Abstract
Soft-bottom macrobenthic invertebrates are sensitive to natural or anthropogenic changes in aquatic ecosystems. The distribution patterns of sublittoral macrobenthic species in Guanabara Bay were studied from 2005 to 2007. Samples were collected at ten stations during six surveys throughout the rainfall regime (dry, early and late rainy). Ten replicates were collected at each station by Gravity corer or skin diving. Van Dorn bottles (bottom water) and by Ekman sediment sampler (granulometry) provided material for abiotic data. Stations were grouped into sectors (Entrance, Intermediary and Inner) based on abiotic data and location. The Redundancy Analysis (RDA) and Parsimonious RDA for all years and each annual cycle showed indicator taxa with high dominance in each sector. PERMANOVA indicated a regular seasonality between the surveys for the first annual cycle (p <0.05), and an atypical pattern for the second (p> 0.05), possibly due the low rainfall observed during this period. The mosaic of soft-bottom substrates infers structural variables, and patterns of temporal distribution were basically influenced by parameters those indicating pollution and the SACW (South Atlantic Central Water) intrusion, as well as ecological attributes among species, such as: predation, competition. The Ervilia concentrica and Cypridinidae could be used as indicators for anthropic and natural impacts in the Guanabara Bay for the Entrance sector, while Cyprideis salebrosa and Cyprideis sp. for the Intermediary sector and Heleobia australis for the Inner sector.
Keywords:
macrobenthic; sediments; climate change; sublittoral; soft-bottom and bioindicators
Resumo
Os invertebrados macrobentônicos são sensíveis as alterações naturais e antrópicas nos ecossistemas aquáticos. O padrão de distribuição das espécies macrobentônicas do infralitoral da Baía de Guanabara foram estudados de 2005 até 2007. Amostras foram coletadas em dez estações durante seis campanhas em todo o regime pluviométrico (seco, pré e pós chuvoso). Dez réplicas foram coletadas em cada estação por meio do amostrador Gravity corer ou por mergulho livre. Os dados abióticos foram coletados por meio de garrafa oceanográfica do tipo van Dorn (água de fundo) e por busca fundo do tipo Ekman (granulometria). As estações foram agrupadas em setores (Entrada, Intermediária e Interna) baseada nos dados abióticos e localização. A Análise de Redundância (RDA) e RDA Parcimoniosa para todos os anos e em cada ano evidenciou taxa indicadores como elevada dominância em cada setor. A PERMANOVA indicou sazonalidade regular entre as campanhas para o primeiro ciclo anual (p<0.05), padrão atípico para o segundo ano (p> 0.05), possivelmente por causa da baixa pluviosidade observada durante esse período. O mosaico do substrato não consolidado infere que as variáveis estruturais, e os padrões de distribuição temporal foram basicamente influenciadas por parâmetros que indicam poluição e intrusão de ACAS (Água Central do Atlântico Sul), bem como atributos ecológicos entre espécies, tais como: predação, competição, entre outros. Ervilia concentrica e Cypridinidae podem ser utilizados como indicadores de alterações naturais e antrópicos no setor da Entrada da Baía de Guanabara, enquanto Cyprideis salebrosa e Cyprideis sp. para o setor Intermediário e Heleobia australis para o setor Interno.
Palavras-chave:
macrobentônicos; sedimentos; mudança climática; infralitoral; fundo não consolidado e bioindicadores
1. Introduction
As sediments are present in almost all aquatic ecosystems (Snelgrove, 1997SNELGROVE, P.V.R., 1997. The importance of marine sediment biodiversity in ecosystem processes. Ambio, vol. 26, pp. 578-583.), all or at least part of the life cycles of a large number of species is associated with them (Alongi, 1989ALONGI, D.M., 1989. Ecology of tropical soft-bottom benthos: a review with emphasis on emerging concepts. Revista de Biología Tropical, vol. 37, no. 1, pp. 85-100.; Day et al., 1989DAY, J., HALL, C., KEMP, W. and YÁÑEZ-ARANCIBIA, A., 1989. Estuarine ecology. 1st ed. New York: John Wiley & Sons.). Invertebrates living under these conditions resort to various strategies for feeding, dispersion, locomotion, among others (Gray and Elliott, 2009GRAY, J.S. and ELLIOTT, M., 2009. Ecology of marine sediments. 2nd ed. Oxford: Oxford Biology, 256 p.), that are relevant in the dynamics of aquatic ecosystems, as a whole. An example of this was observed in deposit feeders, when feeding on organic matter (Lopez and Levinton, 1987LOPEZ, G.R. and LEVINTON, J.S., 1987. Ecology of deposit-feeding animals in marine sediments. The Quarterly Review of Biology, vol. 62, no. 3, pp. 235-260. http://dx.doi.org/10.1086/415511.
http://dx.doi.org/10.1086/415511...
). Macrobenthic samples retained after washing or sieving through a 0.5 mm mesh, are mainly comprised of polychaetes, crustaceans, molluscs and others (Little, 2000LITTLE, C., 2000. The biology of soft shores and estuaries. Oxford: Oxford University Press, 264 p.). Another vital aspect of dynamics is the presence of bioturbinators involved in nutrient recycling and aeration of the sediment. The measures for increasing oxic layers are exerted by the presence of species that rework the sediment through the formation of galleries (Diaz and Rosenberg, 1995DIAZ, R.J. and ROSENBERG, R., 1995. Marine benthic hypoxia: a review of its ecological effects and the behavioral responses of benthic macrofauna. Oceanography and Marine Biology - an Annual Review, vol. 33, pp. 245-303.; Mermillod-Blondin and Rosenberg, 2006MERMILLOD-BLONDIN, F. and ROSENBERG, R., 2006. Ecosystem engineering: the impact of bioturbation on biogeochemical processes in marine and freshwater benthic habitats. Aquatic Sciences, vol. 68, no. 4, pp. 434-442. http://dx.doi.org/10.1007/s00027-006-0858-x.
http://dx.doi.org/10.1007/s00027-006-085...
; Rosenberg, 2001ROSENBERG, R., 2001. Marine benthic faunal successional stages and related sedimentary activity. Scientia Marina, vol. 65, suppl. 2, pp. 107-119. http://dx.doi.org/10.3989/scimar.2001.65s2107.
http://dx.doi.org/10.3989/scimar.2001.65...
).
In soft-bottom environments, the individuals living in the sediment are extremely diverse and with the presence of several taxa (Snelgrove, 1999SNELGROVE, P.V.R., 1999. Getting to the bottom of marine biodiversity: sedimentary habitats. Bioscience, vol. 49, no. 2, pp. 129-138. http://dx.doi.org/10.2307/1313538.
http://dx.doi.org/10.2307/1313538...
). This mixing process facilitates the suspension through the generated turbulence of the nutrients and organic matter in the water column, allowing greater access for producers and detritivores to these compounds compared to other aquatic ecosystems (Day et al., 1989DAY, J., HALL, C., KEMP, W. and YÁÑEZ-ARANCIBIA, A., 1989. Estuarine ecology. 1st ed. New York: John Wiley & Sons.; Gray and Elliott, 2009GRAY, J.S. and ELLIOTT, M., 2009. Ecology of marine sediments. 2nd ed. Oxford: Oxford Biology, 256 p.; Little, 2000LITTLE, C., 2000. The biology of soft shores and estuaries. Oxford: Oxford University Press, 264 p.). Thus, estuaries are considered one of the most productive ecosystems on the planet, with higher productivity rates than tropical forests and coral reefs (Valiela, 1995VALIELA, I., 1995. Marine ecological processes. 2nd ed. New York: Springer-Verlag, 686 p. http://dx.doi.org/10.1007/978-1-4757-4125-4.
http://dx.doi.org/10.1007/978-1-4757-412...
). The spatial and temporal nature of these estuaries are regulated by several environmental variables (ranging from salinity to altering biogeochemical conditions, redox potential, pH, dissolved oxygen and others) in the face of tidal and continental variations (Snelgrove, 1997SNELGROVE, P.V.R., 1997. The importance of marine sediment biodiversity in ecosystem processes. Ambio, vol. 26, pp. 578-583., 1999SNELGROVE, P.V.R., 1999. Getting to the bottom of marine biodiversity: sedimentary habitats. Bioscience, vol. 49, no. 2, pp. 129-138. http://dx.doi.org/10.2307/1313538.
http://dx.doi.org/10.2307/1313538...
).
Benthic communities have a great difference in relation to other communities as to their use as environmental indicators. This characteristic is inherent in most sedentary or sessile groups, when compared to the groups that have the greatest degree of mobility and quickly move to other areas.
The well-defined estuary dynamics involves specific environmental stressors, comprised of a wide range of abiotic variables, such as salinity, dissolved oxygen, pH, and others, whose action is intensified by freshwater flow in rainy periods and tidal action (Perillo et al., 2009PERILLO, G.M.E., PRATOLONGO, P.D., ELIZABETH CARBONE, M. and PICCOLO, M.C., 2009. Biological-physical interactions in estuaries. Estuarine, Coastal and Shelf Science, vol. 85, no. 1, pp. 5-6. http://dx.doi.org/10.1016/j.ecss.2009.08.017.
http://dx.doi.org/10.1016/j.ecss.2009.08...
).
The extremely diverse sediment community is split into various taxa. Several environmental factors determine the structure of a benthic community in marine and estuarine environments (Gray and Elliott, 2009GRAY, J.S. and ELLIOTT, M., 2009. Ecology of marine sediments. 2nd ed. Oxford: Oxford Biology, 256 p.), these especially involving sediment structural elements, such as available organic matter, grain size, among others (Carvalho et al., 2005CARVALHO, S., MOURA, A., GASPAR, M., PEREIRA, P., FONSECA, C., FALCÃO, L., DRAGO, T., LEITÃO, F. and REGALA, F., 2005. Spatial and interannual variability of the macrobenthic communities within a costal lagoon (Obidos Lagoon) and its relationship with environmental parameters. Acta Oceanologica, vol. 27, no. 3, pp. 143-159. http://dx.doi.org/10.1016/j.actao.2004.11.004.
http://dx.doi.org/10.1016/j.actao.2004.1...
; Gray and Elliott, 2009GRAY, J.S. and ELLIOTT, M., 2009. Ecology of marine sediments. 2nd ed. Oxford: Oxford Biology, 256 p.; Snelgrove, 1997SNELGROVE, P.V.R., 1997. The importance of marine sediment biodiversity in ecosystem processes. Ambio, vol. 26, pp. 578-583.). Furthermore, the bottom water-mass also exerts a strong influence on specific community composition. Macrobenthic invertebrates are essential in maintaining matter and energy flow in estuarine regions by assimilating debris. This cycle is fundamental in organic matter processing and nutrient cycling (Snelgrove, 1997SNELGROVE, P.V.R., 1997. The importance of marine sediment biodiversity in ecosystem processes. Ambio, vol. 26, pp. 578-583., 1999SNELGROVE, P.V.R., 1999. Getting to the bottom of marine biodiversity: sedimentary habitats. Bioscience, vol. 49, no. 2, pp. 129-138. http://dx.doi.org/10.2307/1313538.
http://dx.doi.org/10.2307/1313538...
).
The macrobenthos with their populations coexisting with one another in the environment, composing associations of organisms, or high densities of some species, or morphological or behavioral modifications may reflect these local conditions (Snelgrove, 1999SNELGROVE, P.V.R., 1999. Getting to the bottom of marine biodiversity: sedimentary habitats. Bioscience, vol. 49, no. 2, pp. 129-138. http://dx.doi.org/10.2307/1313538.
http://dx.doi.org/10.2307/1313538...
). These responses are mainly due to the mode of locomotion of these individuals, especially those that are sessile or with low mobility, allowing them to be used as good environmental indicators. Variations in diversity, equitability, species richness and density are strong indicators of the quality of the environment and act as parameters to monitor environmental recovery processes (Schindler, 1987SCHINDLER, D.W., 1987. Detecting ecosystem responses to anthropogenics stress. Canadian Journal of Fisheries and Aquatic Sciences, vol. 44, no. S1, pp. 6-25. http://dx.doi.org/10.1139/f87-276.
http://dx.doi.org/10.1139/f87-276...
; Underwood, 1991UNDERWOOD, A.J., 1991. Beyond BACI: experimental designs for detecting human environmental impacts on temporal variations in natural populations. Australian Journal of Marine and Freshwater Research, vol. 42, no. 5, pp. 569-587. http://dx.doi.org/10.1071/MF9910569.
http://dx.doi.org/10.1071/MF9910569...
, 1992UNDERWOOD, A.J., 1992. Beyond BACI: the detection of environmental impact on populations in the real, but variable, world. Journal of Experimental Marine Biology and Ecology, vol. 161, no. 2, pp. 145-178. http://dx.doi.org/10.1016/0022-0981(92)90094-Q.
http://dx.doi.org/10.1016/0022-0981(92)9...
, 1994UNDERWOOD, A.J., 1994. On beyond BACI: sampling designs that might reliably detect environmental disturbances. Ecological Applications, vol. 4, no. 1, pp. 3-15. http://dx.doi.org/10.2307/1942110.
http://dx.doi.org/10.2307/1942110...
).
The broad knowledge of the main groups allows evaluating and identifying pollution and degradation events for higher taxonomic levels, subsidizing monitoring or environmental recovery projects (Dauvin et al., 2003DAUVIN, J.C., GOMEZ GESTEIRA, J.L. and SALVANDE FRAGA, M., 2003. Taxonomic sufficiency: an overview of its use in the monitoring of sublittoral benthic communities after oil spills. Marine Pollution Bulletin, vol. 46, no. 5, pp. 552-555. http://dx.doi.org/10.1016/S0025-326X(03)00033-X. PMid:12735952.
http://dx.doi.org/10.1016/S0025-326X(03)...
