Abstract
The aim of present study is to characterize the resistance and virulence profile of enterococci isolated from aquaculture excavated ponds and masonry tanks (6 samples) in southern Brazil. Samples were cultured in selective medium, 10 colonies were randomly selected from each sample, which were identified by MALDI-TOF and tested against 13 antimicrobials. The presence of resistance (tetL, tetM, tetS, ermB and msrC) and virulence (ace, esp, agg, cylA and gelE) genes were determined by PCR. A total of 79 enterococci were identified, and Entecococcus faecalis (44.3%) and E. casseliflavus (36.7%) were the most prevalent species isolated. Sixty-five strains (82.3%) were resistant to at least one of the antimicrobials tested, whereas 27 (34.2%) strains were multiresistant. The overall percentages of antimicrobial resistant isolates were: 58.2% to rifampicin, 40.5% to fluoroquinolones, 36.7% to erythromycin and 30.4% to tetracycline. The tetL and tetM genes were found in 57.7% of the tetracycline-resistant strains; and msrC in 31.01% of erythromycin-resistant strains. The most frequently detected virulence factors were ace and gelE genes. Although limited to a single farm, these data suggest that aquaculture may be a reservoir of resistant and virulent enterococci. This study is the first step towards enhancing our understandingof distribution, resistance and virulence profile in enterococci isolated from fish farming environments in the south Brazil.
Keywords:
Enterococcus sp.; continental aquaculture; microbial ecology; antimicrobial resistance; virulence genes
Resumo
O objetivo do estudo apresentado é caracterizar o perfil de resistência e virulência de enterococos isolados de viveiros escavados e tanques de alvenaria (6 amostras) de uma pisicultura no Sul do Brasil. As amostras foram cultivadas em meio seletivo, 10 colônias foram selecionadas aleatoriamente de cada amostra, que foram identificadas por MALDI-TOF e testadas contra 13 antimicrobianos. A presença de genes de resistência (tetL, tetM, tetS, ermB e msrC) e virulência (ace, esp, agg, cylA e gelE) foi determinada por PCR. Foram identificados 79 enterococos, sendo Entecococcus faecalis (44,3%) e E. casseliflavus (36,7%) as espécies mais frequentes isoladas. Sessenta e cinco cepas (82,3%) eram resistentes a pelo menos um dos antimicrobianos testados, enquanto 27 (34,2%) eram multirresistentes. As porcentagens gerais de isolados resistentes a antimicrobianos foram: 58,2% para rifampicina, 40,5% para fluoroquinolonas, 36,7% para eritromicina e 30,4% para tetraciclina. Os genes tetL e tetM foram encontrados em 57,7% das cepas resistentes à tetraciclina; e msrC em 31,01% das cepas resistentes à eritromicina. Os fatores de virulência mais comumente detectados foram ace e gelE. Embora limitados a uma única fazenda, esses dados indicam que a aquicultura pode ser uma fonte de enterococos resistentes e virulentos. Este estudo é o primeiro passo para melhorar nosso entendimento da distribuição, resistência e perfil de virulência em enterococos isolados de ambientes de piscicultura no sul do Brasil.
Palavras-chave:
Enterococcus sp.; aquicultura continental; ecologia microbiana; resistência antimicrobiana; genes de virulência
1. Introduction
Aquaculture is the fastest growing sector of world food-production, with a growth rate of over 8.0%. In 2017, world fish production reached 170.9 million tons, roughly 46.8% of all this production derives from aquaculture (FAO, 2018FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS – FAO, 2018. The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals. Rome: FAO, 227 p.). According to PeixeBR data, in 2018 Brazilian fish farm production equaled 722,560 tons of fish, and become the world's fourth biggest producer of Nile tilapia (Oreochromus niloticus). A significant portion of the fish production in Brazil is located in the Rio Grande do Sul, with a total production of 23,000 tons/year, with a high proportion of exotic fishes (74.8%), such as carp (Cyprinus carpio), trout (Oncorhynchus sp.) and panga (Pterogymnus laniarius), followed by Nile tilapia (O. niloticus) (17.8%) and native species (7.4%) (PeixeBR, 2019ASSOCIAÇÃO BRASILEIRA DA PISCICULTURA – PEIXEBR. 2019. Anuário PeixeBR da Piscicultura 2019. São Paulo: PeixeBR, 148 p.).
Fish farming is one of the most valuable solutions to the overexploitation of natural resource (FAO, 2018FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS – FAO, 2018. The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals. Rome: FAO, 227 p.). However, due to the expansion of fish production worldwide, fish waste can impact the local environment by polluting the water and soil, considered one of the major worries of this practice. Therefore, the developments of efficient water reuse systems that reduce the environmental impacts and enable sustainable growth are considered necessary for this activity (Rocha et al., 2016ROCHA, R.S., SOUSA, O.V. and VIEIRA, R.H.S.F., 2016. Multidrug-resistant Vibrio associated with an estuary affected by shrimp farming in Northeastern Brazil. Marine Pollution Bulletin, vol. 105, no. 1, pp. 337-340. http://dx.doi.org/10.1016/j.marpolbul.2016.02.001. PMid:26876560.
http://dx.doi.org/10.1016/j.marpolbul.20...
).
Aquaculture requires large quantities of antimicrobial agents, which are administered as growth promoters (Novais et al., 2018NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
). Effluents from aquaculture contaminated with antibiotic residues and other chemical agents allow selection for antibiotic-resistant organisms (Rocha et al., 2016ROCHA, R.S., SOUSA, O.V. and VIEIRA, R.H.S.F., 2016. Multidrug-resistant Vibrio associated with an estuary affected by shrimp farming in Northeastern Brazil. Marine Pollution Bulletin, vol. 105, no. 1, pp. 337-340. http://dx.doi.org/10.1016/j.marpolbul.2016.02.001. PMid:26876560.
http://dx.doi.org/10.1016/j.marpolbul.20...
). These effluents, when discharged into the water bodies, without a proper water treatment system, favor the process of natural selection of resistant bacteria, serving as a vehicle for transmission of antimicrobial-resistant bacteria in the environment (Di Cesare et al., 2013DI CESARE, A., LUNA, G.M., VIGNAROLI, C., PASQUAROLI, S., TOTA, S., PARONCINI, P. and BIAVASCO, F., 2013. Aquaculture can promote the presence and spread of antibiotic-resistant enterococci in marine sediments. PLoS One, vol. 8, no. 4, pp. 1-12. http://dx.doi.org/10.1371/journal.pone.0062838. PMid:23638152.
http://dx.doi.org/10.1371/journal.pone.0...
; Rocha et al., 2016ROCHA, R.S., SOUSA, O.V. and VIEIRA, R.H.S.F., 2016. Multidrug-resistant Vibrio associated with an estuary affected by shrimp farming in Northeastern Brazil. Marine Pollution Bulletin, vol. 105, no. 1, pp. 337-340. http://dx.doi.org/10.1016/j.marpolbul.2016.02.001. PMid:26876560.
http://dx.doi.org/10.1016/j.marpolbul.20...
). The presence of bacterial species carrying antimicrobial resistance profile has already been detected in fish farming systems, as well as in water effluents (Novais et al., 2018NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
). Among microorganisms resistant isolated from different types of water, highlights the enterococci (Nachtigall et al., 2013NACHTIGALL, G., DE JESUS, A.G., ZVOBODA, D.A., SANTESTEVAN, N.A., MINOTTO, E., DE MOURA, T.M., D’AZEVEDO, J., FRAZZON, J., VAN DER SAND, S. and FRAZZON, A.P.G., 2013. Diversidade e perfil de susceptibilidade antimicrobiana de Enterococcus sp. isolados das águas do Arroio Dilúvio - Porto Alegre, RS, Brasil. Revista Brasileira de Biociências, vol. 11, no. 2, pp. 235-241.; Lebreton et al., 2014LEBRETON, F., WILLEMS, R.J.L. and GILMORE, M.S., 2014. Enterococcus diversity, origins in nature, and gut colonization. In: M.S. GILMORE, D.B. CLEWELL, Y. IKE and N. SHANKAR, eds. Enterococci: from commensals to leading causes of drug resistant infection. New York: Eye and Ear Infirmar, pp. 1-82.).
