisms and by environmental abiotic factors [2525 - Williams P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology. 2007; 153: 3923-3938.], at the same time that is not uncommon to observe that QS signals produced by a microorganism interfere effectively with QS signaling in other organisms in natural environments [3131 - Taga ME, Bassler BL. Chemical communication among bacteria. P Natl Acad Sci USA. 2003; 100: 14549-14554.] and that are even shared sometimes, as has been reported by Wang et al (2004) [2323 - Wang LH, He YW, Gao YF, Wu JE, Dong YH, He C, Wang SX, Weng LX, Xu JL, Tay L, Fang RX, Zhang LH. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol. 2004; 51: 903-912.] through the cross-talk phenomenon. Departing from interference as one the main important ecological ways of evading the QS success, further discussion regarding this and other forms of blocking cell-to-cell communication processes will be held in the following sections of this review.
QS process interference in natural microbial ecosystems
How exactly does QQ occur in microorganisms' natural conditions or free-living styles? Spatial distribution of cells in complex physical environments such as the rhizosphere is far from homogenous, with a suggested diffusion zone of 4-80 mm in soils [3232 - Gantner S, Schmid M, Durr C, Schuhegger R, Steidle A, Hutzler P, Langebartels C, Eberl L, Hartmann A, Dazzo FB. In situ quantitation of the spatial scale of calling distances and population density-independent N-acylhomoserine lactone-mediated communication by rhizobacteria colonized on plant roots. FEMS Microbiol Ecol. 2006; 56: 188-194.]. Instead, cells are localizing where diffusion rate and nutrient availability are temporally changing [2525 - Williams P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology. 2007; 153: 3923-3938.]. Accordingly, given that sufficient autoinducer concentrations and a more heterogeneous distribution are happening, microbial and eukaryotic populations that share habitat can interact with each other and get in direct contact with autoinducer molecules, inducing either the cross-talk phenomenon or the development of interference mechanisms. As Leadbetter & Greenberg (2000) [99 - Leadbetter JR, Greenberg EP. Metabolism of acylhomoserine lactone quorum-sensing signals by Variovorax paradoxus. J Bacteriol. 2000; 182: 6921-6926.] found a soil-born strain of Variovorax paradoxus that achieves QQ by using AHLs as a carbon source, the potential that some organisms have to degrade or interfere with QS signals and the process in general is intriguing and promising, since it confers a competitive advantage for these organisms over the ones that have QS-regulated systems. Other studies have also demonstrated that natural environments are actually polymicrobial communities where bacteria are communicating with neighboring cells or making QS while other cells are interrupting their communication and creating a constantly competitive environment for autoinducers [3333 - Hong KW, Koh CL, Sam CK, Yin WF, Chan KG. Quorum quenching revisited-from signal decays to signalling confusion. Sensors. 2012; 12: 4661-4696.].
Degradation of QS signals
In the search for sustainable and viable applications based on QS interference, the different naturally occurring routes for QQ can be catalogued in two major ones: prevention of accumulation (degradation of the signal) or recognition (interference with cognate receptor) [44 - Zhang LH, Dong YH. Quorum sensing and signal interference: diverse implications. Mol Microbiol. 2004; 53: 1563-1571]. In the case of prevention of signal molecules accumulation, importance has been given to the degradation of signaling molecules. A second alternative refers to the prevention of these autoinducers production or synthesis [3434 - Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013; 31: 224-245.], suggesting a repression at the transcription level of genes involved in the biosynthesis of these compounds. One of the first reports for the phenomenon of autoinducers degradation is the study by Dong et al. (2000) [3535 - Dong YH, Xu JL, Li XZ & Zhang LH. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum sensing signal and attenuates the virulence of Erwinia carotovora. P Natl Acad Sci USA. 2000; 97: 3526-3531.], in which they isolated and identified an AHL-degrading enzyme from a Bacillus sp. strain, described as a 250 aminoacids peptide and termed AiiA. This enzyme has become a cluster model, due to its certain homology with subsequently discovered AHLs-degrading enzymes. The signal-disintegrating phenomenon has been widely studied when regards to AHLs, since they are the most abundant QS signals known up to date. Different classes of inactivating enzymes have been found to be produced, most of them by microorganism and some other by eukaryote fungi, vegetable and mammalian cells, being catalogued in terms of their aminoacids sequence homology. These enzymes have been grouped in clusters with prototypes as the AiiA group, for the first enzyme discovered by Dong et al. (2000) [3535 - Dong YH, Xu JL, Li XZ & Zhang LH. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum sensing signal and attenuates the virulence of Erwinia carotovora. P Natl Acad Sci USA. 2000; 97: 3526-3531.], the AttM for the one discovered in Agrobacterium tumefasciens by Zhang et al. (2002) [3636 - Zhang HB, Wang LH, Zhang LH. Genetic control of quorum-sensing signal turnover in agrobacterium tumefaciens. P Natl Acad Sci USA. 2002; 99: 4638-4643.] and several other kinds [3434 - Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013; 31: 224-245.] generating a considerable repertoire of QQ enzymes that could be useful to industry, health, agriculture [3434 - Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013; 31: 224-245.,99 - Leadbetter JR, Greenberg EP. Metabolism of acylhomoserine lactone quorum-sensing signals by Variovorax paradoxus. J Bacteriol. 2000; 182: 6921-6926.] and other areas in which QS regulated processes cause problems. AHLs-degradation enzymes can also be classified by the cleavage site in the AHL molecule, being either acyl-homoserine lactonase (AHL-lactonase) or acyl-homoserine lactone acylase (AHL-acylase; AHL-amidohydrolases), liberating either AHS for the first class or HSL and an acyl or fatty acid chain for the second ones [3636 - Zhang HB, Wang LH, Zhang LH. Genetic control of quorum-sensing signal turnover in agrobacterium tumefaciens. P Natl Acad Sci USA. 2002; 99: 4638-4643.,3737 - Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, Zhang LH. Quenching quorum sensing dependent bacterial infection by N-acyl homoserine lactonase. Nature. 2001; 411: 813-817.,3838 - Lin YH, Xu JL, Hu J, Wang SL, Ong JR. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represent a novel and potent class of quorum quenching enzymes. Mol Microbiol. 2003; 47:849-860.,3939 - Park SY, Lee SJ, Oh TK, Oh JW, Koo BT, Yum DY, Lee JK. AhID, an N acyl-homoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiology. 2003; 149: 1541-1550.]. The mechanism of this signal degradation case, as well as another one further discussed in following paragraphs, are exemplified in Figure 1. The chemical structures of some autoinducers, with the most abundant and extensively studied AHLs in the center, are also represented in the indicated figure.
Chemical structures of molecules from several autoinducer families, their main QS function and three quorum quenching mechanisms for AHL and FAME autoinducers.
But even though QQ enzymes are known to be produced by many microorganisms and eukaryotic cells, the study by Leadbetter & Greenberg (2000) [99 - Leadbetter JR, Greenberg EP. Metabolism of acylhomoserine lactone quorum-sensing signals by Variovorax paradoxus. J Bacteriol. 2000; 182: 6921-6926.] generates discussion on the production of these compounds in natural ecosystems. This case presents the use of AHLs as carbon and nitrogen sources by a strain of Variovorax paradoxus. Throughout the technique of enrichment selection, the authors could isolate the referred VAI-C strain from soil samples and determine its capacity to produce an enzyme, exactly and AHL-acylase or amydohydrolase, which allowed it to use the AHL molecule for its metabolism, specifically the acyl chain as a carbon source. A second study by Flagan et al. (2003) [4040 - Flagan S, Ching WK, Leadbetter JR. Arthrobacter strain VAI-A utilizes acyl-homoserine lactone inactivation products and stimulates quorum signal biodegradation by Variovorax paradoxus. Appl Environ Microbiol. 2003; 69: 909-916.], could prove that not only the V. paradoxus strain could use QQ by the mechanism of QS signals degradation, but that other representatives of microbial communities from the same soil sample used for enrichment isolation, were capable of using AHLs and its degradation products as sources of nutrients for growth and general metabolism. This was the case of the strain VAI-A belonging to the Arthrobacter genera, which was able to use two nitrogenous degradation products of acyl-HSLs intake by V. Paradoxus, acyl-homoserine and HSL. These results suggest that these two species coexistence in the natural soil ecosystem was not coincidental and that consortia might play an important role in QQ, either for the metabolizing of these organic compounds released in QS process and their further mineralization, or for a phenomenon termed signal turnover, that will be discussed next by using Agrobacterium tumefaciens as the model organism.