; Ellis, 1985ELLIS, D., 1985. Taxonomic sufficiency in pollution assessment. Marine Pollution Bulletin, vol. 16, no. 12, pp. 459p. http://dx.doi.org/10.1016/0025-326X(85)90362-5.
http://dx.doi.org/10.1016/0025-326X(85)9...
; Warwick, 1988WARWICK, R.M., 1988. The level of taxonomic discrimination required to detect pollution effects on marine benthic communities. Marine Pollution Bulletin, vol. 19, no. 6, pp. 259-268. http://dx.doi.org/10.1016/0025-326X(88)90596-6.
http://dx.doi.org/10.1016/0025-326X(88)9...
). In addition, approaches that consider cost-benefit analysis in order to obtain significant results have increased a lot in recent years, through studies that validate the methodology regarding aspects such as mesh size used, taxonomic resolution, sampling effort and seasonal variation (Ammann et al., 1997AMMANN, L.P., WALLER, W.T., KENNEDY, J.H., DICKSON, K.L. and MAYER, F.L., 1997. Power, sample size and taxonomic sufficiency for measures or impact in aquatic systems. Environmental Toxicology and Chemistry, vol. 16, no. 11, pp. 2421-2431. http://dx.doi.org/10.1002/etc.5620161131.
http://dx.doi.org/10.1002/etc.5620161131...
; Ellis, 1985ELLIS, D., 1985. Taxonomic sufficiency in pollution assessment. Marine Pollution Bulletin, vol. 16, no. 12, pp. 459p. http://dx.doi.org/10.1016/0025-326X(85)90362-5.
http://dx.doi.org/10.1016/0025-326X(85)9...
; Thompson et al., 2003THOMPSON, B.W., RIDDLE, M.J. and STARK, J.S., 2003. Cost-efficient methods for marine pollution monitoring at Casey Station, East Antarctica: the choice of sieve mesh-size and taxonomic resolution. Marine Pollution Bulletin, vol. 46, no. 2, pp. 232-243. http://dx.doi.org/10.1016/S0025-326X(02)00366-1. PMid:12586119.
http://dx.doi.org/10.1016/S0025-326X(02)...
).
In tropical regions, the pluviometric regime in estuaries is well defined in rainy and dry seasons. In this way there is a strong influence of the fresh water intake from the estuarine watersheds and an intense hydrodynamic with marked salinity fluctuations (Kjerfve et al., 1997KJERFVE, B., RIBEIRO, C.H.A., DIAS, G.T.M., FILIPPO, A.M. and QUARESMA, V.S., 1997. Oceanographic characteristics of an impacted coastal bay: baía de Guanabara, Rio de Janeiro, Brazil. Continental Shelf Research, vol. 17, no. 13, pp. 1609-1643. http://dx.doi.org/10.1016/S0278-4343(97)00028-9.
http://dx.doi.org/10.1016/S0278-4343(97)...
; Paranhos and Mayr, 1993PARANHOS, R. and MAYR, L.M., 1993. Seasonal patterns of temperature and salinity in Guanabara Bay, Brazil. Fresenius Environmental Bulletin, vol. 2, no. 11, pp. 647-652.; Pritchard, 1967PRITCHARD, D.W., 1967. Observations of circulation in coastal plain estuaries. In: G.H. LAUF, ed. Estuaries. Washington: American Association Advance Science, pp. 37-44.). This characteristic promotes estuaries a high ecological importance as a nursery ground for several species of fish and invertebrates, as well as reproduction and feeding grounds, providing a high production from the input of organic matter and nutrients from its watershed (Gillanders and Kingsford, 2002GILLANDERS, B.M. and KINGSFORD, M.J., 2002. Impact of changes in flow of freshwater on estuarine and open coastal habitats and the associated organisms. Oceanography and Marine Biology - an Annual Review, vol. 40, pp. 233-309. http://dx.doi.org/10.1201/9780203180594.ch5.
http://dx.doi.org/10.1201/9780203180594....
; McLeod and Wing, 2008MCLEOD, R. and WING, S., 2008. Influence of an altered salinity regime on the population structure of two infaunal bivalve species. Estuarine, Coastal and Shelf Science, vol. 78, no. 3, pp. 529-540. http://dx.doi.org/10.1016/j.ecss.2008.01.019.
http://dx.doi.org/10.1016/j.ecss.2008.01...
). Anthropogenic disturbances through modifications in the physical and chemical characteristics of sediment, are reflected in changes in the structure and trophic interactions of benthic communities (Elliott and Quintino, 2007ELLIOTT, M. and QUINTINO, V., 2007. The estuarine quality paradox, environmental homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Marine Pollution Bulletin, vol. 54, no. 6, pp. 640-645. http://dx.doi.org/10.1016/j.marpolbul.2007.02.003. PMid:17418874.
http://dx.doi.org/10.1016/j.marpolbul.20...
). This phenomenon is especially noted in ecosystems recovering from recent activities, such as dredging, landfills, pipeline installation, among others, in which changes in richness, diversity paucity, the appearance of opportunistic species, and morphological and physiological adaptation of the fauna occurs (Coutinho et al., 2014COUTINHO, F.H., SILVEIRA, C.B., PINTO, L.H., SALLOTO, G.R., CARDOSO, A.M., MARTINS, O.B., VIEIRA, R.P. and CLEMENTINO, M.M., 2014. Antibiotic resistance is widespread in urban aquatic environments of Rio de Janeiro. Brazilian Journal of Microbiology, vol. 68, no. 3, pp. 441-452. PMid:24821495.; Kfouri et al., 2005KFOURI, P.B.P., FIGUEIRA, R.C.L., FIGUEIREDO, A.M.G., SOUZA, S.H.M. and EICHLER, B.B., 2005. Metal levels and foraminifera occurrence in sediment cores from Guanabara Bay, Rio de Janeiro. Journal of Radioanalytical and Nuclear Chemistry, vol. 265, no. 3, pp. 459-466. http://dx.doi.org/10.1007/s10967-005-0849-8.
http://dx.doi.org/10.1007/s10967-005-084...
; Lardosa et al., 2013LARDOSA, E.I., SIMÕES, M. and SOARES, M.L.G., 2013. Cartografia das áreas de ocorrência de manguezais no Estado do Rio de Janeiro através da integração de múltiplas fontes de dados. Revista Brasileira de Cartografia, vol. 65, pp. 1-14.; Meniconi et al., 2002MENICONI, M.D.G., GABARDO, I.T., CARNEIRO, M.E.R., BARBANTI, S.M., SILVA, G.C. and MASSONE, C.G., 2002. Brazilian oil spills chemical characterization e case studies. Environmental Forensics, vol. 3, no. 3, pp. 303-321. http://dx.doi.org/10.1080/713848377.
http://dx.doi.org/10.1080/713848377...
). The impacts generated by the development of large economic poles produce profound changes in the environment, whence the extreme importance of evaluating both outcome and recovery (Neves and Valentin, 2011NEVES, R.A.F. and VALENTIN, J.L., 2011. Revisão bibliográfica sobre a macrofauna bentônica de fundos não-consolidados em áreas costeiras prioritárias para a conservação no Brasil. Arquivos de Ciências do Mar, vol. 44, pp. 59-80.; Soares-Gomes et al, 2016SOARES-GOMES, A., GAMA, B.A.P., BAPTISTA NETO, J.A., FREIRE, D.G., CORDEIRO, R.C., MACHADO, W., BERNARDES, M.C., COUTINHO, R., THOMPSON, F. and PEREIRA, R.C., 2016. An environmental overview of Guanabara Bay, Rio de Janeiro. Regional Studies in Marine Science, vol. 8, pp. 319-330. http://dx.doi.org/10.1016/j.rsma.2016.01.009.
http://dx.doi.org/10.1016/j.rsma.2016.01...
).
The sensitivity of ecosystems to certain anthropogenic impacts is influenced by some environmental factors, such as the slope of the coastline, granulometric structure, hydrodynamism, permeability, productivity, water body flow renewal and the specific composition of this ecosystem, among others (Baptista Neto et al., 2005BAPTISTA NETO, J.A., CRAPEZ, M.A.C., MCALISTER, J.J. and VILELA, C.G., 2005. Concentration and availability of heavy metals in sediments from Niterói Harbour (Guanabara Bay, S.E. Brazil). Journal of Coastal Research, vol. 21, pp. 811817., 2006; Borges et al., 2009BORGES, A.C., SANDERS, C.J., SANTOS, H.L.R., ARARIPE, D.R., MACHADO, W. and PATCHINEELAM, S.R., 2009. Eutrophication history of Guanabara Bay (SE Brazil) recorded by phosphorus flux to sediments from a degraded mangrove area. Marine Pollution Bulletin, vol. 58, no. 11, pp. 1750-1765. http://dx.doi.org/10.1016/j.marpolbul.2009.07.025. PMid:19699494.
http://dx.doi.org/10.1016/j.marpolbul.20...
, 2014BORGES, R.C., CALDAS, V.G., SIMÕES FILHO, F.F.L., FERREIRA, M.M. and LAPA, C.M.F., 2014. Use of GIS for the evaluation of heavy metal contamination in the Cunha Canal watershed and west of the Guanabara Bay, Rio de Janeiro, RJ. Marine Pollution Bulletin, vol. 89, no. 1-2, pp. 75-84. http://dx.doi.org/10.1016/j.marpolbul.2014.10.033. PMid:25455374.
http://dx.doi.org/10.1016/j.marpolbul.20...
; Marazzo and Valentin, 2004MARAZZO, A. and VALENTIN, J.L., 2004. Reproductive aspects of marine cladocerans Penilia avirostris and Pseudevadne tergestina (Crustacea, Branchiopoda) in the outer part of Guanabara Bay, Brazil. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 64, no. 3A, pp. 543-549. http://dx.doi.org/10.1590/S1519-69842004000300017. PMid:15622851.
http://dx.doi.org/10.1590/S1519-69842004...
; Soares-Gomes et al., 2010SOARES-GOMES, A., NEVES, R.L., AUCÉLIO, R., VAN DER VEN, P.H., PITOMBO, F.B., MENDES, C.L. and ZIOLLI, R.L., 2010. Changes and variations of polycyclic aromatic hydrocarbon concentrations in fish, barnacles and crabs following an oil spill in a mangrove of Guanabara Bay, Southeast Brazil. Marine Pollution Bulletin, vol. 60, no. 8, pp. 1359-1363. http://dx.doi.org/10.1016/j.marpolbul.2010.05.013. PMid:20538307.
http://dx.doi.org/10.1016/j.marpolbul.20...
; Ventura et al., 2002VENTURA, E.C., GAELZER, L.R., ZANETTE, J., MARQUES, M.R.F. and BAINY, A.C.D., 2002. Biochemichal indicators of contaminant exposure in spotted pigfish (Orthopristis ruber) caught at three bays of Rio de Janeiro coast. Marine Environmental Research, vol. 54, no. 3-5, pp. 775-779. http://dx.doi.org/10.1016/S0141-1136(02)00137-X. PMid:12408649.
http://dx.doi.org/10.1016/S0141-1136(02)...
; Xavier de Brito et al., 2002XAVIER DE BRITO, A.P., BRÜNING, I.M.R. and MOREIRA, I., 2002. Chlorinated pesticides in mussels from Guanabara Bay, Rio de Janeiro, Brazil. Marine Pollution Bulletin, vol. 44, no. 1, pp. 79-81. http://dx.doi.org/10.1016/S0025-326X(01)00222-3. PMid:11885566.
http://dx.doi.org/10.1016/S0025-326X(01)...
).
The aim of this study is to evaluate the influence of seasonality (rainfall) on macrobenthic community within the Guanabara Bay estuary system.
2. Material and Methods
2.1. Study area
Guanabara Bay is located in the State of Rio de Janeiro between the latitudes 22°40’ and 23°00’ S and longitudes 43°00’ and 43°20’ W (Amador, 1997AMADOR, E.S., 1997. Baía de Guanabara e ecossistemas periféricos: homem e natureza. Rio de Janeiro: Reproarte Gráfica e Editora, 539 p., 2012AMADOR, E.S., 2012. Bacia da Baía de Guanabara: características geoambientais e ecossistemas. Rio de Janeiro: Editora Interciência, 406p.; Figure 1).
The bottom grain size pattern of the bay is variable, with a dominance of silt and clay in the interior, where hydrodynamism is reduced, and a gradual change on approaching the entrance, with coarse sand and low concentrations of organic matter (Kjerfve et al., 2001KJERFVE, B., LACERDA, L.D. and DIAS, G.M.T., 2001. Baía de Guanabara, Rio de Janeiro, Brazil. In: U. SEELIGER and B. KJERFVE, eds. Coastal marine ecosystems of Latin America. New York: Springer-Verlag, pp. 107-117.; Quaresma et al., 2000QUARESMA, V.S., DIAS, G.T.M. and BAPTISTA NETO, J.A., 2000. Caracterização da ocorrência de padrões de sonar de varredura lateral e sísmica de alta freqüência (3,5 e 7,0 kHz) na porção sul da Baía de Guanabara – RJ. Brazilian Journal of Geophysics, vol. 18, no. 2, pp. 201-214. http://dx.doi.org/10.1590/S0102-261X2000000200008.
http://dx.doi.org/10.1590/S0102-261X2000...