Enterococcus spp. are considered commensal microbiota of the oral cavity, genitourinary and gastrointestinal tract of humans and animals, and are widely distributed in the environment, occurring in plants, soil and water. Enterococcal species have great genomic plasticity, high versatility to occupy broad ecological roles, able to grow in temperatures ranging from 10 and 45 °C, with optimum growth at 35 °C and salt tolerance (6.5% NaCl) (Lebreton et al., 2014LEBRETON, F., WILLEMS, R.J.L. and GILMORE, M.S., 2014. Enterococcus diversity, origins in nature, and gut colonization. In: M.S. GILMORE, D.B. CLEWELL, Y. IKE and N. SHANKAR, eds. Enterococci: from commensals to leading causes of drug resistant infection. New York: Eye and Ear Infirmar, pp. 1-82.). Currently, the genus is composed of more than 50 species, with Enterococcus faecalis predominant in the gastrointestinal tract of humans and other mammals, followed by E. faecium, E. hirae, E. durans, E. casseliflavus, E. gallinarum and E. mundtii (Cassenego et al., 2011CASSENEGO, A.P.V., D’AZEVEDO, P.A., RIBEIRO, A.M.L., FRAZZON, J., VAN DER SAND, S.T. and FRAZZON, A.P.G., 2011. Species distribution and antimicrobial susceptibility of enterococci isolated from broilers infected experimentally with Eimeria spp and fed with diets containing different supplements. Brazilian Journal of Microbiology, vol. 42, no. 2, pp. 480-488. http://dx.doi.org/10.1590/S1517-83822011000200012. PMid:24031659.
http://dx.doi.org/10.1590/S1517-83822011...
; Novais et al. 2018NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
).
Enterococci are considered opportunistic pathogens, which represent the second most common cause of hospital-acquired infections, particularly affecting the urinary tract, wounds, and soft tissues. The emergence of antibiotic resistant enterococci is a major medical and public health concern. Resistant strains are not exclusively restricted to clinically relevant species, since resistant strains have been investigated and monitored in different environments (Frazzon et al., 2010FRAZZON, A.P.G., GAMA, B.A., HERMES, V., BIERHALS, C.G., PEREIRA, R.I., GUEDES, A.G., D’AZEVEDO, P.A. and FRAZZON, J., 2010. Prevalence of antimicrobial resistance and molecular characterization of tetracycline resistance mediated by tet(M) and tet(L) genes in Enterococcus spp. isolated from food in Southern Brazil. World Journal of Microbiology & Biotechnology, vol. 26, no. 2, pp. 365-370. http://dx.doi.org/10.1007/s11274-009-0160-x.
http://dx.doi.org/10.1007/s11274-009-016...
; Cassenego et al., 2011CASSENEGO, A.P.V., D’AZEVEDO, P.A., RIBEIRO, A.M.L., FRAZZON, J., VAN DER SAND, S.T. and FRAZZON, A.P.G., 2011. Species distribution and antimicrobial susceptibility of enterococci isolated from broilers infected experimentally with Eimeria spp and fed with diets containing different supplements. Brazilian Journal of Microbiology, vol. 42, no. 2, pp. 480-488. http://dx.doi.org/10.1590/S1517-83822011000200012. PMid:24031659.
http://dx.doi.org/10.1590/S1517-83822011...
; Grassotti et al., 2018GRASSOTTI, T.T., DE ANGELIS ZVOBODA, D., DA FONTOURA XAVIER COSTA, L., DE ARAÚJO, A.J.G., PEREIRA, R.I., SOARES, R.O., WAGNER, P.G.C., FRAZZON, J. and FRAZZON, A.P.G., 2018. Antimicrobial Resistance Profiles in Enterococcus spp. Isolates from fecal samples of wild and captive black Capuchin Monkeys (Sapajus nigritus) in South Brazil. Frontiers in Microbiology, vol. 9, pp. 1-10. http://dx.doi.org/10.3389/fmicb.2018.02366. PMid:30356681.
http://dx.doi.org/10.3389/fmicb.2018.023...
; Novais et al., 2018NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
; Huff et al., 2020HUFF, R., INHOQUE PEREIRA, R., PISSETTI, C., MELLENDER DE ARAÚJO, A., ALVES D’AZEVEDO, P., FRAZZON, J. and GUEDESFRAZZON, A.P., 2020. Antimicrobial resistance and genetic relationships of enterococci from siblings and non-siblings Heliconius erato phyllis caterpillars. PeerJ, vol. 8, pp. e8647. http://dx.doi.org/10.7717/peerj.8647. PMid:32149028.
http://dx.doi.org/10.7717/peerj.8647...
). The presence of resistant strains in all environmental, could provide important information of ecological disturbance
Resistance to different classes of antimicrobials is a hallmark of Enterococcus spp., they are intrinsically resistant to sulfonamides, ertapenem, perfloxacin, penicillin and ampicillin and, high-level resistance to most cephalosporins. Enterococcus species are also resistant to low levels of aminoglycosides, due to the decreased uptake of this antibiotic class. Furthermore, they can transfer and acquire antibiotic resistance genes (through the transfer of plasmids and transposons, chromosomal exchange, or mutation) (Lebreton et al., 2014LEBRETON, F., WILLEMS, R.J.L. and GILMORE, M.S., 2014. Enterococcus diversity, origins in nature, and gut colonization. In: M.S. GILMORE, D.B. CLEWELL, Y. IKE and N. SHANKAR, eds. Enterococci: from commensals to leading causes of drug resistant infection. New York: Eye and Ear Infirmar, pp. 1-82.). Indeed, this ability to transfer/acquire resistance and/or virulence genes, offer a selective advantage to Enterococcus spp. survival and dispersal in the environment (Savaşan et al., 2016SAVAŞAN, S., KIRKAN, S., ERBAŞ, G., PARIN, U. and ÇIFTCI, A., 2016. The determination of virulence factors among fish originated enterococci. Etlik Veteriner Mikrobiyoloji Dergisi, vol. 27, no. 2, pp. 98-103. http://dx.doi.org/10.35864/evmd.514501.
http://dx.doi.org/10.35864/evmd.514501...
).
Due to their presence in human and animal faeces, the ability to adapt to different environmental conditions (freshwater and marine environments) and the correlation with human health, allows Enterococcus sp. to be used as sentinels for water quality changes (Di Cesare et al., 2013DI CESARE, A., LUNA, G.M., VIGNAROLI, C., PASQUAROLI, S., TOTA, S., PARONCINI, P. and BIAVASCO, F., 2013. Aquaculture can promote the presence and spread of antibiotic-resistant enterococci in marine sediments. PLoS One, vol. 8, no. 4, pp. 1-12. http://dx.doi.org/10.1371/journal.pone.0062838. PMid:23638152.
http://dx.doi.org/10.1371/journal.pone.0...
, Lebreton et al., 2014LEBRETON, F., WILLEMS, R.J.L. and GILMORE, M.S., 2014. Enterococcus diversity, origins in nature, and gut colonization. In: M.S. GILMORE, D.B. CLEWELL, Y. IKE and N. SHANKAR, eds. Enterococci: from commensals to leading causes of drug resistant infection. New York: Eye and Ear Infirmar, pp. 1-82.). However, specific aquaculture legislation determines standard wastewater-treatment procedure, such as CONAMA Resolutions No. 357/2005 and No. 430/2011 (Brasil, 2005BRASIL, 2005. Resolução CONAMA n° 357. Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e dá outras providências. Diário Oficial da República Federativa do Brasil, Brasília, 17 maio.; Brasil, 2011BRASIL, 2011. Resolução CONAMA n° 430. Dispõe sobre as condições e padrões de lançamento de efluentes, complementa e altera a Resolução no 357, de 17 de março de 2005, do Conselho Nacional do Meio Ambiente-CONAMA. Diário Oficial da República Federativa do Brasil, Brasília, 13 maio.), this requirement does not include enterococci. Based on this, the present study aims to determine the occurrence, the antimicrobial susceptibility profile and the presence of virulence genes in enterococci isolated from a fish farming environment in southern Brazil.