This bacterium, which produces the crown gall disease in many dicotyledonous plants [88 - Dessaux Y, Chapelle E, Faure D. Quorum sensing and quorum quenching in soil ecosystems. In: Witzany G, editor. Biocommunications in Soil Microorganisms. Berlin Heidelberg: Spriger-Verlag; 2011. p. 339-367], is known to produce two AHL-lactonases, encoded by the attM and the aiiB genes, carried on At and Ti plasmids, respectively [4141 - Carlier A, Uroz S, Smadja B, Fray R, Latour X, Dessaux Y, Faure D. The Ti plasmid of Agrobacterium tumefaciens harbors an attM-paralogous gene, aiiB, also encoding N-acyl homoserine lactonase activity. Appl Environ Microb. 2003; 69: 4989-4993.,3636 - Zhang HB, Wang LH, Zhang LH. Genetic control of quorum-sensing signal turnover in agrobacterium tumefaciens. P Natl Acad Sci USA. 2002; 99: 4638-4643.]. In the other hand, the QS signal of the AHLs type, 3OC8HSL, originally known as conjugation factor, regulates Ti plasmid conjugal transfer [4242 - Zhang L, Murphy PJ, Kerr A, Tate ME. Agrobacterium conjugation and gene regulation by N-acyl-L-homoserine lactones. Nature. 1993; 362: 446-448.], meaning that the main virulence factor is regulated through QS. The production of 3OC8HSL is growth phase dependent, with the signal concentration increasing right after the exponential growth, but declining rapidly during the stationary phase [4343 - Dong YH, Zhang LH . Quorum sensing and quorum-quenching enzymes. J Microbiol. 2005; 43: 101-109.,3636 - Zhang HB, Wang LH, Zhang LH. Genetic control of quorum-sensing signal turnover in agrobacterium tumefaciens. P Natl Acad Sci USA. 2002; 99: 4638-4643.], similar to the pattern of Ti plasmid conjugal transfer. The rapid clearance of the AHL signal is attributed to the expression of the mentioned AiiB and AttM lactonases, which is controlled by different environmental signals, including plant compounds and starvation or nutrient exhaustion signals [4141 - Carlier A, Uroz S, Smadja B, Fray R, Latour X, Dessaux Y, Faure D. The Ti plasmid of Agrobacterium tumefaciens harbors an attM-paralogous gene, aiiB, also encoding N-acyl homoserine lactonase activity. Appl Environ Microb. 2003; 69: 4989-4993.,3636 - Zhang HB, Wang LH, Zhang LH. Genetic control of quorum-sensing signal turnover in agrobacterium tumefaciens. P Natl Acad Sci USA. 2002; 99: 4638-4643.]. This signal turnover of QS process in A. tumefaciens, especially the attM lactonase expression, is indicative of a perception by the cells of changes in the conditions of their surrounding environment, making them adjust and adapt their metabolism to for example, starvation stress. Even though in some strains there is evidence of A. tumefaciens terminating the energy-consuming conjugation process through QQ, the study by Khan & Fharrand (2009) [4444 - Khan SR, Farrand SK. The BlcC (AttM) lactonase of Agrobacterium tumefaciens does not quench the quorum-sensing system that regulates Ti plasmid conjugative transfer. J Bacteriol. 2009; 191: 1320-1329.] also states that despite the degradation of the QS signal acting as the essential ligand for TraR, the Ti plasmid activator, the conjugation process is not terminated. This could suggest that, more than terminating the conjugation; the QQ process is activated as a need for alternative carbon sources which provide ecological fitness and the excessive accumulation of AHL signals [99 - Leadbetter JR, Greenberg EP. Metabolism of acylhomoserine lactone quorum-sensing signals by Variovorax paradoxus. J Bacteriol. 2000; 182: 6921-6926.].
Parallel to the last case, multiple strains that produce AHL-degradation and other types of autoinducers-hydrolyzing enzymes, as an esterase active against 3-OH PAME autoinducer of R. solanacearum [4545 - Shinohara M, Nakajima N, Uehara Y. Purification and characterization of a novel esterase (β-hydroxypalmitate methyl ester hydrolase) and prevention of the expression of virulence by Ralstonia solanacearum. J Appl Microbiol. 2007; 103:152-162.], its mode of action also illustrated in Figure 1, and the AHL-acylase form Ralstonia strain XJ12B [3838 - Lin YH, Xu JL, Hu J, Wang SL, Ong JR. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represent a novel and potent class of quorum quenching enzymes. Mol Microbiol. 2003; 47:849-860.] have been identified from soil, plant and biofilm samples [4545 - Shinohara M, Nakajima N, Uehara Y. Purification and characterization of a novel esterase (β-hydroxypalmitate methyl ester hydrolase) and prevention of the expression of virulence by Ralstonia solanacearum. J Appl Microbiol. 2007; 103:152-162.]. Most of the autoinducers-degrading organisms have shown to coexist within the same environment with the ones that use QS, as a study has evidenced within the rhizosphere of tobacco [4646 - d'Angelo-Picard C, Faure D, Penot I, Dessaux Y. Diversity of N-acyl homoserine lactone-producing and degrading bacteria in soil and tobacco rhizosphere. Environ Microbiol. 2005; 7: 1796-1808.] this being a proof of selection pressure's importance for the development of QQ capacities. The human pathogen Pseduomonas aeruginosa, which causes severe infections in immunosuppressed patients, supports the idea that no matter the ecosystems in which the microorganism develops its full potential, there's feasibly to encounter a counterpart for its QS process. It has been encountered that cell membranes from differentiated human airway epithelia, like A549 cells from human lungs, one of the preferred human tissues of P. aeruginosa, produces considerably high concentrations of enzymes that inactivate this pathogen QS signals [4747 - Chun CK, Ozer EA, Welsh MJ, Zabner J, Greenberg EP. Inactivation of a Pseudomonas aeruginosa quorum-sensing signal by human airway epithelia. P Natl Acad Sci USA. 2004; 101: 3587-3590.].