). Composition of the sediment is mainly characterized by mixed fractions combining sand, silt and clay (Amador, 1997AMADOR, E.S., 1997. Baía de Guanabara e ecossistemas periféricos: homem e natureza. Rio de Janeiro: Reproarte Gráfica e Editora, 539 p., 2012AMADOR, E.S., 2012. Bacia da Baía de Guanabara: características geoambientais e ecossistemas. Rio de Janeiro: Editora Interciência, 406p.; Kjerfve et al., 2001KJERFVE, B., LACERDA, L.D. and DIAS, G.M.T., 2001. Baía de Guanabara, Rio de Janeiro, Brazil. In: U. SEELIGER and B. KJERFVE, eds. Coastal marine ecosystems of Latin America. New York: Springer-Verlag, pp. 107-117.; Quaresma et al., 2000QUARESMA, V.S., DIAS, G.T.M. and BAPTISTA NETO, J.A., 2000. Caracterização da ocorrência de padrões de sonar de varredura lateral e sísmica de alta freqüência (3,5 e 7,0 kHz) na porção sul da Baía de Guanabara – RJ. Brazilian Journal of Geophysics, vol. 18, no. 2, pp. 201-214. http://dx.doi.org/10.1590/S0102-261X2000000200008.
http://dx.doi.org/10.1590/S0102-261X2000...
). Sediments at the intermediary and inner stations are fine (silt and clay) (Quaresma et al., 2000QUARESMA, V.S., DIAS, G.T.M. and BAPTISTA NETO, J.A., 2000. Caracterização da ocorrência de padrões de sonar de varredura lateral e sísmica de alta freqüência (3,5 e 7,0 kHz) na porção sul da Baía de Guanabara – RJ. Brazilian Journal of Geophysics, vol. 18, no. 2, pp. 201-214. http://dx.doi.org/10.1590/S0102-261X2000000200008.
http://dx.doi.org/10.1590/S0102-261X2000...
). Ten random replicates of the sediment were collected at each station over six surveys (n = 600).
The bay is classified as a subtropical eutrophic estuary (Paranhos et al., 1993PARANHOS, R., MAYR, L.M., LAVRADO, H.P. and CASTILHO, P.C., 1993. Temperature and salinity trends in Guanabara Bay (Brazil) from 1980 to 1990. Arquivos de Biologia e Tecnologia, vol. 36, no. 4, pp. 685-694.; Paranhos and Mayr, 1993PARANHOS, R. and MAYR, L.M., 1993. Seasonal patterns of temperature and salinity in Guanabara Bay, Brazil. Fresenius Environmental Bulletin, vol. 2, no. 11, pp. 647-652.; Valentin et al., 1999VALENTIN, J.L., TENENBAUM, D.R., BONECKER, A.C.T., BONECKER, S.L.C., NOGUEIRA, C.R. and VILLAC, M.C., 1999. O sistema planctônico da Baía de Guanabara: síntese do conhecimento. In: S.H.G. SILVA and H.P. LAVRADO, eds. Ecologia de Ambientes Costeiros do Estado do Rio de Janeiro. Rio de Janeiro: PPGE-UFRJ, pp. 35-59. Série Oecologia Australis.), with rainy (December, January, February and March) and dry (June, July and August) seasons (Amador, 1997AMADOR, E.S., 1997. Baía de Guanabara e ecossistemas periféricos: homem e natureza. Rio de Janeiro: Reproarte Gráfica e Editora, 539 p., 2012AMADOR, E.S., 2012. Bacia da Baía de Guanabara: características geoambientais e ecossistemas. Rio de Janeiro: Editora Interciência, 406p.; Paranhos and Mayr, 1993PARANHOS, R. and MAYR, L.M., 1993. Seasonal patterns of temperature and salinity in Guanabara Bay, Brazil. Fresenius Environmental Bulletin, vol. 2, no. 11, pp. 647-652.). The constant continental and marine influx of nutrients, plus abundant sunshine, favors the blooming of surface algae (Aguiar et al., 2011AGUIAR, V.M.C., BAPTISTA NETO, J.A. and RANGEL, C.M., 2011. Eutrophication and hypoxia in four streams discharging in Guanabara Bay, RJ, Brazil, a case study. Marine Pollution Bulletin, vol. 62, no. 8, pp. 1915-1919. http://dx.doi.org/10.1016/j.marpolbul.2011.04.035. PMid:21708390.
http://dx.doi.org/10.1016/j.marpolbul.20...
; Valentin et al., 1999VALENTIN, J.L., TENENBAUM, D.R., BONECKER, A.C.T., BONECKER, S.L.C., NOGUEIRA, C.R. and VILLAC, M.C., 1999. O sistema planctônico da Baía de Guanabara: síntese do conhecimento. In: S.H.G. SILVA and H.P. LAVRADO, eds. Ecologia de Ambientes Costeiros do Estado do Rio de Janeiro. Rio de Janeiro: PPGE-UFRJ, pp. 35-59. Série Oecologia Australis.). The highest temperatures occur on the surface during the summer, and the lowest close to the bottom during sporadic SACW (South Atlantic Central Water) intrusion (Paranhos and Mayr, 1993PARANHOS, R. and MAYR, L.M., 1993. Seasonal patterns of temperature and salinity in Guanabara Bay, Brazil. Fresenius Environmental Bulletin, vol. 2, no. 11, pp. 647-652.).
Salinity varies progressively from the continental region (lowest) towards the interior of the bay, where it is highest close to the bottom (higher density) (Amador, 2012AMADOR, E.S., 2012. Bacia da Baía de Guanabara: características geoambientais e ecossistemas. Rio de Janeiro: Editora Interciência, 406p.; Kjerfve et al., 1997KJERFVE, B., RIBEIRO, C.H.A., DIAS, G.T.M., FILIPPO, A.M. and QUARESMA, V.S., 1997. Oceanographic characteristics of an impacted coastal bay: baía de Guanabara, Rio de Janeiro, Brazil. Continental Shelf Research, vol. 17, no. 13, pp. 1609-1643. http://dx.doi.org/10.1016/S0278-4343(97)00028-9.
http://dx.doi.org/10.1016/S0278-4343(97)...
; Paranhos and Mayr, 1993PARANHOS, R. and MAYR, L.M., 1993. Seasonal patterns of temperature and salinity in Guanabara Bay, Brazil. Fresenius Environmental Bulletin, vol. 2, no. 11, pp. 647-652.). Due to the high nutrient load and light availability, Guanabara Bay is considered one of the most productive ecosystems in the world, with high levels of day by day carbon assimilation (Carreira et al., 2002CARREIRA, R.S., WAGENER, A.L.R., READMAN, J.W., FILEMAN, T.W., MACKO, S.A. and VEIGA, A., 2002. Change in the sedimentary organic carbon pool of a fertilized tropical estuary, Guanabara Bay, Brazil: an elemental, isotopic and molecular marker approach. Marine Chemistry, vol. 79, no. 3-4, pp. 207-227. http://dx.doi.org/10.1016/S0304-4203(02)00065-8.
http://dx.doi.org/10.1016/S0304-4203(02)...
, 2004CARREIRA, R.S., WAGENER, A.L.R. and READMAN, J.W., 2004. Sterols as makers of sewage contamination in a tropical urban estuary (Guanabara Bay, Brazil): space-time variations. Estuarine, Coastal and Shelf Science, vol. 60, no. 24, pp. 587-598. http://dx.doi.org/10.1016/j.ecss.2004.02.014.
http://dx.doi.org/10.1016/j.ecss.2004.02...
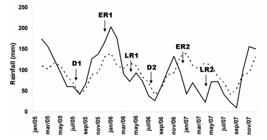
). The seasonal pattern in the estuary of the bay is in accordance with abiotic parameters, with lower temperatures and higher salinity from May to September (dry period), and the inverse from October to April (rainy season) (Paranhos and Mayr, 1993PARANHOS, R. and MAYR, L.M., 1993. Seasonal patterns of temperature and salinity in Guanabara Bay, Brazil. Fresenius Environmental Bulletin, vol. 2, no. 11, pp. 647-652.; Figure 2).
Climate pattern (1961-1990) in the Rio de Janeiro region (dashed line) and the average monthly accumulated rainfall (continuous line) during the study period (2005, 2006 and 2007). Sampling occurred in the dry (D1 – July, 2005; D2 – July, 2006), early rainy (ER1 – December, 2005; ER2 – December, 2006) and late rainy (LR1 – April, 2006; LR2 – April, 2007) seasons. Data modified from INMET (2008)INSTITUTO NACIONAL DE METEREOLOGIA – INMET [online], 2008 [viewed 14 December 2008]. Available from: inmet.gov.br/html/observacoes.php.
2.2. Macrobenthic sampling
The sampling design were carried out in six surveys, determined according to historical rainfall data (normal climatological 61-90) provided by the National Institute of Meteorology (INMET, 2008INSTITUTO NACIONAL DE METEREOLOGIA – INMET [online], 2008 [viewed 14 December 2008]. Available from: inmet.gov.br/html/observacoes.php; Figure 2). Sediment were sampled with Gravity corer (0.0078 m2 per replicate). Sampling occured at 10 georeferenced stations distributed throughout the bay (Figure 1). Sieve patterns at the BG 02, BG 03 and BG 09 (entrance) stations were different from the others. Sediments at the Intermediary and Inner stations were fine (silt and clay) (Quaresma et al., 2000QUARESMA, V.S., DIAS, G.T.M. and BAPTISTA NETO, J.A., 2000. Caracterização da ocorrência de padrões de sonar de varredura lateral e sísmica de alta freqüência (3,5 e 7,0 kHz) na porção sul da Baía de Guanabara – RJ. Brazilian Journal of Geophysics, vol. 18, no. 2, pp. 201-214. http://dx.doi.org/10.1590/S0102-261X2000000200008.
http://dx.doi.org/10.1590/S0102-261X2000...
). Ten random replicates of the sediment were collected at each station over six seasons (n = 600). After washing through 500 μm mesh (macrobenthic), and fixing in alcohol 70%, samples were screened and identified in the laboratory by stereoscope microscopy.
2.3. Bottom water and sediment data
Twice a week samples of background water were retrieved at the stations of original collection. All georeferenced data, are available in the database of the Guanabara Bay Environmental Assessment Program (Petrobras, 2012aPETRÓLEO BRASILEIRO S.A. – PETROBRAS, 2012a. Baía de Guanabara: ambiente e influência antrópica. Rio de Janeiro, 337 p. (vol. I)., bPETRÓLEO BRASILEIRO S.A. – PETROBRAS, 2012b. Baía de Guanabara: síntese do conhecimento ambiental: biodiversidade. Rio de Janeiro, 479 p. (vol. II).). Water chemistry variables were determined in triplicate using standard oceanographic methods (Grasshoff et al., 1999GRASSHOFF, K., KREMLING, K. and ERHARDT, M. 1999. Methods of seawater analysis. 3rd ed. Berlin: Wiley-VCH Verlag, 600 p. http://dx.doi.org/10.1002/9783527613984.
http://dx.doi.org/10.1002/9783527613984...
; Parsons et al., 1984PARSONS, T.R., MAITA, Y. and LALLI, C.M., 1984. A manual of chemical and biological methods for seawater analysis. 2nd ed. Oxford: Pergamon Press, 173 p.). Temperature, salinity, and pH were measured in situ using a Multi Probe System YSI 556 (YSI Incorporated, USA). Salinity was also determined by titration of chlorine against standard seawater (Ocean Scientific International Ltd. - OSIL). Dissolved oxygen was determined by Winkler titration method. Ammonia was measured using the indophenol method, nitrite by diazotation, and nitrate via reduction in a Cd-Cu column followed by diazotation. Total nitrogen (TN) was calculated after alkali digestion to nitrate. Orthophosphate was estimated using the molybdate method, total phosphorus (TP) by acid digestion to phosphate, and silicate using a molybdate reaction. Nutrient standards from OSIL were used in conjunction with calibration curves. Chlorophyll a analyses were performed after gentle vacuum filtration (< 25 cm of Hg) onto cellulose membrane filters (Millipore HAWP 0.45 µm). Filters were extracted overnight in 90% acetone at 4 °C and analyzed with a UV-VIS Lambda 25 spectrophotometer (Perkin Elmer, USA) and a Tuner TD-700 fluorometer both calibrated with pure chlorophyll from Sigma. Sediment variables were analyzed in established periods during the dry and rainy seasons, in all ten. The sediments analysis were elaborated using Wentworth scale was applied to coarse fractions. Fine fractions, characterized by being flaky (<0.0062 mm), were analyzed with the pipetting method (Suguio, 1973SUGUIO, K., 1973. Introdução a sedimentologia. São Paulo, Edgard Blucher/EDUSP, 317 p.). Granulometric classification followed Folk and Ward (1958)FOLK, R. and WARD, W.C., 1958. Brazos River bar: a study in the significance of grain-size parameters. Journal of Sedimentary Petrology, vol. 27, no. 1, pp. 3-26. http://dx.doi.org/10.1306/74D70646-2B21-11D7-8648000102C1865D.
http://dx.doi.org/10.1306/74D70646-2B21-...
, Flemming (2000)FLEMMING, B., 2000. A revised textural classification of gravel-free muddy sediments on the basis of ternary diagrams. Continental Shelf Research, vol. 20, no. 10-11, pp. 1125-1137. http://dx.doi.org/10.1016/S0278-4343(00)00015-7.
http://dx.doi.org/10.1016/S0278-4343(00)...
and Shepard (1954)SHEPARD, F., 1954. Nomenclature based on sand-silt-clay rations. Journal of Sedimentary Research, vol. 24, pp. 151-158..
2.4. Environmental variables
Sixteen environmental variables were collected. These were classified into three groups, structural, pollution indicator and Marine Intrusion (SACW - South Atlantic Central Water) indicator. Structural variables (SV), involved sediment structure features, viz., sand fractions, silt, clay, asymmetry and selection, and are only marginally affected by pollution variables. Pollution Indicator Variables (PIV), basically grouped together are ammonia (NH3), nitrite (NO2), suspended particulate matter (MPS), chlorophyll (CLO), total nitrogen (NTs) and total phosphorus (TP). Marine intrusion (SACW) indicator variables (MIV) are characterized according to high nitrate (NO3), phosphate (PO3) and dissolved oxygen (DO), and low temperature (TEMP), values.