2. Material and Methods
2.1. Sample collection
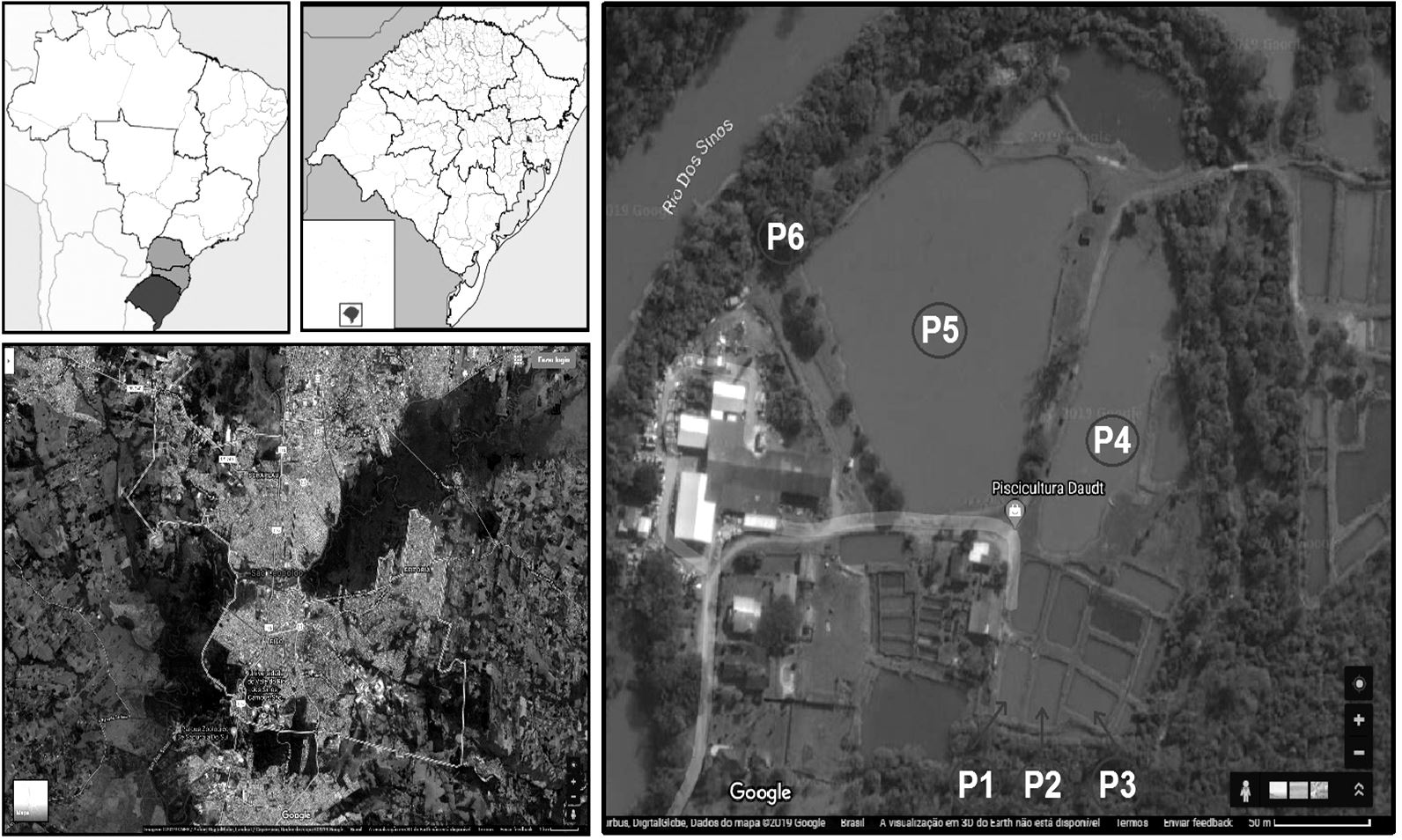
Sediment samples were collected from 6 sites in a fish farm located in the city of São Leopoldo - Rio Grande do Sul, Brazil (-29.736318, - 51.086023). Sediments were collected in 2018, in six different points named: P1, P2, P3 (excavated nurseries covered with masonry and plastic canvas), P4, P5 (excavated uncovered ponds, where water remains constantly contact with the bottom sediment) and P6 (a runoff point of the effluent produced by the farm) (Figure 1). Four replicate sediment samples were taken from each of point in different periods of the year (summer, fall, winter, and spring), totalizing 24 samples. The samples were placed in sterile containers and refrigerated to be transported to the laboratory, where the microbiology analyzes was performed.
Sediment sampling sites along the fish farm, São Leopoldo, Rio Grande do Sul, Brazil. On the left Brazil and Rio Rio Grande do Sul (top) and Fish farm (bottom) maps. On the right-detailed map of Fish farm showing locations of collected samples site. P1, P2 and P3 (excavated nurseries covered with masonry and plastic canvas, average static volume capacity per pond is 80, 90 and 80 m3, respectively); P4, and P5 (excavated uncovered ponds, where water remains constantly contact, average static volume capacity per pond 981 e 1500 m3) and P6 (a runoff point of the effluent produced by the farm, average static volume capacity per pond 256 m3).
Fish farm was located on the bank of the River dos Sinos, considered one of more polluted river in Brazil. The farm is involved in fingerling production and culture of exotic fishes species as carp (C. carpio) and tilapia (O. niloticus), besides native and ornamental fishes. The fishes are fed with the feed. The fish ponds are lined with concrete, earth or plastic, with not interconnection between them. The pond systems are designed to retain or confine water and/or waste within a pond. The fish farm has a water reuse system, where the wastewater is reused to another pond and water purification by water hyacinth (Pontederia crassipes). In the farm area, there is residential property, rearing of livestock (chickens, cattle and horses), and logging.
2.2. Isolation of enterococci
Isolation of enterococci was performed as previously described by Cassenego et al. (2011)CASSENEGO, A.P.V., D’AZEVEDO, P.A., RIBEIRO, A.M.L., FRAZZON, J., VAN DER SAND, S.T. and FRAZZON, A.P.G., 2011. Species distribution and antimicrobial susceptibility of enterococci isolated from broilers infected experimentally with Eimeria spp and fed with diets containing different supplements. Brazilian Journal of Microbiology, vol. 42, no. 2, pp. 480-488. http://dx.doi.org/10.1590/S1517-83822011000200012. PMid:24031659.
http://dx.doi.org/10.1590/S1517-83822011...
, with adaptations. One milliliter of each sample was added in 9 mL Buffered Peptone Water (Himedia, Mumbai, India) and incubated at 35 ± 1 °C for 24 h. After incubation, 1.0 mL was inoculated in 9.0 mL of Azida Dextrose Broth (Himedia, Mumbai, India) and incubated again at 35 ± 1 °C for 24 h. From that, 0.1 mL were inoculated in triplicate in Brain Heart Infusion Agar (Acumedia, Lansing, Michigan) supplemented with 6.5% NaCl and incubated at 35 ± 1 °C for 48 h. Five to ten colony-forming units were randomly selected from each sample. Phenotypic criteria, such as size/volume, shape, color, Gram staining, catalase production, capacity to growth at 45 °C and bile aesculine reaction, were used to separate the enterococci group and the non-enterococcal strains (Teixeira et al., 2011TEIXEIRA, L.M., CARVALHO, M.G.S., SHEWMAKER, P.L. and FACKLAM, R.R., 2011. Manual of clinical microbiology. 10th ed. Washington, DC: American Society for Microbiology, 2630 p.). Selected pure colonies were stored at -20 °C in a 10% (w/v) solution of skim milk (Difco, Sparks, MD, USA) and 10% (v/v) glycerol (Neon Comercial Ltda).
2.3. Species identification using the matrix assisted laser ionization and desorption technique (MALDI-TOF)
Collected bacteria were identified by MALDI-TOF technique applied to Enterococcus, according to Sauget et al. (2017)SAUGET, M., VALOT, B., BERTRAND, X. and HOCQUET, D., 2017. Can Maldi-Tof Mass spectrometry reasonably type bacteria? Trends in Microbiology, vol. 25, no. 6, pp. 447-455. http://dx.doi.org/10.1016/j.tim.2016.12.006. PMid:28094091.
http://dx.doi.org/10.1016/j.tim.2016.12....
. MALDI-TOF analysis was performed using a LT Bruker microflex mass spectrometer (Bruker Daltonik GmbH) and spectra were automatically identified using BrukerBioTyper ™ 1.1 software.
2.4. Antimicrobial susceptibility testing
Antimicrobial susceptibility of all strains was determined by Kirby-Bauer disk diffusion method, recommended by the Clinical and Laboratory Standards Institute (CLSI, 2018CLINICAL AND LABORATORY STANDARDS INSTITUTE – CLSI, 2018. Performance standards for antimicrobial susceptibility testing. 28th ed. Wayne, PA: CLSI, 296 p.). Thirteen antimicrobial agents were tested: Ampicillin 10 μg (AMP), Ciprofloxacin 5 μg (CIP), Chloramphenicol 30 μg (CHL), Erythromycin 15 μg (ERY), Streptomycin 300 μg (STR), Gentamicin 120 μg (GEN), Linezolid 30 μg (LNZ), Nitrofurantoin 300 μg (NIT), Norfloxacin 10 μg (NOR), Oxitetracycline 30 μg (OTC), Rifampicin 5 μg (RIF), Tetracycline 30 μg (TET) and Vancomycin 30 μg (VAN). Reference strains E. faecalis ATCC 29212 was used as control.
Intermediate and resistant-strains were included in a single category as resistant-strains. Strains were classified has single (SR), double (DR) or multidrug-resistant (MDR) when showned resistance for one, two, and three or more antibiotics, respectively (EFSA and ECDC, 2013EUROPEAN FOOD SAFETY AUTHORITY – EFSA and EUROPEAN CENTRE FOR DISEASE PREVENTION AND CONTROL – ECDC, 2013. The European Union Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2011. EFSA Journal, vol. 11, no. 5, pp. 359.).