In conclusion, through the study of the first case of AHL degradation by an AHL-lactonase by Dong et al. (2000) [3535 - Dong YH, Xu JL, Li XZ & Zhang LH. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum sensing signal and attenuates the virulence of Erwinia carotovora. P Natl Acad Sci USA. 2000; 97: 3526-3531.], the self-defense basis of QQ appears as a reality, since the producing strain belongs to Bacillus genus and it's known that long-chain 3-oxo-AHL signals, which spontaneously form rearrangement compounds, are toxic to several Bacillus species [4848 - Kaufmann GF, Sartorio R, Lee SH, Rogers CJ, Meijler MM, Moss JA, Clapham B, Brogam AP, Dickerson TB, Janda KD. Revisiting quorum sensing: discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. P Natl Acad Sci USA. 2005; 102: 309-314.]. The signal turnover systems of A. tumefaciens [3636 - Zhang HB, Wang LH, Zhang LH. Genetic control of quorum-sensing signal turnover in agrobacterium tumefaciens. P Natl Acad Sci USA. 2002; 99: 4638-4643.], as well as the one from Erwinia carotovora [2626 - Kai K, Ohnishi H, Shimatani M, Ishikawa S, Mori Y, Kiba A, Ohnishi K, Tabuchi M, Hikichi Y. Methyl 3-hydroxymyristate, a diffusible signal mediating phc quorum sensing in Ralstonia solanacearum. Chembiochem. 2015; 16: 2309-2318.], are proper illustrations of how bacteria and perhaps other microorganisms can take QQ as a way of controlling their infection or symbiotic processes, either activating or stopping them by a controlled interchange of QS and QQ mechanisms, which can be biological like the production of degrading enzymes in the first case, for certain A. tumefaciens strains, or physicochemical, as the one observed for E. carotovora. In the later case, the rapid diminution of the AHL QS signal observed during the stationary-phase was caused by a non-enzymatic degradation of the molecule. It was rather due to a rise of the pH in the fermentation, which increased from 7 to 8.5 as the growth curve progressed in cultures grown in Luria-Bertani medium, evidencing as well an ecosystemical interaction between the QS mechanism and abiotic factors in the environment which derive in the occurrence of QQ. This case accordingly introduces the last ecological reason for QQ discussed in this review, which is the use of signals as nutrient sources especially for carbon and nitrogen intake, since any degradation product of QS signals, either enzymatic or physicochemical, leads to an increase in nutrient sources availability in natural and artificial ecosystems. This has been well illustrated by Leadbetter & Greenberg (2000) [99 - Leadbetter JR, Greenberg EP. Metabolism of acylhomoserine lactone quorum-sensing signals by Variovorax paradoxus. J Bacteriol. 2000; 182: 6921-6926.] and Flagan et al. (2003) [4040 - Flagan S, Ching WK, Leadbetter JR. Arthrobacter strain VAI-A utilizes acyl-homoserine lactone inactivation products and stimulates quorum signal biodegradation by Variovorax paradoxus. Appl Environ Microbiol. 2003; 69: 909-916.] throughout their discoveries of QS signals as nutrient sources being used by soil bacteria isolates. These three arguments, which have been discussed so far, support an ecological basis of QQ and true cases for each of them are summarized and referred to in Table 1.