2.5. Redundancy Analysis and PERMANOVA
This procedure facilitates visualizing holistically the characteristics of the ecosystem, as well as the spatial and temporal correlations of fauna to seasonality (abiotic variables). It comprises a combination of multiple regression and Principal Component Analysis (PCA), a direct extension of regression analysis to model multivariate response data (Borcard et al., 2011BORCARD, D., GILLET, F. and LEGENDRE, P., 2011. Numerical ecology with R. New York: Springer, 306 p. http://dx.doi.org/10.1007/978-1-4419-7976-6.
http://dx.doi.org/10.1007/978-1-4419-797...
), with the same assumptions for PCA with explanatory variables. However, in order to eliminate variable excess and select only the explanatory, Parsimonious RDA was also applied, in which only the important variables selected by multiple regression were included in the model. These results are interesting, since by demonstrating the most important explanatory variables for the model, they comprise a highly significant model with no harmful collinearity (Borcard et al., 2011BORCARD, D., GILLET, F. and LEGENDRE, P., 2011. Numerical ecology with R. New York: Springer, 306 p. http://dx.doi.org/10.1007/978-1-4419-7976-6.
http://dx.doi.org/10.1007/978-1-4419-797...
). The PERMANOVA (Permutational Multivariate Analysis of Variance) is a nonparametric method to test multivariate differences among spatial, temporal and null hypothesis (Anderson, 2001ANDERSON, M.J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology, vol. 26, pp. 32-46.).
3. Results
The 18,108 collected specimens were distributed among 124 taxa. The species Heleobia australis (Gastropoda) was the most dominant (10,403 ind. ~58%). The others dominants were Cyprideis sp. (Ostracoda/1,802 ind. ~ 10%), Americuna besnardi (Gastropoda/768 ind. ~4.5%), Cyprideis salebrosa (Ostracoda/486 ind. 2.7%), Ervilia concentrica (Gastropoda/377 ind. 2.1%), Mytilidae (350 ind. ~2%), Cypridinidae (347 ind. ~2%) and the remaining taxa a total of the 3,575 ind. distributed in 117 taxa with percentages below 2%.
The seven dominant species totalized 14.533 individuals and represented 81.3% with indicator species in each sector of the Guanabara Bay.
The canonical ordering analyses (redundancy analysis) with abiotic variables and all taxa evidencing a distribution by sectors (Figure 3).
RDA of abiotic and biotic data for two years (Acronyms in appendix A Appendix A Acronyms taxa/species list. LIS Listriella titinga AMPHIPODA MIC Microphoxus breviramus AMPHIPODA TIB Tiburonella viscana AMPHIPODA GIB Giberosus sp. AMPHIPODA BIR Birubius sp. AMPHIPODA COR Corophiidae AMPHIPODA EUD Eudevenopus sp. AMPHIPODA EUR Eurydice sp. AMPHIPODA MCR Macrochiridothea sp. AMPHIPODA ERI Ericthonius brasiliensis BIVALVIA NUC Nucula semiornata BIVALVIA CAR Carditamera micella BIVALVIA AME Americuna besnardi BIVALVIA CRA1 Crassinella marplatensis BIVALVIA CRA2 Crassinella martinicensis BIVALVIA ERV Ervilia concentrica BIVALVIA SEM1 Semele nuculoides BIVALVIA SEM2 Semele purpurascens BIVALVIA CHI Chione cancellata BIVALVIA MUS Musculus lateralis BIVALVIA BOT Botula fusca BIVALVIA ANO Anomalocardia brasiliana BIVALVIA GOU Gouldia cerina BIVALVIA TRA1 Transennella cubaniana BIVALVIA TRA2 Transennella stimpsoni BIVALVIA THR Thracia similis BIVALVIA LAS Lasaea adansoni BIVALVIA ABR Abra cf uruguayensis BIVALVIA TEL Tellina exerythra BIVALVIA COR Corbula cubaniana BIVALVIA LUC Lucina pectinata BIVALVIA CTE Ctena pectinella BIVALVIA HIA Hiatella arctica BIVALVIA MOD Modiolus carvalhoi BIVALVIA PIC Pinctada imbricata BIVALVIA MDL Modiolus sp. BIVALVIA CTN Ctena sp. BIVALVIA SML Semele sp. BIVALVIA TLN Tellina sp. BIVALVIA OLV Olivella sp. BIVALVIA MTL Mytilidae BIVALVIA HUT Hutchinsoniella macracantha CEPHALOCARIDA NEB Neballa sp. CEPHALOCARIDA CUM Cumacea CUMACEA PIN Pinnixa chaetopterana DECAPODA POR Portunus ventralis DECAPODA PRO Processa hemphilli DECAPODA UPO Upogebia omissa DECAPODA PAG Paguridae DECAPODA ALB Albunea paretti DECAPODA CRO Cronius sp. DECAPODA CAE1 Caecum brasilicum GASTROPODA GAB Gabrielona sulcifera GASTROPODA BIT Bittiolum varium GASTROPODA CAE2 Caecum someri GASTROPODA CAE3 Caecum ryssotitum GASTROPODA FIN Finella dubia GASTROPODA HEL Heleobia australis GASTROPODA NAT Natica pusilla GASTROPODA OLI Olivella minuta GASTROPODA TEI Teinostoma cocolitoris GASTROPODA PAR Parviturboides interruptus GASTROPODA AES Aesopus stearnsii GASTROPODA MEL Melanella arcuata GASTROPODA ALV Alvania faberi GASTROPODA ANA Anachis isabellei GASTROPODA ACT1 Acteocina bidentata GASTROPODA ACT2 Acteocina bullata GASTROPODA NAS Nassarius vibex GASTROPODA CRY Chrysallida sp. GASTROPODA ODS Odostomia sp. GASTROPODA TRB Turbonilla sp. GASTROPODA CRT Cerithiopsis sp. GASTROPODA EPT Epitonium sp. GASTROPODA MLN Melanella sp. GASTROPODA NTC Natica sp. GASTROPODA RSN Rissoina sp. GASTROPODA MYS Mysidacea MYSIDACEA AUR Aurila ornellasae OSTRACODA CSA Cyprideis salebrosa OSTRACODA CYP Cyprideis sp. OSTRACODA BAR Bairdiidae OSTRACODA CYT Cytherideidae OSTRACODA CYL Cylindroleberididae OSTRACODA MAC Macrocyprina sp. OSTRACODA CYP Cypridinidae OSTRACODA URO Urocythereis sp. OSTRACODA HEM Hemicytheridae OSTRACODA CAP Capitella capitata POLYCHAETA ARI Aricidea (Acmira) taylori POLYCHAETA GYP Gyptis callithrix POLYCHAETA ORB Orbinia johnsoni POLYCHAETA PAR Paraprionospio pinnata POLYCHAETA SPI1 Spio quadrisetosa POLYCHAETA OWE Owenia fusiformis POLYCHAETA NAI Naineris setosa POLYCHAETA SIG Sigalion taquari POLYCHAETA MAG Magelona crenulata POLYCHAETA GLY Glycera americana POLYCHAETA GON Goniadides carolinae POLYCHAETA SPI2 Spiochaetopterus nonatoi POLYCHAETA POL Polydora websteri POLYCHAETA STR Streblospio benedicti POLYCHAETA SCO Scoloplos sp. POLYCHAETA ALL Allia sp. POLYCHAETA ARC Aricidea sp. POLYCHAETA HMP Hemipodia sp. POLYCHAETA GND Goniada sp. POLYCHAETA ONP Onuphidae POLYCHAETA KIN Kinbergonuphis sp. POLYCHAETA PIO Pionosyllis sp. POLYCHAETA MSC Mesochaetopterus sp. POLYCHAETA THR Tharyx sp. POLYCHAETA MGL Magelona sp. POLYCHAETA PCL Poecilochaetus sp. POLYCHAETA SAB Sabellidae POLYCHAETA SPN Spionidae POLYCHAETA APS Apoprionospio sp. POLYCHAETA DSP Dispio sp. POLYCHAETA PNS Prionospio sp POLYCHAETA LMP Limopsis sp POLYCHAETA KAL Kalliapseudes schubarti TANAIDACEA SKU Skuphonura sp. TANAIDACEA TAN Tanaidacea TANAIDACEA and B Appendix B Acronyms environmental variables list. TEMP Temperature DO Dissolved Oxygen ORT Orthophosphate NH4 Ammonia NO2 Nitrite NO3 Nitrate CLO Chlorophyll SAL Salinity TPs Total Phosphorus NTs Total Nitrogen SPM Suspended Particulate Material FSAN Fine sand MSAN Medium sand SOR Sorting SKW Skewness ).
The RDA for the two annual cycles defined three areas, such as: Entrance, Intermediary and Inner. The entrance sector was characterized for eigenvalues of the nitrate (NO3), salinity (SAL), dissolved oxygen (OD) and middle (MD_SAND) and fine sand (FINE_SAND) with Ervilia concentrica (ERV) and Cypridinidae (CYP1) are indicators taxa. The Intermediary sector had eigenvalues of the total phosphate (FTs), chlorophyll (CLO) and sorting sediment (SOR) with Cyprideis salebrosa (CSA) and Cyprideis sp. (CYP) as dominant taxa. The Inner sector had eigenvalues of the total nitrogen (NTs), ammonia (NH4), clay (CLAY) and skewness (SKW) with Heleobia australis (HEL) as dominant species.
The RDA partially identified only seven explanatory variables (Figure 4). At the Entrance sector, these variables were fine sand (FINE_SAND) and nitrate (NO3) and dissolved oxygen (OD). There was aggregation of stations BG 02 and BG 03 into different rainfall periods, and the most dominant/indicator was the Cypridinidae and Ervilia concentrica. At the Intermediary sector, suspended particulate material (MPS) and skewness (SKW) were the only explanatory variables that most defined a sector comprising stations BG 09, BG 10, BG 13 and BG 14, and the most dominant/indicator species was Cyprideis salebrosa and Cyprideis sp. While at the Inner sector, had clay (CLAY) and Heleobia australis as the most dominant/indicator species.
Partially RDA for two years showing the most significant explanatory variables were FINE SAND, NO3, OD, MPS, SKW, NO2 and CLAY.
The Redundancy Analysis of the first annual cycle (2005-2006) (Figure 5) continues to indicated three sectors (Entrance, Intermediary and Inner). Ervilia concentrica (ERV) and Cypridinidae (CYP1) was indicator in Entrance sector, as indicated by dissolved oxygen (DO), fine sand (FSAN) and nitrate (NO3). Cyprideis salebrosa and Cyprideis sp. were indicators at the Intermediary sector with high values of suspended particulate matter (MPS) and skewness (SKW). At the Inner sector, the explanatory variables were nitrate (NO2) and clay (CLAY), with Heleobia australis as indicator species.
The Partially RDA of the first annual cycle (2005-2006) (Figure 6) evidenced five explanatory variables, as follows: sorting (SOR), dissolved oxygen (OD), salinity (SAL), middle sand (MD_SAND) and nitrite (NO2). The Entrance sector was characterized by high concentrations of dissolved oxygen (DO), salinity (SAL) and middle sand (MD_SAND), while Ervilia concentrica (ERV) and Cypridinidae (CYP1) were indicator species. At the Intermediary sector, sorting (SOR) and Cyprideis salebrosa and Cyprideis sp. as indicator species, and, at the Inner sector, nitrite (NO2) was the unique explanatory variable observed with Heleobia australis (HEL) as species indicator.
Partially RDA of the first year (2005-2006) showing the most significant explanatory variables were SOR, OD, SAL, MD_SAND and NO2.
The RDA of the second cycle (2006-2007) (Figure 7) showed three sectors well defined. The Entrance sector had middle sand (MD_SAND), fine sand (FINE_SAND), nitrate (NO3) and salinity (SAL) as explanatory variables, while Ervilia concentrica (ERV) and Cypridinidae (CIP) as indicator species. At the Intermediary sector, skewness (SKW) was the explanatory variable, with Cyprideis salebrosa and Cyprideis sp. as indicator species, and, at the Inner sector, phosphate (TFs) and Heleobia australis as indicator species.
The Partially RDA for the second annual cycle (2006-2007) (Figure 8) evidenced only four explanatory variables, as follows, middle sand (MD_SAND), fine sand (FINE_SAND), nitrate (NO3) and skewness (SKW). Thus, the Entrance sector was characterized by middle and fine sand, and nitrate, while Ervilia concentrica (ERV) and Cypridinidae (CYP) were the indicator species. At the Intermediary sector, Cyprideis salebrosa (CSA) Cyprideis sp. (CYP) were the indicator species, and finally, at the Inner sector, skewness (SKW) and Heleobia australis, the indicator species.
Partially RDA of the second year (2006-2007) showing the most significant explanatory variables were MD_SAND, FINE_SAND, NO3 and SKW).
Exploratory analysis showed the existence of patterns and associations. Thus, PERMANOVA was applied to evaluate the existence of intra and inter-annual variations within the three sectors, during the periods (dry, early rainy and late rainy), along both annual cycles (2005-2006 and 2006-2007) (Table 1). It was possible to note significant differences between the sectors as proposed, and the surveys (respectively, p<0.001 and p=0.002). On the other hand, temporal analysis indicated cycles extremely well defined (p <0.001), even though surveys of the first cycle presented significant differences, thereby corroborating the hypothesis that rainfall acts as a regulator in estuarine dynamics. In the second annual cycle (2006-2007), there were also indications of significant differences between the sectors, although not observed in the surveys.
Macrobenthhos soft-bottom between the years according to the proposed sectors (External, Intermediary and Inner) along Guanabara Bay.