2.5. Detection of resistance and virulence related genes.
Genomic DNA was extracted by physicochemical method as previously described by Depardieu et al., (2004)DEPARDIEU, F., PERICHON, B. and COURVALIN, P., 2004. Detection of the van Alphabet and Identification of Enterococci and Staphylococci at the Species Level by Multiplex PCR. Journal of Clinical Microbiology, vol. 42, no. 12, pp. 5857-5860. http://dx.doi.org/10.1128/JCM.42.12.5857-5860.2004. PMid:15583325.
http://dx.doi.org/10.1128/JCM.42.12.5857...
. The detection of resistance-related genes commonly observed in clinical and environmental enterococci: ermB, which encodes a ribosomal methylase that mediates macrolides, lincosamides and type B streptogramins resistance; msrC, which encodes for a macrolide and streptogramin B efflux pump; tetM and tetS, which encodes for tetracycline resistance via a ribosomal protection protein mechanism, and tetL which encodes for tetracycline resistance via efflux pumps proteins was performed using the Polymerase Chain Reaction (PCR) (Sutcliffe et al., 1996SUTCLIFFE, J., GREBE, T., TAIT-KAMRADT, A. and WONDRACK, L., 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrobial Agents and Chemotherapy, vol. 40, no. 11, pp. 2562-2566. http://dx.doi.org/10.1128/AAC.40.11.2562. PMid:8913465.
http://dx.doi.org/10.1128/AAC.40.11.2562...
; Aarestrup et al., 2000AARESTRUP, F.M., AGERSO, Y., GERNER-SMIDT, P., MADSEN, M. and JENSEN, L.B., 2000. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagnostic Microbiology and Infectious Disease, vol. 37, no. 2, pp. 127-137. http://dx.doi.org/10.1016/S0732-8893(00)00130-9. PMid:10863107.
http://dx.doi.org/10.1016/S0732-8893(00)...
; Werner et al., 2001WERNER, G., HILDEBRANDT, B., WITTE, W., MURRAY, B.E. and SINGH, K.V., 2001. The newly described msrC gene is not equally distributed among all isolates of Enterococcus faecium. Antimicrobial Agents and Chemotherapy, vol. 45, no. 12, pp. 3672-3673. http://dx.doi.org/10.1128/AAC.45.12.3672-3673.2001. PMid:11724034.
http://dx.doi.org/10.1128/AAC.45.12.3672...
; Frazzon et al., 2010FRAZZON, A.P.G., GAMA, B.A., HERMES, V., BIERHALS, C.G., PEREIRA, R.I., GUEDES, A.G., D’AZEVEDO, P.A. and FRAZZON, J., 2010. Prevalence of antimicrobial resistance and molecular characterization of tetracycline resistance mediated by tet(M) and tet(L) genes in Enterococcus spp. isolated from food in Southern Brazil. World Journal of Microbiology & Biotechnology, vol. 26, no. 2, pp. 365-370. http://dx.doi.org/10.1007/s11274-009-0160-x.
http://dx.doi.org/10.1007/s11274-009-016...
, Rathnayake et al., 2011RATHNAYAKE, I., HARGREAVES, M. and HUYGENS, F., 2011. SNP diversity of Enterococcus faecalis and Enterococcus faecium in a South East Queensland waterway, Australia, and associated antibiotic resistance gene profiles. BMC Microbiology, vol. 11, no. 1, pp. 1-13. http://dx.doi.org/10.1186/1471-2180-11-201. PMid:21910889.
http://dx.doi.org/10.1186/1471-2180-11-2...
). In addition, PCR reactions were performed to detected virulence encoding genes: ace (collagen adhesin), agg (aggregating substance), cylA (cytolysin), esp (enterococcal surface protein) and gelE (gelatinase) (Mannu et al., 2003MANNU, L., PABA, A., DAGA, E., COMUNIAN, R., ZANETTI, S., DUPRÉ, I. and SECHI, L.A., 2003. Comparison of the incidence of virulence determinants and antibiotic resistance between Enterococcus faecium strains of dairy, animal and clinical origin. International Journal of Food Microbiology, vol. 88, no. 2-3, pp. 291-304. http://dx.doi.org/10.1016/S0168-1605(03)00191-0. PMid:14597001.
http://dx.doi.org/10.1016/S0168-1605(03)...
; Eaton and Gasson 2001EATON, T.J. and GASSON, M.J., 2001. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Applied and Environmental Microbiology, vol. 67, no. 4, pp. 1628-1635. http://dx.doi.org/10.1128/AEM.67.4.1628-1635.2001. PMid:11282615.
http://dx.doi.org/10.1128/AEM.67.4.1628-...
; Shankar et al., 1999SHANKAR, V., BAGHDAYAN, A.S., HUYCKE, M.M., LINDAHL, G. and GILMORE, M.S., 1999. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infection and Immunity, vol. 67, no. 1, pp. 193-200. http://dx.doi.org/10.1128/IAI.67.1.193-200.1999. PMid:9864215.
http://dx.doi.org/10.1128/IAI.67.1.193-2...
).
3. Results and Discussion
3.1. Enterococcus spp. distribution in sediments of fish farm
The number of colony-forming units of Enterococcus spp. in sediment samples ranged from 1.9 x 103 to 2.0 x 107 CFU.g-1. The current agriculture, livestock and food supply legislation in Brazil does not determine the enterococci limits in fish or aquaculture environments, but ensures values of this bacterial in recreational water use for primary contact activities (Brasil, 2005BRASIL, 2005. Resolução CONAMA n° 357. Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e dá outras providências. Diário Oficial da República Federativa do Brasil, Brasília, 17 maio.). Nevertheless, fish farming is not part of the primary contact activity; however, practices developed in fish farming can expose workers to the risk of direct contact with water for a considerable period.
The distribution of Enterococcus species recovered from sediment samples is shown in Table 1. The most common species found in our samples were E. faecalis (n = 35; 44.3%) and E. casseliflavus (n = 29; 36.7%), followed by E. faecium (n = 11; 13.9%), E. gallinarum (n = 2; 2.5%) and E. hirae (n = 2; 2.5%). These results corroborate with Di Cesare et al. (2013), DI CESARE, A., LUNA, G.M., VIGNAROLI, C., PASQUAROLI, S., TOTA, S., PARONCINI, P. and BIAVASCO, F., 2013. Aquaculture can promote the presence and spread of antibiotic-resistant enterococci in marine sediments. PLoS One, vol. 8, no. 4, pp. 1-12. http://dx.doi.org/10.1371/journal.pone.0062838. PMid:23638152.
http://dx.doi.org/10.1371/journal.pone.0...
Pereira et al. (2017)PEREIRA, G.V., CUNHA, D.G., MOURINO, J.L.P., RODILES, A., JARAMILLO-TORRES, A. and MERRIFIELD, D.L., 2017. Characterization of microbiota in Arapaima gigas intestine and isolation of potential probiotic bacteria. Journal of Applied Microbiology, vol. 123, no. 5, pp. 1298-1311. http://dx.doi.org/10.1111/jam.13572. PMid:28833934.
http://dx.doi.org/10.1111/jam.13572...
and Novais et al. (2018)NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
, which found these species in water and sediment samples from freshwater and marine aquatic environments. Besides, these studies reported E. faecalis as the abundance specie in these environments (Di Cesare et al., 2013DI CESARE, A., LUNA, G.M., VIGNAROLI, C., PASQUAROLI, S., TOTA, S., PARONCINI, P. and BIAVASCO, F., 2013. Aquaculture can promote the presence and spread of antibiotic-resistant enterococci in marine sediments. PLoS One, vol. 8, no. 4, pp. 1-12. http://dx.doi.org/10.1371/journal.pone.0062838. PMid:23638152.
http://dx.doi.org/10.1371/journal.pone.0...
; Pereira et al., 2017PEREIRA, G.V., CUNHA, D.G., MOURINO, J.L.P., RODILES, A., JARAMILLO-TORRES, A. and MERRIFIELD, D.L., 2017. Characterization of microbiota in Arapaima gigas intestine and isolation of potential probiotic bacteria. Journal of Applied Microbiology, vol. 123, no. 5, pp. 1298-1311. http://dx.doi.org/10.1111/jam.13572. PMid:28833934.
http://dx.doi.org/10.1111/jam.13572...
; Novais et al., 2018NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
).