Inhibition of QS process
The second major QS interference process is related to the preclusion of QS signal sensing process by its cognate protein receptor. It is known as QS inhibition, since it's based on blocking signals detection sites, rather than degrading them [4949 - Zhang LH. Quorum quenching and proactive host defense. Trends Plant Sci. 2003; 8: 238-244.]. QS inhibitors are often also called antagonists and both terms are more commonly used for compounds that derive from eukaryote-prokaryote interactions, specially the host-pathogen interaction kind. It has been observed, that this kind of interactions between eukaryotic host and pathogenic bacteria provoke a wide range of reactions especially in the presence of QS molecules [2424 - Oh KB, Miyazawa H, Naito T, Matsuoka H. Purification and characterization of an autoregulatory substance capable of regulating the morphological transition in Candida albicans. P Natl Acad Sci USA. 2001; 98: 4664-4668.,44 - Zhang LH, Dong YH. Quorum sensing and signal interference: diverse implications. Mol Microbiol. 2004; 53: 1563-1571]. Several studies have demonstrated that eukaryotic organisms have possibly co-evolved with microbial pathogens or communities and accordingly, this adapting process originated a variety of compounds or mechanisms to protect themselves from negative QS regulated traits [2424 - Oh KB, Miyazawa H, Naito T, Matsuoka H. Purification and characterization of an autoregulatory substance capable of regulating the morphological transition in Candida albicans. P Natl Acad Sci USA. 2001; 98: 4664-4668.]. The halogenated furanones produced by the marine macro-algae Delisea pulchra is perhaps one the most discussed cases [5050 - Givskov M, DeNys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg PD, Kjelleberg S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J Bacteriol. 1996; 178: 6618-6622.]. These molecules were the first metabolites found to interfere with QS-regulated phenotypes and inhibit QS by directly interacting with LuxR homologues, triggering LuxR turnover [5151 - Manefield M, de Nys R, Kumar N, Read R, Givskov M, Steinberg P, Kjelleberg S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology. 1999; 145: 283-291.]. The results, reported by Givskov et al. (1996) [5050 - Givskov M, DeNys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg PD, Kjelleberg S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J Bacteriol. 1996; 178: 6618-6622.] suggest that the interaction between higher organisms and their associated microflora may be mediated by interference with bacterial regulatory systems.
Studies on plant, fungi and animal cells have also rendered evidence on signal interfere metabolites production, as a natural way to prevent microbial infections or negative relations with them. Several plants produce not only potential inhibitors that interfere with bacterial QS systems but also, in several occasions, AHL-mimics that stimulate them. This mimic compounds are normally nontoxic secondary metabolites that may act as QS antagonists by competing with AHL for receptor binding [5252 - Teasdale ME, Liu J, Wallace J, Akhlaghi F, Rowley DC. Secondary metabolites produced by the marine bacterium Halobacillus salinus that inhibit quorum sensing-controlled phenotypes in gram-negative bacteria. Appl Environ Microbiol. 2009; 75: 567-572.]. This is the case of the phenethylamide metabolites produced by the marine gram-positive bacterium Halobacillus salinus, which inhibit QS-regulated phenotypes, such as bioluminescence in Vibrio harveyi and violacein production in Chromobacterium violaceum. These mimic compounds seem to be highly promising for developing new antimicrobial solutions. In the other hand, different inhibitors or antagonist from plant extracts have been found to act as QS inhibitors because of their similar chemical structures to those of QS signals and also because of their ability to degrade signal receptors (LuxR/LasR) [5353 - Teplitski M, Robinson JB, Bauer WD. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol Plant Microbe In. 2000; 13: 637-648.]. In brief, these QS inhibitors have been proven to be as effective in exerting QQ, as are the autoinducer-degradation enzymes.
Ecologically based bioprospection of quorum quenchers
To consider the ecological nature and implications of QQ, before realizing bioprospection of QS interfering microorganisms or its metabolites, can be time and energy saving. Focus should be positioned on the three main ecological reasons for QQ occurrence, to try and adapt them to the ecosystem from where the QQ activity wants to be grabbed or isolated. It is proposed then, after this review, a strategy for achieving an ecological-focused experimental design, answering the following questions: 1- which of the different niches has the greatest population of the QS producing microbe? 2- Is the QS microorganism autoregulating its communication process by producing some autoinducer-degrading enzymes itself? 3- Are there reports of known QQ phyla in the niches of the selected sampling ecosystem? Bringing out these main questions before starting the bioprospection experimental procedures can avoid unnecessary sampling and collecting. The researcher will be aware of the most promising niches to sample in order to encounter QQ activity and of how to process these samples in the laboratory, either by enrichment selection or by selective isolation, without doing redundant procedures or replicates. Plus, different strategies have also been proposed by experimented researchers in the subject, to increase the percentage of QQ-bacteria obtained from natural samples, specially to develop crop protection approaches [88 - Dessaux Y, Chapelle E, Faure D. Quorum sensing and quorum quenching in soil ecosystems. In: Witzany G, editor. Biocommunications in Soil Microorganisms. Berlin Heidelberg: Spriger-Verlag; 2011. p. 339-367], these strategies being: 1) introduction of selected signal in soil (2) introduction of biodegradable compounds that stimulate the growth of natural QQ-bacteria; (3) a combination of both.