The observed results of RDAs and the high representation of dominant taxa suggested very similar results for PERMANOVA (Table 2), where sectors are well defined and, moreover, shows the setorization defined along the years.
Dominant macrobenthos soft-bottom PERMANOVA (Heleobia australis, Cyprideis salebrosa, Americuna besnardii, Ervilia concentrica, Cyprideis sp. and Cypridinidae) between the years according to the proposed sectors (External, Intermediary and Inner) along Guanabara Bay.
4. Discussion
Seasonality in Guanabara Bay bottom water is defined by two natural forces, SACW (South Atlantic Central Water) marine intrusion during the summer months, and a well-defined rainfall regime, split into dry and rainy periods (INMET, 2008INSTITUTO NACIONAL DE METEREOLOGIA – INMET [online], 2008 [viewed 14 December 2008]. Available from: inmet.gov.br/html/observacoes.php; Amador, 1997AMADOR, E.S., 1997. Baía de Guanabara e ecossistemas periféricos: homem e natureza. Rio de Janeiro: Reproarte Gráfica e Editora, 539 p.; Filippo, 1997FILIPPO, A.M., 1997. Passagem de frente frias na Baía de Guanabara, RJ – Brasil: impacto no nível do mar. Niterói: Universidade Federal Fluminense. Dissertação de Mestrado em Geoquímica Ambiental, 79 p.; Kjerfve et al., 1997KJERFVE, B., RIBEIRO, C.H.A., DIAS, G.T.M., FILIPPO, A.M. and QUARESMA, V.S., 1997. Oceanographic characteristics of an impacted coastal bay: baía de Guanabara, Rio de Janeiro, Brazil. Continental Shelf Research, vol. 17, no. 13, pp. 1609-1643. http://dx.doi.org/10.1016/S0278-4343(97)00028-9.
http://dx.doi.org/10.1016/S0278-4343(97)...
; Valentin et al., 1999VALENTIN, J.L., TENENBAUM, D.R., BONECKER, A.C.T., BONECKER, S.L.C., NOGUEIRA, C.R. and VILLAC, M.C., 1999. O sistema planctônico da Baía de Guanabara: síntese do conhecimento. In: S.H.G. SILVA and H.P. LAVRADO, eds. Ecologia de Ambientes Costeiros do Estado do Rio de Janeiro. Rio de Janeiro: PPGE-UFRJ, pp. 35-59. Série Oecologia Australis.; Figure 2).
SACW intrusion during the summer (November to March) gives rise to drops in temperatures (<15 °C), high dissolved oxygen, nitrate, phosphate and silicate rates, and pronounced bottom water eutrophication (Mendes et al., 2012MENDES, F., FIGUEIREDO, G.M. and VALENTIN, J.L., 2012. Reproduction and structure of the population of the chaetognath Parasagitta friderici in Guanabara Bay (Brazil) based on short term sampling. Anais da Academia Brasileira de Ciências, vol. 84, no. 1, pp. 103-112. http://dx.doi.org/10.1590/S0001-37652012005000008. PMid:22441599.
http://dx.doi.org/10.1590/S0001-37652012...
; Paranhos and Mayr, 1993PARANHOS, R. and MAYR, L.M., 1993. Seasonal patterns of temperature and salinity in Guanabara Bay, Brazil. Fresenius Environmental Bulletin, vol. 2, no. 11, pp. 647-652.; Petrobras 2012aPETRÓLEO BRASILEIRO S.A. – PETROBRAS, 2012a. Baía de Guanabara: ambiente e influência antrópica. Rio de Janeiro, 337 p. (vol. I)., bPETRÓLEO BRASILEIRO S.A. – PETROBRAS, 2012b. Baía de Guanabara: síntese do conhecimento ambiental: biodiversidade. Rio de Janeiro, 479 p. (vol. II).; Santi and Tavares, 2009SANTI, L. and TAVARES, M., 2009. Polychaeta assemblage of an impacted estuary, Guanabara Bay, Rio de Janeiro, Brazil. Brazilian Journal of Oceanography, vol. 57, no. 4, pp. 287-303. http://dx.doi.org/10.1590/S1679-87592009000400004.
http://dx.doi.org/10.1590/S1679-87592009...
; Silva and Valentin, 1988SILVA, M. N. L. J. L. and VALENTIN, J. L., 1988. O Microfitoplâncton das Águas Costeiras do Litoral Fluminense (Estado do Rio de Janeiro): Lista de Espécies e Aspectos Ecológicos. Boletim Do Instituto Oceanográfico, vol. 36, no.1, pp. 1-16.; Villac et al., 1991VILLAC, M.C., MAYR, L.M., TENENBAUM, D.R. and PARANHOS, R., 1991. Sampling strategies proposed to monitor Guanabara Bay, RJ, Brazil. In: O.T. MAGOON, H.V. CONVERSE, V. TIPPIE, L.T. TOBIN and D. CLARCK, eds. Coastal Zone’91. New York: American Society of Civil Engineers, pp. 1168-1182.). Hence, during this period, a temporary dry season may occur, with low rainfall throughout the bay (Filippo, 1997FILIPPO, A.M., 1997. Passagem de frente frias na Baía de Guanabara, RJ – Brasil: impacto no nível do mar. Niterói: Universidade Federal Fluminense. Dissertação de Mestrado em Geoquímica Ambiental, 79 p.; Mayr et al., 1989MAYR, L.M., TENENBAUM, D.R., VILLAC, M.C., PARANHOS, R., NOGUEIRA, C.R., BONECKER, S. and BONECKER, A., 1989. Hidrobiological characterization of Guanabara Bay. In: O. MAGOON and C. NEVES, eds. Costalines of Brazil. New York: American Society of Civil Engineers, pp. 124-138.). Under these conditions, variation between surface and bottom layer temperatures can induce strong stratification (Mendes et al., 2012MENDES, F., FIGUEIREDO, G.M. and VALENTIN, J.L., 2012. Reproduction and structure of the population of the chaetognath Parasagitta friderici in Guanabara Bay (Brazil) based on short term sampling. Anais da Academia Brasileira de Ciências, vol. 84, no. 1, pp. 103-112. http://dx.doi.org/10.1590/S0001-37652012005000008. PMid:22441599.
http://dx.doi.org/10.1590/S0001-37652012...
), as recorded during the dry periods of both annual cycles studied herein.
Apart from summer SACW stratification (Mayr et al., 1989MAYR, L.M., TENENBAUM, D.R., VILLAC, M.C., PARANHOS, R., NOGUEIRA, C.R., BONECKER, S. and BONECKER, A., 1989. Hidrobiological characterization of Guanabara Bay. In: O. MAGOON and C. NEVES, eds. Costalines of Brazil. New York: American Society of Civil Engineers, pp. 124-138.), seasonal patterns are also evident by the more intensive biological activities, as expressed by higher chlorophyll and bacterial production, normal for the warmer months of the year (Paranhos et al., 2001PARANHOS, R., ANDRADE, L., MENDONÇA-HAGLER, L.C. and PFEIFFER, W.C. 2001. Coupling bacterial density with activity in a tropical polluted coastal bay. In: B.M. FARIA, V. FARJALLA and F.A. ESTEVES, eds. Aquatic microbial ecology in Brazil. Rio de Janeiro: PPGE-UFRJ, pp. 117-132. Serie Oecologia Brasiliensis, no. 9.). The estuary of the bay is composed by a variety of sediments (Amador, 1997AMADOR, E.S., 1997. Baía de Guanabara e ecossistemas periféricos: homem e natureza. Rio de Janeiro: Reproarte Gráfica e Editora, 539 p., 2012AMADOR, E.S., 2012. Bacia da Baía de Guanabara: características geoambientais e ecossistemas. Rio de Janeiro: Editora Interciência, 406p.; Catanzaro et al., 2004CATANZARO, L.F., BAPTISTA NETO, J.A., GUIMARÃES, M.S.D. and SILVA, C.G., 2004. Distinctive Sedimentary Processes in Guanabara Bay – SE/Brazil, based on the analysis of echo-character (7.0 khz). Revista Brasileira de Geofísica, vol. 22, no. 1, pp. 69-83. http://dx.doi.org/10.1590/S0102-261X2004000100006.
http://dx.doi.org/10.1590/S0102-261X2004...
; Kjerfve et al., 1997KJERFVE, B., RIBEIRO, C.H.A., DIAS, G.T.M., FILIPPO, A.M. and QUARESMA, V.S., 1997. Oceanographic characteristics of an impacted coastal bay: baía de Guanabara, Rio de Janeiro, Brazil. Continental Shelf Research, vol. 17, no. 13, pp. 1609-1643. http://dx.doi.org/10.1016/S0278-4343(97)00028-9.
http://dx.doi.org/10.1016/S0278-4343(97)...
; Quaresma et al., 2000QUARESMA, V.S., DIAS, G.T.M. and BAPTISTA NETO, J.A., 2000. Caracterização da ocorrência de padrões de sonar de varredura lateral e sísmica de alta freqüência (3,5 e 7,0 kHz) na porção sul da Baía de Guanabara – RJ. Brazilian Journal of Geophysics, vol. 18, no. 2, pp. 201-214. http://dx.doi.org/10.1590/S0102-261X2000000200008.
http://dx.doi.org/10.1590/S0102-261X2000...
). Granulometric structures with the dominance of silt and clay occurred at all the stations located in the Intermediary and Inner sectors (BG 10, BG 13, BG 14, BG 18, BG 19, BG 25 and BG 28) (Petrobras, 2012aPETRÓLEO BRASILEIRO S.A. – PETROBRAS, 2012a. Baía de Guanabara: ambiente e influência antrópica. Rio de Janeiro, 337 p. (vol. I).). Here, hydrodynamics was reduced, and sediment richness in organic matter low, as were dissolved oxygen levels, with well defined anoxic layers (Baptista Neto and Silva, 1996BAPTISTA NETO, J.A. and SILVA, M.A.M., 1996. Caracterização dos sedimentos de fundo e dinâmica sedimentar da Enseada de Jurujuba (Baía de Guanabara), Niterói, RJ. Pesquisas, vol. 23, no. 1-2, pp. 7-16.; Carreira et al., 2004CARREIRA, R.S., WAGENER, A.L.R. and READMAN, J.W., 2004. Sterols as makers of sewage contamination in a tropical urban estuary (Guanabara Bay, Brazil): space-time variations. Estuarine, Coastal and Shelf Science, vol. 60, no. 24, pp. 587-598. http://dx.doi.org/10.1016/j.ecss.2004.02.014.
http://dx.doi.org/10.1016/j.ecss.2004.02...
; Kjerfve et al., 2001KJERFVE, B., LACERDA, L.D. and DIAS, G.M.T., 2001. Baía de Guanabara, Rio de Janeiro, Brazil. In: U. SEELIGER and B. KJERFVE, eds. Coastal marine ecosystems of Latin America. New York: Springer-Verlag, pp. 107-117.). Nonetheless, at BG 09 station, even though located within the Intermediary sector, the singular granulometric structure was composed of low selective sediments, with the dominance of medium and fine sand fractions, silt and clay (Catanzaro et al., 2004CATANZARO, L.F., BAPTISTA NETO, J.A., GUIMARÃES, M.S.D. and SILVA, C.G., 2004. Distinctive Sedimentary Processes in Guanabara Bay – SE/Brazil, based on the analysis of echo-character (7.0 khz). Revista Brasileira de Geofísica, vol. 22, no. 1, pp. 69-83. http://dx.doi.org/10.1590/S0102-261X2004000100006.
http://dx.doi.org/10.1590/S0102-261X2004...
; Gray and Elliott, 2009GRAY, J.S. and ELLIOTT, M., 2009. Ecology of marine sediments. 2nd ed. Oxford: Oxford Biology, 256 p.). This structure favors an environment with a deeper oxide layer combined with high concentrations of organic matter.
In addition to environmental conditions, community dynamics, and biological pressures exerted by species from other ecological compartments, specific strategies, such as dispersion, tube formation, intra and interspecific relations can also contribute to characterize these communities (Gray and Elliott, 2009GRAY, J.S. and ELLIOTT, M., 2009. Ecology of marine sediments. 2nd ed. Oxford: Oxford Biology, 256 p.; Echeverría et al., 2010ECHEVERRÍA, C.A., NEVES, R.A.F., PESSOA, L.A. and PAIVA, P.C., 2010. Spatial and temporal distribution of the gastropod Heleobia australis in an eutrophic estuarine system suggests a metapopulation dynamics. Nature Science, vol. 2, no. 8, pp. 860-867.; Neves et al., 2013NEVES, R.A.F., ECHEVERRÍA, C.A., PESSOA, L.A., PAIVA, P.C., PARANHOS, R. and VALENTIN, J.L., 2013. Factors influencing spatial patterns of mollusks in a eutrophic tropical bay. Journal of the Marine Biological Association of the United Kingdom, vol. 93, no. 3, pp. 577-589. http://dx.doi.org/10.1017/S0025315412001105.
http://dx.doi.org/10.1017/S0025315412001...
; Pereira et al., 2013PEREIRA, V.P.P., PESSOA, L.A., FREITAS, M.A.V. and ECHEVERRÍA, C.A., 2013. Recovery process on soft-bottom macrobenthic communities after artificial disturbance in tropical polluted estuary (Guanabara Bay, Rio de Janeiro, Brazil). Open Journal of Marine Science, vol. 3, no. 04, pp. 161-166. http://dx.doi.org/10.4236/ojms.2013.34018.
http://dx.doi.org/10.4236/ojms.2013.3401...
; Negrello-Filho et al., 2018NEGRELLO-FILHO, O.A., UGAZ-CODINA, J.C., OLIVEIRA, L.H.S., SOUZA, M.C. and ÂNGULO, R.J., 2018. Subtidal soft sediments of the Paranaguá Bay inlet: mapping habitats and species distribution at a landscape scale. Brazilian Journal of Oceanography, vol. 66, no. 3, pp. 255-266. http://dx.doi.org/10.1590/s1679-875920180192406603.
http://dx.doi.org/10.1590/s1679-87592018...