Analyzing the distribution of species by collection point, it was observed that in P4 site there was a predominance of E. faecium (13/35; 37.1%) and E. faecalis (7/11; 63.3%) (Table 1). The P4 site is one of the largest nursery ponds for farming of carp (C. carpio) and tilapia (O. niloticus), with a high density of animals. E. faecalis and E. faecium are commonly described in human fecal samples and considered as fecal contaminants in water samples. Similar results were reported for rainbow trout (Oncorhyncus mykiss) in Portugal, pirarucu (Arapaima gigas) in Brazil and sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) in Italy, where these two species also prevailed over the isolates, further revalidating their occurrences in these environments, regardless of the fish species cultivated (Di Cesare et al., 2013DI CESARE, A., LUNA, G.M., VIGNAROLI, C., PASQUAROLI, S., TOTA, S., PARONCINI, P. and BIAVASCO, F., 2013. Aquaculture can promote the presence and spread of antibiotic-resistant enterococci in marine sediments. PLoS One, vol. 8, no. 4, pp. 1-12. http://dx.doi.org/10.1371/journal.pone.0062838. PMid:23638152.
http://dx.doi.org/10.1371/journal.pone.0...
; Pereira et al., 2017PEREIRA, G.V., CUNHA, D.G., MOURINO, J.L.P., RODILES, A., JARAMILLO-TORRES, A. and MERRIFIELD, D.L., 2017. Characterization of microbiota in Arapaima gigas intestine and isolation of potential probiotic bacteria. Journal of Applied Microbiology, vol. 123, no. 5, pp. 1298-1311. http://dx.doi.org/10.1111/jam.13572. PMid:28833934.
http://dx.doi.org/10.1111/jam.13572...
; Novais et al., 2018NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
). It is important to highlights that these species are the main agents of hospital-acquired infections (Lebreton et al., 2014LEBRETON, F., WILLEMS, R.J.L. and GILMORE, M.S., 2014. Enterococcus diversity, origins in nature, and gut colonization. In: M.S. GILMORE, D.B. CLEWELL, Y. IKE and N. SHANKAR, eds. Enterococci: from commensals to leading causes of drug resistant infection. New York: Eye and Ear Infirmar, pp. 1-82.).
The presence of E. casseliflavus, E. gallinarum and E. hirae in our samples might be justified by the occurrence of the vegetation, birds and bats near to the ponds (Lebreton et al., 2014LEBRETON, F., WILLEMS, R.J.L. and GILMORE, M.S., 2014. Enterococcus diversity, origins in nature, and gut colonization. In: M.S. GILMORE, D.B. CLEWELL, Y. IKE and N. SHANKAR, eds. Enterococci: from commensals to leading causes of drug resistant infection. New York: Eye and Ear Infirmar, pp. 1-82.). However, Novais et al. (2018)NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
, showed results contrary to that for E. casseliflavus in water samples collected from trout sample that reported the lower presence of this species. The high frequency of this species in the study may be associated by the fact E. casseliflavus is usually isolated from larval feces of insect species. In fish farming environments there are dragonflies eggs, these animals depend on water in the larval and adult phase, also serving as food for fishes.
Our results show a low incidence of E. gallinarum and E. hirae in sediment samples from the fish farm. These results corroborate those observed by Novais et al. (2018)NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
in sediment samples of cultivation of trout (O. mykiss) in southern Europe. Although not common, both species have already been reported to be responsible for human infections (Lebreton et al., 2014LEBRETON, F., WILLEMS, R.J.L. and GILMORE, M.S., 2014. Enterococcus diversity, origins in nature, and gut colonization. In: M.S. GILMORE, D.B. CLEWELL, Y. IKE and N. SHANKAR, eds. Enterococci: from commensals to leading causes of drug resistant infection. New York: Eye and Ear Infirmar, pp. 1-82.).
3.2. Antimicrobial susceptibility profile and resistance gene frequencies
Of the 79 strains of enterococci tested, 65 (82.3%) were resistant to at least one antimicrobial agent tested (Table 2). Isolates were susceptible to STR, GEN, LNZ and VAN. In the samples collected from fish ponds, it was possible to recover Enterococcus spp. with resistance profile of different antimicrobial agents, such as beta-lactams, phenolics, macrolides, fluoroquinolones, nitrofurans, rifampicin and tetracyclines, considered important by the World Health Organization. Previous studies on different fish farms systems have found antimicrobial resistance strains (Di Cesare et al., 2013DI CESARE, A., LUNA, G.M., VIGNAROLI, C., PASQUAROLI, S., TOTA, S., PARONCINI, P. and BIAVASCO, F., 2013. Aquaculture can promote the presence and spread of antibiotic-resistant enterococci in marine sediments. PLoS One, vol. 8, no. 4, pp. 1-12. http://dx.doi.org/10.1371/journal.pone.0062838. PMid:23638152.
http://dx.doi.org/10.1371/journal.pone.0...
; Novais et al., 2018NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
). These data can be considered worrying since these environmental bacterial populations may evolve throughout secondary genetic events to resistance levels with human clinical impact (Andersson and Hughes, 2014ANDERSSON, D.I. and HUGHES, D., 2014. Microbiological effects of sublethal levels of antibiotics. Nature Reviews. Microbiology, vol. 12, no. 7, pp. 465-478. http://dx.doi.org/10.1038/nrmicro3270. PMid:24861036.
http://dx.doi.org/10.1038/nrmicro3270...
).
Resistance to RIF was mainly found among E. casseliflavus (100%) and E. hirae (100%), while resistance to OTC or/and TET was observed in E. faecium (100%), E. hirae (100%), E. faecalis (44%) and E. casseliflavus (24.13%). More than forty percent of the E. casseliflavus and E. faecalis analyzed are resistant to ERY and CIP, respectively. Similar results have been reported by Novais et al. (2018)NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
who found high rates of resistance to CIP, ERY and TET in enterococci isolated from water and sediment samples from trout farming in Southern Europe, while Di Cesare et al. (2013)DI CESARE, A., LUNA, G.M., VIGNAROLI, C., PASQUAROLI, S., TOTA, S., PARONCINI, P. and BIAVASCO, F., 2013. Aquaculture can promote the presence and spread of antibiotic-resistant enterococci in marine sediments. PLoS One, vol. 8, no. 4, pp. 1-12. http://dx.doi.org/10.1371/journal.pone.0062838. PMid:23638152.
http://dx.doi.org/10.1371/journal.pone.0...
found TET resistant enterococci in sediments collected at a fish farm in Varano lagoon. Although the farm owner denied all antibiotic use in the pond, either for therapeutic or for growth promotion purposes, the use of OTC in previous years, could partially explain the tetracyclines resistance observed in our study. Tetracycline and erythromycin are prescribed in human and veterinary medicine, and are excreted as active metabolites and remain stable in the environment (Rahardjo et al., 2011RAHARDJO, A.K., SUSANTO, M.J., KURNIAWAN, A., INDRASWATI, N. and ISMADJI, S., 2011. Modified ponorogo bentonite for the removal of ampicillin from wastewater. Journal of Hazardous Materials, vol. 190, no. 1-3, pp. 1001-1008. http://dx.doi.org/10.1016/j.jhazmat.2011.04.052. PMid:21550716.
http://dx.doi.org/10.1016/j.jhazmat.2011...
; Schafhauser et al., 2018SCHAFHAUSER, B.H., KRISTOFCO, L.A., DE OLIVEIRA, C.M.R. and BROOKS, B.W., 2018. Global review and analysis of erythromycin in the environment: occurrence, bioaccumulation and antibiotic resistance hazards. Environmental Pollution, vol. 238, pp. 440-451. http://dx.doi.org/10.1016/j.envpol.2018.03.052. PMid:29587215.
http://dx.doi.org/10.1016/j.envpol.2018....
; Rudra et al., 2018RUDRA, P., HURST-HESS, K., LAPPIERRE, P. and GHOSH, P., 2018. High levels of intrinsic tetracycline resistance in Mycobacterium abscessus is conferred by a tetracycline - modifying monooxygenase. Antimicrobial Agents and Chemotherapy, vol. 62, no. 6, pp. e00119-e18. http://dx.doi.org/10.1128/AAC.00119-18. PMid:29632012.
http://dx.doi.org/10.1128/AAC.00119-18...
). Consequently, this class of antibiotic is considered modern pollutants in the soil and aquatic environment (Gothwal and Shashidhar, 2014GOTHWAL, R. and SHASHIDHAR, T., 2014. Antibiotic pollution in the environment: a review. Clean, vol. 43, no. 4, pp. 479-489. http://dx.doi.org/10.1002/clen.201300989.
http://dx.doi.org/10.1002/clen.201300989...