Concluding remarks
QS seems to be done by microbial cells in a way that encompasses with their physiological equilibrium and that allows them to gain fitness in their niches. This constant physiological regulation implies signal-controlling procedures, where QQ appears as a clear and necessary ecological strategy. Studies have demonstrated the use of QS autoinducers as nutrient sources or the use of QQ mechanisms to degrade potentially dangerous traits regulated by QS in nearby microbial communities. Furthermore, there is mounting data demonstrating that autoinducer signals elicit specific responses from eukaryotic hosts, unlocking reactions that inhibit QS processes, resulting in another QQ approximation. Evolutionary pressure and coexistence of microorganisms with other communities of microbes or higher organisms is a major force on the natural occurrence and function of QQ, rendering the overall picture of QS far more complex than it has thought to be until know: QS genes are embedded in a network of global regulation which includes QQ, whether realized by the same QS realizer microorganisms or by surrounding communities, where synthesis and environmental concentration of the autoinducers signal is highly responsive to the growth phase and to environmental factors. Emphasis should be placed now in the development of rigorous analysis and studies on how QQ affects natural ecosystems equilibrium, through transcriptomic studies or in-situ fluorescence to observe real expression levels of significant genes, in order to establish their future employment as microbial control agents. Their effectiveness in a minor scale has been already elucidated but much more complex consequences are pending to be submitted, in order to enhance research on QQ molecules as future antimicrobial agents.
REFERENCES
-
1- Nealson KH, Platt T, Hastings JW. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol 1970; 104: 313-322.
-
2- Tommonaro G, Abbamondi GR, Toksoy OE, Nicolaus, B. Investigating the quorum sensing system in halophilic bacteria. In: Maheshwari DK, Saraf M, editors Halophiles, Sustainable Development and Biodiversity Switzerland: Springer; 2015. p. 189-207.
-
3- Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 1994; 176: 269-275.
-
4- Zhang LH, Dong YH. Quorum sensing and signal interference: diverse implications. Mol Microbiol 2004; 53: 1563-1571
-
5- Garg N, Manchanda G, Kumar A. Bacterial quorum sensing: circuits and applications. A Van Leeuw J Microb. 2014; 105: 289-305.
-
6- Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. Quorum-sensing in gram-negative bacteria. FEMS Microbiol Rev 2001; 25: 365-404.
-
7- Bassler BL. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol. 1999; 2: 582-587.
-
8- Dessaux Y, Chapelle E, Faure D. Quorum sensing and quorum quenching in soil ecosystems. In: Witzany G, editor. Biocommunications in Soil Microorganisms. Berlin Heidelberg: Spriger-Verlag; 2011. p. 339-367
-
9- Leadbetter JR, Greenberg EP. Metabolism of acylhomoserine lactone quorum-sensing signals by Variovorax paradoxus J Bacteriol 2000; 182: 6921-6926.
-
10- Schell M. Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network. Annu Rev Phytopathol 2000; 38:263-292.
-
11- Uroz S, Chhabra SR, Camara M, Williams P, Oger P, Dessaux Y. N-acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology 2005; 151: 3313-3322.
-
12- Boyer M, Wisniewski-Dye F. Cell-cell signalling in bacteria: not simply a matter of quorum. FEMS Microbiol Ecol 2009; 70:1-19.
-
13- Von Bodman SB, Bauer WD, Coplin DL. Quorum sensing in plant-pathogenic bacteria. Annu Rev Phytopathol. 2003; 41: 455-482.
-
14- Holden MT, Ram Chhabra S, De Nys R, Stead P, Bainton NJ, Hill PJ, Manefield M, Kumar N, Labatte M, England D, Rice S, Givskov M, Salmond GP, Stewart GS, Bycroft BW, Kjelleberg S, Williams P. Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram-negative bacteria. Mol Microbiol 1999; 33: 1254-1266.
-
15- Dubern JF, Diggle SP. Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species. Mol Biosyst 2008; 4: 882-888.
-
16- Schaefer AL, Greenberg EP, Oliver CM, Oda Y, Huang JJ, Bittan-Banin G, Peres CM, Schmidt S, Juhaszova K, Sufrin JR, Harwood CS. A new class of homoserine lactone quorum-sensing signals. Nature 2008; 454: 595-599.