; Pessoa et al., 2020PESSOA, L.A., PAIVA, P.C., PARANHOS, R., FREITAS, M.A.V. and ECHEVERRÍA, C.A., 2020. Intra-annual variation in rainfall and it’s influence of the adult’s Cyprideis spp (Ostracoda, Crustacea) on a eutrophic estuary (Guanabara Bay, Rio de Janeiro, Brazil). Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 80, no. 2, pp. 449-459. http://dx.doi.org/10.1590/1519-6984.216263. PMid:31482973.
http://dx.doi.org/10.1590/1519-6984.2162...
).
The composition of the macrobenthos of Guanabara Bay is composed of 124 taxa, with 7 taxa responsible for more than 80% of the total abundance. Most of species had low frequency and abundance. This pattern of few dominant species is found in highly impacted environments, reflecting the high dominance of opportunistic species (Echeverría et al., 2010ECHEVERRÍA, C.A., NEVES, R.A.F., PESSOA, L.A. and PAIVA, P.C., 2010. Spatial and temporal distribution of the gastropod Heleobia australis in an eutrophic estuarine system suggests a metapopulation dynamics. Nature Science, vol. 2, no. 8, pp. 860-867.; Neves et al., 2013NEVES, R.A.F., ECHEVERRÍA, C.A., PESSOA, L.A., PAIVA, P.C., PARANHOS, R. and VALENTIN, J.L., 2013. Factors influencing spatial patterns of mollusks in a eutrophic tropical bay. Journal of the Marine Biological Association of the United Kingdom, vol. 93, no. 3, pp. 577-589. http://dx.doi.org/10.1017/S0025315412001105.
http://dx.doi.org/10.1017/S0025315412001...
; Pereira et al., 2013PEREIRA, V.P.P., PESSOA, L.A., FREITAS, M.A.V. and ECHEVERRÍA, C.A., 2013. Recovery process on soft-bottom macrobenthic communities after artificial disturbance in tropical polluted estuary (Guanabara Bay, Rio de Janeiro, Brazil). Open Journal of Marine Science, vol. 3, no. 04, pp. 161-166. http://dx.doi.org/10.4236/ojms.2013.34018.
http://dx.doi.org/10.4236/ojms.2013.3401...
; Pessoa et al., 2020PESSOA, L.A., PAIVA, P.C., PARANHOS, R., FREITAS, M.A.V. and ECHEVERRÍA, C.A., 2020. Intra-annual variation in rainfall and it’s influence of the adult’s Cyprideis spp (Ostracoda, Crustacea) on a eutrophic estuary (Guanabara Bay, Rio de Janeiro, Brazil). Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 80, no. 2, pp. 449-459. http://dx.doi.org/10.1590/1519-6984.216263. PMid:31482973.
http://dx.doi.org/10.1590/1519-6984.2162...
).
The results evidence a distinct spatial distribution among the sectors with differences in the composition of the assemblages because of the heterogeneity of abiotic conditions (sediment, bottom water, continental input and others). The sectorization observed in the results is widely discussed in other works due to its oceanographic and biological characteristics (Echeverría et al., 2010ECHEVERRÍA, C.A., NEVES, R.A.F., PESSOA, L.A. and PAIVA, P.C., 2010. Spatial and temporal distribution of the gastropod Heleobia australis in an eutrophic estuarine system suggests a metapopulation dynamics. Nature Science, vol. 2, no. 8, pp. 860-867.; Neves et al., 2013NEVES, R.A.F., ECHEVERRÍA, C.A., PESSOA, L.A., PAIVA, P.C., PARANHOS, R. and VALENTIN, J.L., 2013. Factors influencing spatial patterns of mollusks in a eutrophic tropical bay. Journal of the Marine Biological Association of the United Kingdom, vol. 93, no. 3, pp. 577-589. http://dx.doi.org/10.1017/S0025315412001105.
http://dx.doi.org/10.1017/S0025315412001...
; Pereira et al., 2013PEREIRA, V.P.P., PESSOA, L.A., FREITAS, M.A.V. and ECHEVERRÍA, C.A., 2013. Recovery process on soft-bottom macrobenthic communities after artificial disturbance in tropical polluted estuary (Guanabara Bay, Rio de Janeiro, Brazil). Open Journal of Marine Science, vol. 3, no. 04, pp. 161-166. http://dx.doi.org/10.4236/ojms.2013.34018.
http://dx.doi.org/10.4236/ojms.2013.3401...
; Francisco and Netto, 2020FRANCISCO, A.S. and NETTO, S.A., 2020. El niño-southern oscillations and pacific decadal oscillation as drivers of the decadal dynamics of benthic macrofauna in two subtropical estuaries (Southern Brazil). Ecosystems. In press. http://dx.doi.org/10.1007/s10021-019-00475-6.
http://dx.doi.org/10.1007/s10021-019-004...
; Pessoa et al., 2020PESSOA, L.A., PAIVA, P.C., PARANHOS, R., FREITAS, M.A.V. and ECHEVERRÍA, C.A., 2020. Intra-annual variation in rainfall and it’s influence of the adult’s Cyprideis spp (Ostracoda, Crustacea) on a eutrophic estuary (Guanabara Bay, Rio de Janeiro, Brazil). Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 80, no. 2, pp. 449-459. http://dx.doi.org/10.1590/1519-6984.216263. PMid:31482973.
http://dx.doi.org/10.1590/1519-6984.2162...
). Our results provide a sectorization based not only on abiotic characteristics, but also on the distribution of fauna by their possible indicators.
At the Entrance sector it was possible to observe the predominance of Cypridinidae and Ervilia concentrica taxa over the years. This sector was defined by the predominance of biotic forcing with oceanic characteristics, evidenced by high salinity values, dissolved oxygen, nitrate and orthophosphate for the water column due to the high renewal rate and predominance of medium and fine sand for sediment due to high hydrodynamism. These abiotic conditions were remarkable in the separating analyses for the respective years (2005 to 2006 and 2006 to 2007).
For the Intermediary sector it was possible to observe the predominance of the taxa Cyprideis salebrosa and Cyprideis sp. over the years. The sector had abiotic forcings that evidence a mixing zone of oceanic influence with the runnoff from watershed of the Guanabara Bay. These variables were presented with high chlorophyll, nitrite values and granulometry with silt and clay medium sand fractions.
The Inner sector was exclusively represented by high density values of the gastropod Heleobia australis. In this sector there was a high predominance of abiotic variables of strong influence of the watershed, such as high temperature, suspended particulate matter, nitrite, ammonia, chlorophyll and low values of dissolved oxygen and salinity and, for sediment, there was a predominance of silt and clay fractions, besides high concentrations of organic matter.
Circulation dynamics throughout the renewal water body, which, thereby facilitate the benthic invertebrate dispersion, comprises a typical example of metapopulation source-sink dynamics (Echeverría et al., 2010ECHEVERRÍA, C.A., NEVES, R.A.F., PESSOA, L.A. and PAIVA, P.C., 2010. Spatial and temporal distribution of the gastropod Heleobia australis in an eutrophic estuarine system suggests a metapopulation dynamics. Nature Science, vol. 2, no. 8, pp. 860-867.). This was the case among Heleobia australis (Gastropoda) in Guanabara Bay. Metapopulation dynamics also seems to occur among other taxonomic groups, although other mechanisms might be involved (Pereira et al., 2013PEREIRA, V.P.P., PESSOA, L.A., FREITAS, M.A.V. and ECHEVERRÍA, C.A., 2013. Recovery process on soft-bottom macrobenthic communities after artificial disturbance in tropical polluted estuary (Guanabara Bay, Rio de Janeiro, Brazil). Open Journal of Marine Science, vol. 3, no. 04, pp. 161-166. http://dx.doi.org/10.4236/ojms.2013.34018.
http://dx.doi.org/10.4236/ojms.2013.3401...
).
Unlike Heleobia australis, endowed with various strategies for quick opportunistic dispersal, ostracods of the genus Cyprideis, deprived of this capacity, are either stationary. They directly reflect impacts since they are already in the sediment before changes occur. Over time, both portray heterogeneity in the bay. On analyzing the rainfall cycles, it was possible to observe a regular cycle for the first annual cycle (2005-2006) and an unusual one for the second annual cycle (2006-2007), characterized by periods of low rainfall followed by high rainfall. There are indications that this atypical rainfall may have caused the observed structural changes in the community. When clustering the two cycles, subsequent analysis showed the sectors and the surveys as being well defined over a wide temporal scale.
It was possible to assume the influence of seasonality on macrobenthic communities. According to the prevailing rainfall regime, seasonality clearly defines the periods and their influence. Taxa were distributed across sectors on a declining scale of richness and diversity towards the bottom of the bay. The species Heleobia australis was the only abundantly well distributed one in the Inner sector. Intra and inter-annual variations were well defined and observed during an atypical rainfall regime. The mosaic of soft-bottom substrates infers structural variables. Thus, patterns of temporal distribution were basically influenced by those indicating pollution and SACW intrusion. Ervilia concentrica and family Cypridinidae could be indicators for the Entrance sector and the species Cyprideis salebrosa and Cyprideis sp. for the Intermediary sector. The Americuna besnardii (present only at station BG 02 and with high density in survey III) and Mytilidae (present only at station BG 09 and with high density in survey IV) taxa were dominant due their high density values. However, these occurrences were restricted to just one station in a few surveys, this condition underpins their nature of aggregated distribution.
Population coexistence of macrobenthic species, high species density, and morphological and behavioral modifications, may all reflect local conditions. Longer term sampling and dedication to experiments, such as ecotoxicology, dispersion, bioaccumulation, predation, etc., could better identify environmental indicators for Guanabara Bay. As responses often depend on individual mobility, especially as to sessile level or restricted capacity, this aspect could be useful as a reliable environment indicator. Variations in diversity, equitability, species richness and density are efficient indicators of the quality of the environment and assume the role of parameters for monitoring environmental recovery.
Appendix A Acronyms taxa/species list.