; Dizavandi et al., 2016DIZAVANDI, Z.R., ALIAKBAR, A. and SHEYKHAN, M., 2016. A novel Pb-poly aminophenol glassy carbon electrode for determination of tetracycline by adsorptive differential pulse cathodic stripping voltammetry. Electrochimica Acta, vol. 227, pp. 345-356. http://dx.doi.org/10.1016/j.electacta.2016.12.167.
http://dx.doi.org/10.1016/j.electacta.20...
).
In this study, 34.2% (n=27) of strains were multi-resistant (Table 3). The detection of multidrug-resistant enterococci isolated from the sediments of fish ponds might represent potential environmental contamination since this fish farm is near to River of Sinos, considered one of the more polluted rivers in Brazil. Human negligence with waste, sewage, wastewater, and also industrial waste and agricultural irrigation is the main environmental impacts within the River of Sinos (Figueiredo et al., 2010FIGUEIREDO, J.A.S., DRUMM, E., RODRIGUES, M.A.S. and SPILKI, F.R., 2010. The Rio dos Sinos watershed: an economic and social space and its interface with environmental status. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 70, no. 4, suppl., pp. 1131-1136. http://dx.doi.org/10.1590/S1519-69842010000600001. PMid:21225153.
http://dx.doi.org/10.1590/S1519-69842010...
). Also, Di Cesare et al. (2013)DI CESARE, A., LUNA, G.M., VIGNAROLI, C., PASQUAROLI, S., TOTA, S., PARONCINI, P. and BIAVASCO, F., 2013. Aquaculture can promote the presence and spread of antibiotic-resistant enterococci in marine sediments. PLoS One, vol. 8, no. 4, pp. 1-12. http://dx.doi.org/10.1371/journal.pone.0062838. PMid:23638152.
http://dx.doi.org/10.1371/journal.pone.0...
suggested that feed supplied to captive fish contributed to the presence of multidrug-resistant strains.
Antimicrobial resistance phenotypic profile of Enterococcus spp. isolated from sediments isolated in the fish farm.
Among the antimicrobial resistance genes found in this study, the tetL and tetM genes were observed in 57.7% of the tetracycline-resistant strains; and the msrC gene (encoding) was positive in 31.01% of the erythromycin-resistant strains. None of the strains tested were positive to tetS and ermB genes. Similar results were found by Di Cesare et al. (2013)DI CESARE, A., LUNA, G.M., VIGNAROLI, C., PASQUAROLI, S., TOTA, S., PARONCINI, P. and BIAVASCO, F., 2013. Aquaculture can promote the presence and spread of antibiotic-resistant enterococci in marine sediments. PLoS One, vol. 8, no. 4, pp. 1-12. http://dx.doi.org/10.1371/journal.pone.0062838. PMid:23638152.
http://dx.doi.org/10.1371/journal.pone.0...
and Novais et al. (2018)NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
in studies conducted in southern European. Authors pointed out that the tetM gene is of great recurrence in enterococci isolated from environmental samples, including marine aquaculture, coinciding with those found in the present study. In relation to species, the msrC gene was carried by all erythromycin-resistant E. faecium and two E. casseliflavus; and the tetL and tetM genes in six tetracycline-resistant E. faecium, five E. faecalis, two E. casseliflavus and two E. hirae. In spite of the fact that msrC gene is considered intrinsic to E. faecium,Werner et al. (2001)WERNER, G., HILDEBRANDT, B., WITTE, W., MURRAY, B.E. and SINGH, K.V., 2001. The newly described msrC gene is not equally distributed among all isolates of Enterococcus faecium. Antimicrobial Agents and Chemotherapy, vol. 45, no. 12, pp. 3672-3673. http://dx.doi.org/10.1128/AAC.45.12.3672-3673.2001. PMid:11724034.
http://dx.doi.org/10.1128/AAC.45.12.3672...
, demonstrated that this gene is not an intrinsic property of all E. faecium isolates, and other studies have been showed the presence of this gene in other enterococci species, such as E. hirae, and E. faecalis (Grassotti et al., 2018GRASSOTTI, T.T., DE ANGELIS ZVOBODA, D., DA FONTOURA XAVIER COSTA, L., DE ARAÚJO, A.J.G., PEREIRA, R.I., SOARES, R.O., WAGNER, P.G.C., FRAZZON, J. and FRAZZON, A.P.G., 2018. Antimicrobial Resistance Profiles in Enterococcus spp. Isolates from fecal samples of wild and captive black Capuchin Monkeys (Sapajus nigritus) in South Brazil. Frontiers in Microbiology, vol. 9, pp. 1-10. http://dx.doi.org/10.3389/fmicb.2018.02366. PMid:30356681.
http://dx.doi.org/10.3389/fmicb.2018.023...
).
Aquaculture is believed to contribute to the spread and persistence of antimicrobial resistance in the environment, and resistant bacteria have been recovered from aquaculture production areas, as previously mentioned by Di Cesare et al. (2013)DI CESARE, A., LUNA, G.M., VIGNAROLI, C., PASQUAROLI, S., TOTA, S., PARONCINI, P. and BIAVASCO, F., 2013. Aquaculture can promote the presence and spread of antibiotic-resistant enterococci in marine sediments. PLoS One, vol. 8, no. 4, pp. 1-12. http://dx.doi.org/10.1371/journal.pone.0062838. PMid:23638152.
http://dx.doi.org/10.1371/journal.pone.0...
, Pereira et al. (2017)PEREIRA, G.V., CUNHA, D.G., MOURINO, J.L.P., RODILES, A., JARAMILLO-TORRES, A. and MERRIFIELD, D.L., 2017. Characterization of microbiota in Arapaima gigas intestine and isolation of potential probiotic bacteria. Journal of Applied Microbiology, vol. 123, no. 5, pp. 1298-1311. http://dx.doi.org/10.1111/jam.13572. PMid:28833934.
http://dx.doi.org/10.1111/jam.13572...
and, Novais et al. (2018)NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
. Not surprisingly, research reports not only resistant strains, but also resistance genes and remnants of the drug itself, reiterating that the discharge of antibiotics through effluents into natural water bodies, can lead to selective pressure and consequent increased antimicrobial resistance (Rocha et al., 2016ROCHA, R.S., SOUSA, O.V. and VIEIRA, R.H.S.F., 2016. Multidrug-resistant Vibrio associated with an estuary affected by shrimp farming in Northeastern Brazil. Marine Pollution Bulletin, vol. 105, no. 1, pp. 337-340. http://dx.doi.org/10.1016/j.marpolbul.2016.02.001. PMid:26876560.
http://dx.doi.org/10.1016/j.marpolbul.20...
).
3.3. Virulence profile of enterococcal strains
The gelE was the most frequently detected gene (64.6%) in enterococci strains isolated from fish farm, followed by ace (51.9%), agg (13.9%) and cylA (3.8%) genes (Table 4). The esp gene was not detected. Strains carrying virulence genes in water could be a risk to the health of humans and other animals that inhabit aquatic environments. Ahmad et al. (2014)AHMAD, A., DADA, A.C., USUP, G. and HENG, L.Y., 2014. Occurrence of Enterococcus species with virulence markers in an urban flow-influenced tropical recreational beach. Marine Pollution Bulletin, vol. 82, no. 1-2, pp. 26-38. http://dx.doi.org/10.1016/j.marpolbul.2014.03.028. PMid:24725825.
http://dx.doi.org/10.1016/j.marpolbul.20...
compared the presence of these virulence genes between enterococci isolated from seawater and river on a Malaysian beach. The authors reported that in river water samples, the gelE, asa, esp and cylA genes were detected in 100%, 63.41%, 21.95% and 7.32% of the strains, respectively; and in beach water samples a lower frequency of these genes was observed, being present in 67.27%, 41.82%, 20% and 0% of the strains.