-
17- Bassler BL, Wright M, Silverman MR. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol 1994; 13: 273-286
-
18- Yamada Y, Nihira T. Microbial hormones and microbial chemical ecology. In: Barton D, Nakanishi K, editors. Comprehensive natural products chemistry volume 8. Oxford, UK: Elsevier; 1998. p. 377-413.
-
19- Dunny GM, Winans SC. Bacterial life: neither lonely nor boring. In: Dunny GM, Winans SC, editors. Cell-Cell signaling in bacteria. Washington DC: ASM Press; 1999. p. 1-5
-
20- Loh J, Carlson RW, York WS, Stacey G. Bradyoxetin, a unique chemical signal involved in symbiotic gene regulation. P Natl Acad Sci USA 2002; 99: 14446-14451.
-
21- Chun W, Cui J, Poplawsky A. Purification, characterization and biological role of a pheromone produced by Xanthomonas campestris pv. campestris. Physiol Mol Plant P 1997; 51: 1-14.
-
22- Flavier AB, Clough SJ, Schell MA, Denny TP. Identification of 3-hidroxypalmitic acid methyl ester as a novel autoregulator controlling virulence in Ralstonia solanacearum Mol Microbiol 1997; 26: 251-259.
-
23- Wang LH, He YW, Gao YF, Wu JE, Dong YH, He C, Wang SX, Weng LX, Xu JL, Tay L, Fang RX, Zhang LH. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol 2004; 51: 903-912.
-
24- Oh KB, Miyazawa H, Naito T, Matsuoka H. Purification and characterization of an autoregulatory substance capable of regulating the morphological transition in Candida albicans P Natl Acad Sci USA 2001; 98: 4664-4668.
-
25- Williams P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 2007; 153: 3923-3938.
-
26- Kai K, Ohnishi H, Shimatani M, Ishikawa S, Mori Y, Kiba A, Ohnishi K, Tabuchi M, Hikichi Y. Methyl 3-hydroxymyristate, a diffusible signal mediating phc quorum sensing in Ralstonia solanacearum Chembiochem 2015; 16: 2309-2318.
-
27- Byers JT, Lucas C, Salmond GP, Welch M. Nonenzymatic turnover of an Erwinia carotovora quorum-sensing signaling molecule. J Bacteriol 2002; 184: 1163-1171.
-
28- Denny TP. Plant Pathogenic Ralstonia Species. In: Gnanamanickam SS, editor. Plant-associated bacteria. The Netherlands: Springer; 2006. p. 573-644.
-
29- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev 2005; 21: 319-346.
-
30- Dourado MN, Bogas AC, Pomini AM, Andreote FD, Quecine MC, Marsaioli AJ, Araújo WL. Methylobacterium-plant interaction genes regulated by plant exudate and quorum sensing molecules. Braz J Microbiol 2013; 44: 1331-1339.
-
31- Taga ME, Bassler BL. Chemical communication among bacteria. P Natl Acad Sci USA 2003; 100: 14549-14554.
-
32- Gantner S, Schmid M, Durr C, Schuhegger R, Steidle A, Hutzler P, Langebartels C, Eberl L, Hartmann A, Dazzo FB. In situ quantitation of the spatial scale of calling distances and population density-independent N-acylhomoserine lactone-mediated communication by rhizobacteria colonized on plant roots. FEMS Microbiol Ecol 2006; 56: 188-194.
-
33- Hong KW, Koh CL, Sam CK, Yin WF, Chan KG. Quorum quenching revisited-from signal decays to signalling confusion. Sensors 2012; 12: 4661-4696.
-
34- Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv 2013; 31: 224-245.
-
35- Dong YH, Xu JL, Li XZ & Zhang LH. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum sensing signal and attenuates the virulence of Erwinia carotovora P Natl Acad Sci USA. 2000; 97: 3526-3531.
-
36- Zhang HB, Wang LH, Zhang LH. Genetic control of quorum-sensing signal turnover in agrobacterium tumefaciens P Natl Acad Sci USA 2002; 99: 4638-4643.
-
37- Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, Zhang LH. Quenching quorum sensing dependent bacterial infection by N-acyl homoserine lactonase. Nature 2001; 411: 813-817.
-
38- Lin YH, Xu JL, Hu J, Wang SL, Ong JR. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represent a novel and potent class of quorum quenching enzymes. Mol Microbiol 2003; 47:849-860.
-
39- Park SY, Lee SJ, Oh TK, Oh JW, Koo BT, Yum DY, Lee JK. AhID, an N acyl-homoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiology 2003; 149: 1541-1550.