| LIS | Listriella titinga | AMPHIPODA |
| MIC | Microphoxus breviramus | AMPHIPODA |
| TIB | Tiburonella viscana | AMPHIPODA |
| GIB | Giberosus sp. | AMPHIPODA |
| BIR | Birubius sp. | AMPHIPODA |
| COR | Corophiidae | AMPHIPODA |
| EUD | Eudevenopus sp. | AMPHIPODA |
| EUR | Eurydice sp. | AMPHIPODA |
| MCR | Macrochiridothea sp. | AMPHIPODA |
| ERI | Ericthonius brasiliensis | BIVALVIA |
| NUC | Nucula semiornata | BIVALVIA |
| CAR | Carditamera micella | BIVALVIA |
| AME | Americuna besnardi | BIVALVIA |
| CRA1 | Crassinella marplatensis | BIVALVIA |
| CRA2 | Crassinella martinicensis | BIVALVIA |
| ERV | Ervilia concentrica | BIVALVIA |
| SEM1 | Semele nuculoides | BIVALVIA |
| SEM2 | Semele purpurascens | BIVALVIA |
| CHI | Chione cancellata | BIVALVIA |
| MUS | Musculus lateralis | BIVALVIA |
| BOT | Botula fusca | BIVALVIA |
| ANO | Anomalocardia brasiliana | BIVALVIA |
| GOU | Gouldia cerina | BIVALVIA |
| TRA1 | Transennella cubaniana | BIVALVIA |
| TRA2 | Transennella stimpsoni | BIVALVIA |
| THR | Thracia similis | BIVALVIA |
| LAS | Lasaea adansoni | BIVALVIA |
| ABR | Abra cf uruguayensis | BIVALVIA |
| TEL | Tellina exerythra | BIVALVIA |
| COR | Corbula cubaniana | BIVALVIA |
| LUC | Lucina pectinata | BIVALVIA |
| CTE | Ctena pectinella | BIVALVIA |
| HIA | Hiatella arctica | BIVALVIA |
| MOD | Modiolus carvalhoi | BIVALVIA |
| PIC | Pinctada imbricata | BIVALVIA |
| MDL | Modiolus sp. | BIVALVIA |
| CTN | Ctena sp. | BIVALVIA |
| SML | Semele sp. | BIVALVIA |
| TLN | Tellina sp. | BIVALVIA |
| OLV | Olivella sp. | BIVALVIA |
| MTL | Mytilidae | BIVALVIA |
| HUT | Hutchinsoniella macracantha | CEPHALOCARIDA |
| NEB | Neballa sp. | CEPHALOCARIDA |
| CUM | Cumacea | CUMACEA |
| PIN | Pinnixa chaetopterana | DECAPODA |
| POR | Portunus ventralis | DECAPODA |
| PRO | Processa hemphilli | DECAPODA |
| UPO | Upogebia omissa | DECAPODA |
| PAG | Paguridae | DECAPODA |
| ALB | Albunea paretti | DECAPODA |
| CRO | Cronius sp. | DECAPODA |
| CAE1 | Caecum brasilicum | GASTROPODA |
| GAB | Gabrielona sulcifera | GASTROPODA |
| BIT | Bittiolum varium | GASTROPODA |
| CAE2 | Caecum someri | GASTROPODA |
| CAE3 | Caecum ryssotitum | GASTROPODA |
| FIN | Finella dubia | GASTROPODA |
| HEL | Heleobia australis | GASTROPODA |
| NAT | Natica pusilla | GASTROPODA |
| OLI | Olivella minuta | GASTROPODA |
| TEI | Teinostoma cocolitoris | GASTROPODA |
| PAR | Parviturboides interruptus | GASTROPODA |
| AES | Aesopus stearnsii | GASTROPODA |
| MEL | Melanella arcuata | GASTROPODA |
| ALV | Alvania faberi | GASTROPODA |
| ANA | Anachis isabellei | GASTROPODA |
| ACT1 | Acteocina bidentata | GASTROPODA |
| ACT2 | Acteocina bullata | GASTROPODA |
| NAS | Nassarius vibex | GASTROPODA |
| CRY | Chrysallida sp. | GASTROPODA |
| ODS | Odostomia sp. | GASTROPODA |
| TRB | Turbonilla sp. | GASTROPODA |
| CRT | Cerithiopsis sp. | GASTROPODA |
| EPT | Epitonium sp. | GASTROPODA |
| MLN | Melanella sp. | GASTROPODA |
| NTC | Natica sp. | GASTROPODA |
| RSN | Rissoina sp. | GASTROPODA |
| MYS | Mysidacea | MYSIDACEA |
| AUR | Aurila ornellasae | OSTRACODA |
| CSA | Cyprideis salebrosa | OSTRACODA |
| CYP | Cyprideis sp. | OSTRACODA |
| BAR | Bairdiidae | OSTRACODA |
| CYT | Cytherideidae | OSTRACODA |
| CYL | Cylindroleberididae | OSTRACODA |
| MAC | Macrocyprina sp. | OSTRACODA |
| CYP | Cypridinidae | OSTRACODA |
| URO | Urocythereis sp. | OSTRACODA |
| HEM | Hemicytheridae | OSTRACODA |
| CAP | Capitella capitata | POLYCHAETA |
| ARI | Aricidea (Acmira) taylori | POLYCHAETA |
| GYP | Gyptis callithrix | POLYCHAETA |
| ORB | Orbinia johnsoni | POLYCHAETA |
| PAR | Paraprionospio pinnata | POLYCHAETA |
| SPI1 | Spio quadrisetosa | POLYCHAETA |
| OWE | Owenia fusiformis | POLYCHAETA |
| NAI | Naineris setosa | POLYCHAETA |
| SIG | Sigalion taquari | POLYCHAETA |
| MAG | Magelona crenulata | POLYCHAETA |
| GLY | Glycera americana | POLYCHAETA |
| GON | Goniadides carolinae | POLYCHAETA |
| SPI2 | Spiochaetopterus nonatoi | POLYCHAETA |
| POL | Polydora websteri | POLYCHAETA |
| STR | Streblospio benedicti | POLYCHAETA |
| SCO | Scoloplos sp. | POLYCHAETA |
| ALL | Allia sp. | POLYCHAETA |
| ARC | Aricidea sp. | POLYCHAETA |
| HMP | Hemipodia sp. | POLYCHAETA |
| GND | Goniada sp. | POLYCHAETA |
| ONP | Onuphidae | POLYCHAETA |
| KIN | Kinbergonuphis sp. | POLYCHAETA |
| PIO | Pionosyllis sp. | POLYCHAETA |
| MSC | Mesochaetopterus sp. | POLYCHAETA |
| THR | Tharyx sp. | POLYCHAETA |
| MGL | Magelona sp. | POLYCHAETA |
| PCL | Poecilochaetus sp. | POLYCHAETA |
| SAB | Sabellidae | POLYCHAETA |
| SPN | Spionidae | POLYCHAETA |
| APS | Apoprionospio sp. | POLYCHAETA |
| DSP | Dispio sp. | POLYCHAETA |
| PNS | Prionospio sp | POLYCHAETA |
| LMP | Limopsis sp | POLYCHAETA |
| KAL | Kalliapseudes schubarti | TANAIDACEA |
| SKU | Skuphonura sp. | TANAIDACEA |
| TAN | Tanaidacea | TANAIDACEA |
Appendix B Acronyms environmental variables list.
| TEMP | Temperature |
| DO | Dissolved Oxygen |
| ORT | Orthophosphate |
| NH4 | Ammonia |
| NO2 | Nitrite |
| NO3 | Nitrate |
| CLO | Chlorophyll |
| SAL | Salinity |
| TPs | Total Phosphorus |
| NTs | Total Nitrogen |
| SPM | Suspended Particulate Material |
| FSAN | Fine sand |
| MSAN | Medium sand |
| SOR | Sorting |
| SKW | Skewness |
Acknowledgements
The authors are grateful to the researchers Mariana Melão, Bruna Tovar Faro, Ricardo Bastos, Raquel Neves, Érico Demari e Silva and Fernanda Lana, and anonymous reviewers for useful comments. The present work was part of the subproject “Estrutura das Comunidades da Macroendofauna Bentônica de Substrato Inconsolidado do Infralitoral” of the Project “Avaliação Ambiental da Baía de Guanabara, Rio de Janeiro, Brasil”, coordinated by CENPES/Petrobrás.
-
(With 8 figures)
References
- AGUIAR, V.M.C., BAPTISTA NETO, J.A. and RANGEL, C.M., 2011. Eutrophication and hypoxia in four streams discharging in Guanabara Bay, RJ, Brazil, a case study. Marine Pollution Bulletin, vol. 62, no. 8, pp. 1915-1919. http://dx.doi.org/10.1016/j.marpolbul.2011.04.035 PMid:21708390.
» http://dx.doi.org/10.1016/j.marpolbul.2011.04.035 - ALONGI, D.M., 1989. Ecology of tropical soft-bottom benthos: a review with emphasis on emerging concepts. Revista de Biología Tropical, vol. 37, no. 1, pp. 85-100.
- AMADOR, E.S., 1997. Baía de Guanabara e ecossistemas periféricos: homem e natureza Rio de Janeiro: Reproarte Gráfica e Editora, 539 p.
- AMADOR, E.S., 2012. Bacia da Baía de Guanabara: características geoambientais e ecossistemas Rio de Janeiro: Editora Interciência, 406p.
- AMMANN, L.P., WALLER, W.T., KENNEDY, J.H., DICKSON, K.L. and MAYER, F.L., 1997. Power, sample size and taxonomic sufficiency for measures or impact in aquatic systems. Environmental Toxicology and Chemistry, vol. 16, no. 11, pp. 2421-2431. http://dx.doi.org/10.1002/etc.5620161131
» http://dx.doi.org/10.1002/etc.5620161131 - ANDERSON, M.J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology, vol. 26, pp. 32-46.
- BAPTISTA NETO, J.A. and SILVA, M.A.M., 1996. Caracterização dos sedimentos de fundo e dinâmica sedimentar da Enseada de Jurujuba (Baía de Guanabara), Niterói, RJ. Pesquisas, vol. 23, no. 1-2, pp. 7-16.
- BAPTISTA NETO, J.A., CRAPEZ, M.A.C., MCALISTER, J.J. and VILELA, C.G., 2005. Concentration and availability of heavy metals in sediments from Niterói Harbour (Guanabara Bay, S.E. Brazil). Journal of Coastal Research, vol. 21, pp. 811817.
- BAPTISTA-NETO, J.A., GINGELE, F.X., LEIPE, T. and BREHME, I., 2006. Spatial distribution of heavy metals in superficial sediments from Guanabara Bay: Rio de Janeiro, Brazil. Environmental Geology, vol. 49, no. 7, pp. 1051-1063. http://dx.doi.org/10.1007/s00254-005-0149-1
» http://dx.doi.org/10.1007/s00254-005-0149-1 - BORCARD, D., GILLET, F. and LEGENDRE, P., 2011. Numerical ecology with R New York: Springer, 306 p. http://dx.doi.org/10.1007/978-1-4419-7976-6
» http://dx.doi.org/10.1007/978-1-4419-7976-6 - BORGES, A.C., SANDERS, C.J., SANTOS, H.L.R., ARARIPE, D.R., MACHADO, W. and PATCHINEELAM, S.R., 2009. Eutrophication history of Guanabara Bay (SE Brazil) recorded by phosphorus flux to sediments from a degraded mangrove area. Marine Pollution Bulletin, vol. 58, no. 11, pp. 1750-1765. http://dx.doi.org/10.1016/j.marpolbul.2009.07.025 PMid:19699494.
» http://dx.doi.org/10.1016/j.marpolbul.2009.07.025 - BORGES, R.C., CALDAS, V.G., SIMÕES FILHO, F.F.L., FERREIRA, M.M. and LAPA, C.M.F., 2014. Use of GIS for the evaluation of heavy metal contamination in the Cunha Canal watershed and west of the Guanabara Bay, Rio de Janeiro, RJ. Marine Pollution Bulletin, vol. 89, no. 1-2, pp. 75-84. http://dx.doi.org/10.1016/j.marpolbul.2014.10.033 PMid:25455374.
» http://dx.doi.org/10.1016/j.marpolbul.2014.10.033 - CARREIRA, R.S., WAGENER, A.L.R. and READMAN, J.W., 2004. Sterols as makers of sewage contamination in a tropical urban estuary (Guanabara Bay, Brazil): space-time variations. Estuarine, Coastal and Shelf Science, vol. 60, no. 24, pp. 587-598. http://dx.doi.org/10.1016/j.ecss.2004.02.014
» http://dx.doi.org/10.1016/j.ecss.2004.02.014 - CARREIRA, R.S., WAGENER, A.L.R., READMAN, J.W., FILEMAN, T.W., MACKO, S.A. and VEIGA, A., 2002. Change in the sedimentary organic carbon pool of a fertilized tropical estuary, Guanabara Bay, Brazil: an elemental, isotopic and molecular marker approach. Marine Chemistry, vol. 79, no. 3-4, pp. 207-227. http://dx.doi.org/10.1016/S0304-4203(02)00065-8
» http://dx.doi.org/10.1016/S0304-4203(02)00065-8 - CARVALHO, S., MOURA, A., GASPAR, M., PEREIRA, P., FONSECA, C., FALCÃO, L., DRAGO, T., LEITÃO, F. and REGALA, F., 2005. Spatial and interannual variability of the macrobenthic communities within a costal lagoon (Obidos Lagoon) and its relationship with environmental parameters. Acta Oceanologica, vol. 27, no. 3, pp. 143-159. http://dx.doi.org/10.1016/j.actao.2004.11.004
» http://dx.doi.org/10.1016/j.actao.2004.11.004 - CATANZARO, L.F., BAPTISTA NETO, J.A., GUIMARÃES, M.S.D. and SILVA, C.G., 2004. Distinctive Sedimentary Processes in Guanabara Bay – SE/Brazil, based on the analysis of echo-character (7.0 khz). Revista Brasileira de Geofísica, vol. 22, no. 1, pp. 69-83. http://dx.doi.org/10.1590/S0102-261X2004000100006
» http://dx.doi.org/10.1590/S0102-261X2004000100006 - COUTINHO, F.H., SILVEIRA, C.B., PINTO, L.H., SALLOTO, G.R., CARDOSO, A.M., MARTINS, O.B., VIEIRA, R.P. and CLEMENTINO, M.M., 2014. Antibiotic resistance is widespread in urban aquatic environments of Rio de Janeiro. Brazilian Journal of Microbiology, vol. 68, no. 3, pp. 441-452. PMid:24821495.
- DAUVIN, J.C., GOMEZ GESTEIRA, J.L. and SALVANDE FRAGA, M., 2003. Taxonomic sufficiency: an overview of its use in the monitoring of sublittoral benthic communities after oil spills. Marine Pollution Bulletin, vol. 46, no. 5, pp. 552-555. http://dx.doi.org/10.1016/S0025-326X(03)00033-X PMid:12735952.
» http://dx.doi.org/10.1016/S0025-326X(03)00033-X - DAY, J., HALL, C., KEMP, W. and YÁÑEZ-ARANCIBIA, A., 1989. Estuarine ecology 1st ed. New York: John Wiley & Sons.
- DIAZ, R.J. and ROSENBERG, R., 1995. Marine benthic hypoxia: a review of its ecological effects and the behavioral responses of benthic macrofauna. Oceanography and Marine Biology - an Annual Review, vol. 33, pp. 245-303.
- ECHEVERRÍA, C.A., NEVES, R.A.F., PESSOA, L.A. and PAIVA, P.C., 2010. Spatial and temporal distribution of the gastropod Heleobia australis in an eutrophic estuarine system suggests a metapopulation dynamics. Nature Science, vol. 2, no. 8, pp. 860-867.
- ELLIOTT, M. and QUINTINO, V., 2007. The estuarine quality paradox, environmental homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Marine Pollution Bulletin, vol. 54, no. 6, pp. 640-645. http://dx.doi.org/10.1016/j.marpolbul.2007.02.003 PMid:17418874.
» http://dx.doi.org/10.1016/j.marpolbul.2007.02.003 - ELLIS, D., 1985. Taxonomic sufficiency in pollution assessment. Marine Pollution Bulletin, vol. 16, no. 12, pp. 459p. http://dx.doi.org/10.1016/0025-326X(85)90362-5
» http://dx.doi.org/10.1016/0025-326X(85)90362-5 - FILIPPO, A.M., 1997. Passagem de frente frias na Baía de Guanabara, RJ – Brasil: impacto no nível do mar Niterói: Universidade Federal Fluminense. Dissertação de Mestrado em Geoquímica Ambiental, 79 p.