The gelE gene was observed at high frequency among strains. This result agrees with those obtained by Novais et al. (2018)NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
who also detected high percentages of this gene in E. faecalis isolated from water and sediment samples from a trout fish farm in Europe. On the contrary, Savaşan et al. (2016)SAVAŞAN, S., KIRKAN, S., ERBAŞ, G., PARIN, U. and ÇIFTCI, A., 2016. The determination of virulence factors among fish originated enterococci. Etlik Veteriner Mikrobiyoloji Dergisi, vol. 27, no. 2, pp. 98-103. http://dx.doi.org/10.35864/evmd.514501.
http://dx.doi.org/10.35864/evmd.514501...
found low frequencies of gelE gene in Enterococcus spp. isolated from fish samples in Turkey. The ace gene was one of the more prevalent in our samples. The ace gene contributes to the microorganisms attach to surfaces and develop biofilms (Lebreton et al., 2014LEBRETON, F., WILLEMS, R.J.L. and GILMORE, M.S., 2014. Enterococcus diversity, origins in nature, and gut colonization. In: M.S. GILMORE, D.B. CLEWELL, Y. IKE and N. SHANKAR, eds. Enterococci: from commensals to leading causes of drug resistant infection. New York: Eye and Ear Infirmar, pp. 1-82.). Aquaculture environments present a large flow of water movement, necessary for the renovation or filling of the ponds. Thus, it is possible to assume that the occurrence of this gene is useful to bacterial maintenance in this environment. The agg gene, responsible for Enterococcus aggregation on host surfaces, occurred in 13.9% of the isolates. Similar results were observed by Savaşan et al. (2016)SAVAŞAN, S., KIRKAN, S., ERBAŞ, G., PARIN, U. and ÇIFTCI, A., 2016. The determination of virulence factors among fish originated enterococci. Etlik Veteriner Mikrobiyoloji Dergisi, vol. 27, no. 2, pp. 98-103. http://dx.doi.org/10.35864/evmd.514501.
http://dx.doi.org/10.35864/evmd.514501...
with samples from farmed fish. Novais et al. (2018)NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265. PMid:29996407.
http://dx.doi.org/10.1016/j.scitotenv.20...
did not observe the presence of the agg gene in fish samples in Portugal, but detected the presence of another aggregation gene in 50% of the isolates they studied. The presence of virulence factor in clinical strains of Enterococcus spp. is associated with the occurrence of infections; however, other authors associate the occurrence of these genes in non-clinical strains as a common characteristic, which probably contributes to enterococci persistence in the environment (Lebreton et al., 2014LEBRETON, F., WILLEMS, R.J.L. and GILMORE, M.S., 2014. Enterococcus diversity, origins in nature, and gut colonization. In: M.S. GILMORE, D.B. CLEWELL, Y. IKE and N. SHANKAR, eds. Enterococci: from commensals to leading causes of drug resistant infection. New York: Eye and Ear Infirmar, pp. 1-82.).
In conclusion, this study showed the occurrence of resistant enterococci as well as multiresistant strains in a fish pond that do not use antibiotics, either for therapeutic or for growth promotion purposes. Suggesting that the resistance found in these strains may be associated with environmental pollution. This study is the first step towards enhancing our understandingof distribution, resistance and virulence profile in enterococci isolated from fish farming environments in the south Brazil. The presence of resistant enterococci in waters and sediment is not only indicative of faecal contamination but may also a public health problem whether these microorganisms come into contact with humans and other animals. Our results are promising and should be validated by a large sample size.
Acknowledgements
The authors are grateful to Mr. Mauricio Daudt, owner of the fish farm, for his generously allowing access to the site and acquisition of material to be analyzed and occasional technical support.
This work was performed within the framework of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), a foundation linked to the Brazilian Ministry of Education that operates in the expansion and consolidation of stricto sensu graduate programs in all Brazilian states and sponsored by Conselho Nacional de Desenvolvimento Científico (CNPq - # 407886 /2018-4, and # 302574 / 2017-4).
-
(With 1 figure)
References
- AARESTRUP, F.M., AGERSO, Y., GERNER-SMIDT, P., MADSEN, M. and JENSEN, L.B., 2000. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagnostic Microbiology and Infectious Disease, vol. 37, no. 2, pp. 127-137. http://dx.doi.org/10.1016/S0732-8893(00)00130-9 PMid:10863107.
» http://dx.doi.org/10.1016/S0732-8893(00)00130-9 - AHMAD, A., DADA, A.C., USUP, G. and HENG, L.Y., 2014. Occurrence of Enterococcus species with virulence markers in an urban flow-influenced tropical recreational beach. Marine Pollution Bulletin, vol. 82, no. 1-2, pp. 26-38. http://dx.doi.org/10.1016/j.marpolbul.2014.03.028 PMid:24725825.
» http://dx.doi.org/10.1016/j.marpolbul.2014.03.028 - ANDERSSON, D.I. and HUGHES, D., 2014. Microbiological effects of sublethal levels of antibiotics. Nature Reviews. Microbiology, vol. 12, no. 7, pp. 465-478. http://dx.doi.org/10.1038/nrmicro3270 PMid:24861036.
» http://dx.doi.org/10.1038/nrmicro3270 - ASSOCIAÇÃO BRASILEIRA DA PISCICULTURA – PEIXEBR. 2019. Anuário PeixeBR da Piscicultura 2019 São Paulo: PeixeBR, 148 p.
- BRASIL, 2005. Resolução CONAMA n° 357. Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e dá outras providências. Diário Oficial da República Federativa do Brasil, Brasília, 17 maio.
- BRASIL, 2011. Resolução CONAMA n° 430. Dispõe sobre as condições e padrões de lançamento de efluentes, complementa e altera a Resolução no 357, de 17 de março de 2005, do Conselho Nacional do Meio Ambiente-CONAMA Diário Oficial da República Federativa do Brasil, Brasília, 13 maio.
- CASSENEGO, A.P.V., D’AZEVEDO, P.A., RIBEIRO, A.M.L., FRAZZON, J., VAN DER SAND, S.T. and FRAZZON, A.P.G., 2011. Species distribution and antimicrobial susceptibility of enterococci isolated from broilers infected experimentally with Eimeria spp and fed with diets containing different supplements. Brazilian Journal of Microbiology, vol. 42, no. 2, pp. 480-488. http://dx.doi.org/10.1590/S1517-83822011000200012 PMid:24031659.
» http://dx.doi.org/10.1590/S1517-83822011000200012 - CLINICAL AND LABORATORY STANDARDS INSTITUTE – CLSI, 2018. Performance standards for antimicrobial susceptibility testing 28th ed. Wayne, PA: CLSI, 296 p.
- DEPARDIEU, F., PERICHON, B. and COURVALIN, P., 2004. Detection of the van Alphabet and Identification of Enterococci and Staphylococci at the Species Level by Multiplex PCR. Journal of Clinical Microbiology, vol. 42, no. 12, pp. 5857-5860. http://dx.doi.org/10.1128/JCM.42.12.5857-5860.2004 PMid:15583325.
» http://dx.doi.org/10.1128/JCM.42.12.5857-5860.2004 - DI CESARE, A., LUNA, G.M., VIGNAROLI, C., PASQUAROLI, S., TOTA, S., PARONCINI, P. and BIAVASCO, F., 2013. Aquaculture can promote the presence and spread of antibiotic-resistant enterococci in marine sediments. PLoS One, vol. 8, no. 4, pp. 1-12. http://dx.doi.org/10.1371/journal.pone.0062838 PMid:23638152.
» http://dx.doi.org/10.1371/journal.pone.0062838 - DIZAVANDI, Z.R., ALIAKBAR, A. and SHEYKHAN, M., 2016. A novel Pb-poly aminophenol glassy carbon electrode for determination of tetracycline by adsorptive differential pulse cathodic stripping voltammetry. Electrochimica Acta, vol. 227, pp. 345-356. http://dx.doi.org/10.1016/j.electacta.2016.12.167
» http://dx.doi.org/10.1016/j.electacta.2016.12.167 - EATON, T.J. and GASSON, M.J., 2001. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Applied and Environmental Microbiology, vol. 67, no. 4, pp. 1628-1635. http://dx.doi.org/10.1128/AEM.67.4.1628-1635.2001 PMid:11282615.
» http://dx.doi.org/10.1128/AEM.67.4.1628-1635.2001 - EUROPEAN FOOD SAFETY AUTHORITY – EFSA and EUROPEAN CENTRE FOR DISEASE PREVENTION AND CONTROL – ECDC, 2013. The European Union Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2011. EFSA Journal, vol. 11, no. 5, pp. 359.
- FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS – FAO, 2018. The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals Rome: FAO, 227 p.
- FRAZZON, A.P.G., GAMA, B.A., HERMES, V., BIERHALS, C.G., PEREIRA, R.I., GUEDES, A.G., D’AZEVEDO, P.A. and FRAZZON, J., 2010. Prevalence of antimicrobial resistance and molecular characterization of tetracycline resistance mediated by tet(M) and tet(L) genes in Enterococcus spp. isolated from food in Southern Brazil. World Journal of Microbiology & Biotechnology, vol. 26, no. 2, pp. 365-370. http://dx.doi.org/10.1007/s11274-009-0160-x
» http://dx.doi.org/10.1007/s11274-009-0160-x - FIGUEIREDO, J.A.S., DRUMM, E., RODRIGUES, M.A.S. and SPILKI, F.R., 2010. The Rio dos Sinos watershed: an economic and social space and its interface with environmental status. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 70, no. 4, suppl., pp. 1131-1136. http://dx.doi.org/10.1590/S1519-69842010000600001 PMid:21225153.