-
40- Flagan S, Ching WK, Leadbetter JR. Arthrobacter strain VAI-A utilizes acyl-homoserine lactone inactivation products and stimulates quorum signal biodegradation by Variovorax paradoxus Appl Environ Microbiol 2003; 69: 909-916.
-
41- Carlier A, Uroz S, Smadja B, Fray R, Latour X, Dessaux Y, Faure D. The Ti plasmid of Agrobacterium tumefaciens harbors an attM-paralogous gene, aiiB, also encoding N-acyl homoserine lactonase activity. Appl Environ Microb 2003; 69: 4989-4993.
-
42- Zhang L, Murphy PJ, Kerr A, Tate ME. Agrobacterium conjugation and gene regulation by N-acyl-L-homoserine lactones. Nature 1993; 362: 446-448.
-
43- Dong YH, Zhang LH . Quorum sensing and quorum-quenching enzymes. J Microbiol 2005; 43: 101-109.
-
44- Khan SR, Farrand SK. The BlcC (AttM) lactonase of Agrobacterium tumefaciens does not quench the quorum-sensing system that regulates Ti plasmid conjugative transfer. J Bacteriol 2009; 191: 1320-1329.
-
45- Shinohara M, Nakajima N, Uehara Y. Purification and characterization of a novel esterase (β-hydroxypalmitate methyl ester hydrolase) and prevention of the expression of virulence by Ralstonia solanacearum J Appl Microbiol 2007; 103:152-162.
-
46- d'Angelo-Picard C, Faure D, Penot I, Dessaux Y. Diversity of N-acyl homoserine lactone-producing and degrading bacteria in soil and tobacco rhizosphere. Environ Microbiol. 2005; 7: 1796-1808.
-
47- Chun CK, Ozer EA, Welsh MJ, Zabner J, Greenberg EP. Inactivation of a Pseudomonas aeruginosa quorum-sensing signal by human airway epithelia. P Natl Acad Sci USA 2004; 101: 3587-3590.
-
48- Kaufmann GF, Sartorio R, Lee SH, Rogers CJ, Meijler MM, Moss JA, Clapham B, Brogam AP, Dickerson TB, Janda KD. Revisiting quorum sensing: discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. P Natl Acad Sci USA 2005; 102: 309-314.
-
49- Zhang LH. Quorum quenching and proactive host defense. Trends Plant Sci 2003; 8: 238-244.
-
50- Givskov M, DeNys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg PD, Kjelleberg S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J Bacteriol 1996; 178: 6618-6622.
-
51- Manefield M, de Nys R, Kumar N, Read R, Givskov M, Steinberg P, Kjelleberg S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 1999; 145: 283-291.
-
52- Teasdale ME, Liu J, Wallace J, Akhlaghi F, Rowley DC. Secondary metabolites produced by the marine bacterium Halobacillus salinus that inhibit quorum sensing-controlled phenotypes in gram-negative bacteria. Appl Environ Microbiol. 2009; 75: 567-572.
-
53- Teplitski M, Robinson JB, Bauer WD. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol Plant Microbe In 2000; 13: 637-648.
-
54- Chan KG, Wong CS, Yin WF, Sam CK, Koh CL. Rapid degradation of N-3-oxo-acylhomoserine lactones by a Bacillus cereus isolate from Malaysian rainforest soil. Anton Leeuw Int J G 2010; 98: 299-305.
-
55- Park SY, Kang HO, Jang HS, Lee JK, Koo BT, Yum DY. Identification of extracellular N-acylhomoserine lactone acylase from a Streptomyces sp and its application to quorum quenching. Appl Environ Microbiol 2005; 71: 2632-2641.
-
56- Romero M, Diggle SP, Heeb S, Camara M, Otero A. Quorum quenching activity in Anabaena sp. PCC 7120: identification of AiiC, a novel AHL-acylase. FEMS Microbiol Lett 2008; 280:73-80.
-
57- Romero M, Avendano-Herrara R, Magarinos B, Camara M, Otero A. Acylhomoserine lactone production and degradation by the fish pathogen Tenacibaculum maritimum, a member of the Cytophaga-Flavobacterium-Bacteroides (CFB) group. FEMS Microbiol Lett. 2010; 304:131-139.
-
58- Gonzalez JE, Keshavan ND. Messing with bacterial quorum sensing. Microbiol Mol Biol R. 2006; 70: 859-875.
-
59- Scutera S, Zucca M, Savoia D. Novel approaches for the design and discovery of quorum-sensing inhibitors. Expert Opin Drug Discov 2014; 9: 353-66.
Publication Dates
-
Publication in this collection
2017
History
-
Received
03 Feb 2016 -
Accepted
14 July 2016