» http://dx.doi.org/10.1590/S1519-69842010000600001 - GOTHWAL, R. and SHASHIDHAR, T., 2014. Antibiotic pollution in the environment: a review. Clean, vol. 43, no. 4, pp. 479-489. http://dx.doi.org/10.1002/clen.201300989
» http://dx.doi.org/10.1002/clen.201300989 - GRASSOTTI, T.T., DE ANGELIS ZVOBODA, D., DA FONTOURA XAVIER COSTA, L., DE ARAÚJO, A.J.G., PEREIRA, R.I., SOARES, R.O., WAGNER, P.G.C., FRAZZON, J. and FRAZZON, A.P.G., 2018. Antimicrobial Resistance Profiles in Enterococcus spp. Isolates from fecal samples of wild and captive black Capuchin Monkeys (Sapajus nigritus) in South Brazil. Frontiers in Microbiology, vol. 9, pp. 1-10. http://dx.doi.org/10.3389/fmicb.2018.02366 PMid:30356681.
» http://dx.doi.org/10.3389/fmicb.2018.02366 - HUFF, R., INHOQUE PEREIRA, R., PISSETTI, C., MELLENDER DE ARAÚJO, A., ALVES D’AZEVEDO, P., FRAZZON, J. and GUEDESFRAZZON, A.P., 2020. Antimicrobial resistance and genetic relationships of enterococci from siblings and non-siblings Heliconius erato phyllis caterpillars. PeerJ, vol. 8, pp. e8647. http://dx.doi.org/10.7717/peerj.8647 PMid:32149028.
» http://dx.doi.org/10.7717/peerj.8647 - LEBRETON, F., WILLEMS, R.J.L. and GILMORE, M.S., 2014. Enterococcus diversity, origins in nature, and gut colonization. In: M.S. GILMORE, D.B. CLEWELL, Y. IKE and N. SHANKAR, eds. Enterococci: from commensals to leading causes of drug resistant infection New York: Eye and Ear Infirmar, pp. 1-82.
- MANNU, L., PABA, A., DAGA, E., COMUNIAN, R., ZANETTI, S., DUPRÉ, I. and SECHI, L.A., 2003. Comparison of the incidence of virulence determinants and antibiotic resistance between Enterococcus faecium strains of dairy, animal and clinical origin. International Journal of Food Microbiology, vol. 88, no. 2-3, pp. 291-304. http://dx.doi.org/10.1016/S0168-1605(03)00191-0 PMid:14597001.
» http://dx.doi.org/10.1016/S0168-1605(03)00191-0 - NACHTIGALL, G., DE JESUS, A.G., ZVOBODA, D.A., SANTESTEVAN, N.A., MINOTTO, E., DE MOURA, T.M., D’AZEVEDO, J., FRAZZON, J., VAN DER SAND, S. and FRAZZON, A.P.G., 2013. Diversidade e perfil de susceptibilidade antimicrobiana de Enterococcus sp. isolados das águas do Arroio Dilúvio - Porto Alegre, RS, Brasil. Revista Brasileira de Biociências, vol. 11, no. 2, pp. 235-241.
- NOVAIS, C., CAMPOS, J., FREITAS, A.R., BARROS, M., SILVEIRA, E., COQUE, T.M., ANTUNES, P. and PEIXE, L., 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. The Science of the Total Environment, vol. 625, pp. 1102-1112. http://dx.doi.org/10.1016/j.scitotenv.2017.12.265 PMid:29996407.
» http://dx.doi.org/10.1016/j.scitotenv.2017.12.265 - PEREIRA, G.V., CUNHA, D.G., MOURINO, J.L.P., RODILES, A., JARAMILLO-TORRES, A. and MERRIFIELD, D.L., 2017. Characterization of microbiota in Arapaima gigas intestine and isolation of potential probiotic bacteria. Journal of Applied Microbiology, vol. 123, no. 5, pp. 1298-1311. http://dx.doi.org/10.1111/jam.13572 PMid:28833934.
» http://dx.doi.org/10.1111/jam.13572 - RAHARDJO, A.K., SUSANTO, M.J., KURNIAWAN, A., INDRASWATI, N. and ISMADJI, S., 2011. Modified ponorogo bentonite for the removal of ampicillin from wastewater. Journal of Hazardous Materials, vol. 190, no. 1-3, pp. 1001-1008. http://dx.doi.org/10.1016/j.jhazmat.2011.04.052 PMid:21550716.
» http://dx.doi.org/10.1016/j.jhazmat.2011.04.052 - RATHNAYAKE, I., HARGREAVES, M. and HUYGENS, F., 2011. SNP diversity of Enterococcus faecalis and Enterococcus faecium in a South East Queensland waterway, Australia, and associated antibiotic resistance gene profiles. BMC Microbiology, vol. 11, no. 1, pp. 1-13. http://dx.doi.org/10.1186/1471-2180-11-201 PMid:21910889.
» http://dx.doi.org/10.1186/1471-2180-11-201 - ROCHA, R.S., SOUSA, O.V. and VIEIRA, R.H.S.F., 2016. Multidrug-resistant Vibrio associated with an estuary affected by shrimp farming in Northeastern Brazil. Marine Pollution Bulletin, vol. 105, no. 1, pp. 337-340. http://dx.doi.org/10.1016/j.marpolbul.2016.02.001 PMid:26876560.
» http://dx.doi.org/10.1016/j.marpolbul.2016.02.001 - RUDRA, P., HURST-HESS, K., LAPPIERRE, P. and GHOSH, P., 2018. High levels of intrinsic tetracycline resistance in Mycobacterium abscessus is conferred by a tetracycline - modifying monooxygenase. Antimicrobial Agents and Chemotherapy, vol. 62, no. 6, pp. e00119-e18. http://dx.doi.org/10.1128/AAC.00119-18 PMid:29632012.
» http://dx.doi.org/10.1128/AAC.00119-18 - SAUGET, M., VALOT, B., BERTRAND, X. and HOCQUET, D., 2017. Can Maldi-Tof Mass spectrometry reasonably type bacteria? Trends in Microbiology, vol. 25, no. 6, pp. 447-455. http://dx.doi.org/10.1016/j.tim.2016.12.006 PMid:28094091.
» http://dx.doi.org/10.1016/j.tim.2016.12.006 - SAVAŞAN, S., KIRKAN, S., ERBAŞ, G., PARIN, U. and ÇIFTCI, A., 2016. The determination of virulence factors among fish originated enterococci. Etlik Veteriner Mikrobiyoloji Dergisi, vol. 27, no. 2, pp. 98-103. http://dx.doi.org/10.35864/evmd.514501
» http://dx.doi.org/10.35864/evmd.514501 - SCHAFHAUSER, B.H., KRISTOFCO, L.A., DE OLIVEIRA, C.M.R. and BROOKS, B.W., 2018. Global review and analysis of erythromycin in the environment: occurrence, bioaccumulation and antibiotic resistance hazards. Environmental Pollution, vol. 238, pp. 440-451. http://dx.doi.org/10.1016/j.envpol.2018.03.052 PMid:29587215.
» http://dx.doi.org/10.1016/j.envpol.2018.03.052 - SHANKAR, V., BAGHDAYAN, A.S., HUYCKE, M.M., LINDAHL, G. and GILMORE, M.S., 1999. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infection and Immunity, vol. 67, no. 1, pp. 193-200. http://dx.doi.org/10.1128/IAI.67.1.193-200.1999 PMid:9864215.
» http://dx.doi.org/10.1128/IAI.67.1.193-200.1999 - SUTCLIFFE, J., GREBE, T., TAIT-KAMRADT, A. and WONDRACK, L., 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrobial Agents and Chemotherapy, vol. 40, no. 11, pp. 2562-2566. http://dx.doi.org/10.1128/AAC.40.11.2562 PMid:8913465.
» http://dx.doi.org/10.1128/AAC.40.11.2562 - TEIXEIRA, L.M., CARVALHO, M.G.S., SHEWMAKER, P.L. and FACKLAM, R.R., 2011. Manual of clinical microbiology 10th ed. Washington, DC: American Society for Microbiology, 2630 p.
- WERNER, G., HILDEBRANDT, B., WITTE, W., MURRAY, B.E. and SINGH, K.V., 2001. The newly described msrC gene is not equally distributed among all isolates of Enterococcus faecium. Antimicrobial Agents and Chemotherapy, vol. 45, no. 12, pp. 3672-3673. http://dx.doi.org/10.1128/AAC.45.12.3672-3673.2001 PMid:11724034.
» http://dx.doi.org/10.1128/AAC.45.12.3672-3673.2001
Publication Dates
-
Publication in this collection
12 Oct 2020 -
Date of issue
Oct-Dec 2021
History
-
Received
23 Dec 2019 -
Accepted
27 May 2020