- FLEMMING, B., 2000. A revised textural classification of gravel-free muddy sediments on the basis of ternary diagrams. Continental Shelf Research, vol. 20, no. 10-11, pp. 1125-1137. http://dx.doi.org/10.1016/S0278-4343(00)00015-7
» http://dx.doi.org/10.1016/S0278-4343(00)00015-7 - FOLK, R. and WARD, W.C., 1958. Brazos River bar: a study in the significance of grain-size parameters. Journal of Sedimentary Petrology, vol. 27, no. 1, pp. 3-26. http://dx.doi.org/10.1306/74D70646-2B21-11D7-8648000102C1865D
» http://dx.doi.org/10.1306/74D70646-2B21-11D7-8648000102C1865D - FRANCISCO, A.S. and NETTO, S.A., 2020. El niño-southern oscillations and pacific decadal oscillation as drivers of the decadal dynamics of benthic macrofauna in two subtropical estuaries (Southern Brazil). Ecosystems In press. http://dx.doi.org/10.1007/s10021-019-00475-6
» http://dx.doi.org/10.1007/s10021-019-00475-6 - GILLANDERS, B.M. and KINGSFORD, M.J., 2002. Impact of changes in flow of freshwater on estuarine and open coastal habitats and the associated organisms. Oceanography and Marine Biology - an Annual Review, vol. 40, pp. 233-309. http://dx.doi.org/10.1201/9780203180594.ch5
» http://dx.doi.org/10.1201/9780203180594.ch5 - GRASSHOFF, K., KREMLING, K. and ERHARDT, M. 1999. Methods of seawater analysis 3rd ed. Berlin: Wiley-VCH Verlag, 600 p. http://dx.doi.org/10.1002/9783527613984
» http://dx.doi.org/10.1002/9783527613984 - GRAY, J.S. and ELLIOTT, M., 2009. Ecology of marine sediments 2nd ed. Oxford: Oxford Biology, 256 p.
- INSTITUTO NACIONAL DE METEREOLOGIA – INMET [online], 2008 [viewed 14 December 2008]. Available from: inmet.gov.br/html/observacoes.php
- KFOURI, P.B.P., FIGUEIRA, R.C.L., FIGUEIREDO, A.M.G., SOUZA, S.H.M. and EICHLER, B.B., 2005. Metal levels and foraminifera occurrence in sediment cores from Guanabara Bay, Rio de Janeiro. Journal of Radioanalytical and Nuclear Chemistry, vol. 265, no. 3, pp. 459-466. http://dx.doi.org/10.1007/s10967-005-0849-8
» http://dx.doi.org/10.1007/s10967-005-0849-8 - KJERFVE, B., LACERDA, L.D. and DIAS, G.M.T., 2001. Baía de Guanabara, Rio de Janeiro, Brazil. In: U. SEELIGER and B. KJERFVE, eds. Coastal marine ecosystems of Latin America New York: Springer-Verlag, pp. 107-117.
- KJERFVE, B., RIBEIRO, C.H.A., DIAS, G.T.M., FILIPPO, A.M. and QUARESMA, V.S., 1997. Oceanographic characteristics of an impacted coastal bay: baía de Guanabara, Rio de Janeiro, Brazil. Continental Shelf Research, vol. 17, no. 13, pp. 1609-1643. http://dx.doi.org/10.1016/S0278-4343(97)00028-9
» http://dx.doi.org/10.1016/S0278-4343(97)00028-9 - LARDOSA, E.I., SIMÕES, M. and SOARES, M.L.G., 2013. Cartografia das áreas de ocorrência de manguezais no Estado do Rio de Janeiro através da integração de múltiplas fontes de dados. Revista Brasileira de Cartografia, vol. 65, pp. 1-14.
- LITTLE, C., 2000. The biology of soft shores and estuaries Oxford: Oxford University Press, 264 p.
- LOPEZ, G.R. and LEVINTON, J.S., 1987. Ecology of deposit-feeding animals in marine sediments. The Quarterly Review of Biology, vol. 62, no. 3, pp. 235-260. http://dx.doi.org/10.1086/415511
» http://dx.doi.org/10.1086/415511 - MARAZZO, A. and VALENTIN, J.L., 2004. Reproductive aspects of marine cladocerans Penilia avirostris and Pseudevadne tergestina (Crustacea, Branchiopoda) in the outer part of Guanabara Bay, Brazil. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 64, no. 3A, pp. 543-549. http://dx.doi.org/10.1590/S1519-69842004000300017 PMid:15622851.
» http://dx.doi.org/10.1590/S1519-69842004000300017 - MAYR, L.M., TENENBAUM, D.R., VILLAC, M.C., PARANHOS, R., NOGUEIRA, C.R., BONECKER, S. and BONECKER, A., 1989. Hidrobiological characterization of Guanabara Bay. In: O. MAGOON and C. NEVES, eds. Costalines of Brazil New York: American Society of Civil Engineers, pp. 124-138.
- MCLEOD, R. and WING, S., 2008. Influence of an altered salinity regime on the population structure of two infaunal bivalve species. Estuarine, Coastal and Shelf Science, vol. 78, no. 3, pp. 529-540. http://dx.doi.org/10.1016/j.ecss.2008.01.019
» http://dx.doi.org/10.1016/j.ecss.2008.01.019 - MENDES, F., FIGUEIREDO, G.M. and VALENTIN, J.L., 2012. Reproduction and structure of the population of the chaetognath Parasagitta friderici in Guanabara Bay (Brazil) based on short term sampling. Anais da Academia Brasileira de Ciências, vol. 84, no. 1, pp. 103-112. http://dx.doi.org/10.1590/S0001-37652012005000008 PMid:22441599.
» http://dx.doi.org/10.1590/S0001-37652012005000008 - MENICONI, M.D.G., GABARDO, I.T., CARNEIRO, M.E.R., BARBANTI, S.M., SILVA, G.C. and MASSONE, C.G., 2002. Brazilian oil spills chemical characterization e case studies. Environmental Forensics, vol. 3, no. 3, pp. 303-321. http://dx.doi.org/10.1080/713848377
» http://dx.doi.org/10.1080/713848377 - MERMILLOD-BLONDIN, F. and ROSENBERG, R., 2006. Ecosystem engineering: the impact of bioturbation on biogeochemical processes in marine and freshwater benthic habitats. Aquatic Sciences, vol. 68, no. 4, pp. 434-442. http://dx.doi.org/10.1007/s00027-006-0858-x
» http://dx.doi.org/10.1007/s00027-006-0858-x - NEGRELLO-FILHO, O.A., UGAZ-CODINA, J.C., OLIVEIRA, L.H.S., SOUZA, M.C. and ÂNGULO, R.J., 2018. Subtidal soft sediments of the Paranaguá Bay inlet: mapping habitats and species distribution at a landscape scale. Brazilian Journal of Oceanography, vol. 66, no. 3, pp. 255-266. http://dx.doi.org/10.1590/s1679-875920180192406603
» http://dx.doi.org/10.1590/s1679-875920180192406603 - NEVES, R.A.F. and VALENTIN, J.L., 2011. Revisão bibliográfica sobre a macrofauna bentônica de fundos não-consolidados em áreas costeiras prioritárias para a conservação no Brasil. Arquivos de Ciências do Mar, vol. 44, pp. 59-80.
- NEVES, R.A.F., ECHEVERRÍA, C.A., PESSOA, L.A., PAIVA, P.C., PARANHOS, R. and VALENTIN, J.L., 2013. Factors influencing spatial patterns of mollusks in a eutrophic tropical bay. Journal of the Marine Biological Association of the United Kingdom, vol. 93, no. 3, pp. 577-589. http://dx.doi.org/10.1017/S0025315412001105
» http://dx.doi.org/10.1017/S0025315412001105 - PARANHOS, R. and MAYR, L.M., 1993. Seasonal patterns of temperature and salinity in Guanabara Bay, Brazil. Fresenius Environmental Bulletin, vol. 2, no. 11, pp. 647-652.
- PARANHOS, R., ANDRADE, L., MENDONÇA-HAGLER, L.C. and PFEIFFER, W.C. 2001. Coupling bacterial density with activity in a tropical polluted coastal bay. In: B.M. FARIA, V. FARJALLA and F.A. ESTEVES, eds. Aquatic microbial ecology in Brazil Rio de Janeiro: PPGE-UFRJ, pp. 117-132. Serie Oecologia Brasiliensis, no. 9.
- PARANHOS, R., MAYR, L.M., LAVRADO, H.P. and CASTILHO, P.C., 1993. Temperature and salinity trends in Guanabara Bay (Brazil) from 1980 to 1990. Arquivos de Biologia e Tecnologia, vol. 36, no. 4, pp. 685-694.
- PARSONS, T.R., MAITA, Y. and LALLI, C.M., 1984. A manual of chemical and biological methods for seawater analysis 2nd ed. Oxford: Pergamon Press, 173 p.
- PEREIRA, V.P.P., PESSOA, L.A., FREITAS, M.A.V. and ECHEVERRÍA, C.A., 2013. Recovery process on soft-bottom macrobenthic communities after artificial disturbance in tropical polluted estuary (Guanabara Bay, Rio de Janeiro, Brazil). Open Journal of Marine Science, vol. 3, no. 04, pp. 161-166. http://dx.doi.org/10.4236/ojms.2013.34018
» http://dx.doi.org/10.4236/ojms.2013.34018 - PERILLO, G.M.E., PRATOLONGO, P.D., ELIZABETH CARBONE, M. and PICCOLO, M.C., 2009. Biological-physical interactions in estuaries. Estuarine, Coastal and Shelf Science, vol. 85, no. 1, pp. 5-6. http://dx.doi.org/10.1016/j.ecss.2009.08.017
» http://dx.doi.org/10.1016/j.ecss.2009.08.017 - PESSOA, L.A., PAIVA, P.C., PARANHOS, R., FREITAS, M.A.V. and ECHEVERRÍA, C.A., 2020. Intra-annual variation in rainfall and it’s influence of the adult’s Cyprideis spp (Ostracoda, Crustacea) on a eutrophic estuary (Guanabara Bay, Rio de Janeiro, Brazil). Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 80, no. 2, pp. 449-459. http://dx.doi.org/10.1590/1519-6984.216263 PMid:31482973.
» http://dx.doi.org/10.1590/1519-6984.216263 - PETRÓLEO BRASILEIRO S.A. – PETROBRAS, 2012a. Baía de Guanabara: ambiente e influência antrópica Rio de Janeiro, 337 p. (vol. I).
- PETRÓLEO BRASILEIRO S.A. – PETROBRAS, 2012b. Baía de Guanabara: síntese do conhecimento ambiental: biodiversidade Rio de Janeiro, 479 p. (vol. II).
- PRITCHARD, D.W., 1967. Observations of circulation in coastal plain estuaries. In: G.H. LAUF, ed. Estuaries Washington: American Association Advance Science, pp. 37-44.
- QUARESMA, V.S., DIAS, G.T.M. and BAPTISTA NETO, J.A., 2000. Caracterização da ocorrência de padrões de sonar de varredura lateral e sísmica de alta freqüência (3,5 e 7,0 kHz) na porção sul da Baía de Guanabara – RJ. Brazilian Journal of Geophysics, vol. 18, no. 2, pp. 201-214. http://dx.doi.org/10.1590/S0102-261X2000000200008
» http://dx.doi.org/10.1590/S0102-261X2000000200008 - ROSENBERG, R., 2001. Marine benthic faunal successional stages and related sedimentary activity. Scientia Marina, vol. 65, suppl. 2, pp. 107-119. http://dx.doi.org/10.3989/scimar.2001.65s2107
» http://dx.doi.org/10.3989/scimar.2001.65s2107 - SANTI, L. and TAVARES, M., 2009. Polychaeta assemblage of an impacted estuary, Guanabara Bay, Rio de Janeiro, Brazil. Brazilian Journal of Oceanography, vol. 57, no. 4, pp. 287-303. http://dx.doi.org/10.1590/S1679-87592009000400004
» http://dx.doi.org/10.1590/S1679-87592009000400004 - SCHINDLER, D.W., 1987. Detecting ecosystem responses to anthropogenics stress. Canadian Journal of Fisheries and Aquatic Sciences, vol. 44, no. S1, pp. 6-25. http://dx.doi.org/10.1139/f87-276
» http://dx.doi.org/10.1139/f87-276 - SHEPARD, F., 1954. Nomenclature based on sand-silt-clay rations. Journal of Sedimentary Research, vol. 24, pp. 151-158.
- SILVA, M. N. L. J. L. and VALENTIN, J. L., 1988. O Microfitoplâncton das Águas Costeiras do Litoral Fluminense (Estado do Rio de Janeiro): Lista de Espécies e Aspectos Ecológicos. Boletim Do Instituto Oceanográfico, vol. 36, no.1, pp. 1-16.
- SNELGROVE, P.V.R., 1997. The importance of marine sediment biodiversity in ecosystem processes. Ambio, vol. 26, pp. 578-583.
- SNELGROVE, P.V.R., 1999. Getting to the bottom of marine biodiversity: sedimentary habitats. Bioscience, vol. 49, no. 2, pp. 129-138. http://dx.doi.org/10.2307/1313538
» http://dx.doi.org/10.2307/1313538 - SOARES-GOMES, A., GAMA, B.A.P., BAPTISTA NETO, J.A., FREIRE, D.G., CORDEIRO, R.C., MACHADO, W., BERNARDES, M.C., COUTINHO, R., THOMPSON, F. and PEREIRA, R.C., 2016. An environmental overview of Guanabara Bay, Rio de Janeiro. Regional Studies in Marine Science, vol. 8, pp. 319-330. http://dx.doi.org/10.1016/j.rsma.2016.01.009
» http://dx.doi.org/10.1016/j.rsma.2016.01.009 - SOARES-GOMES, A., NEVES, R.L., AUCÉLIO, R., VAN DER VEN, P.H., PITOMBO, F.B., MENDES, C.L. and ZIOLLI, R.L., 2010. Changes and variations of polycyclic aromatic hydrocarbon concentrations in fish, barnacles and crabs following an oil spill in a mangrove of Guanabara Bay, Southeast Brazil. Marine Pollution Bulletin, vol. 60, no. 8, pp. 1359-1363. http://dx.doi.org/10.1016/j.marpolbul.2010.05.013 PMid:20538307.
» http://dx.doi.org/10.1016/j.marpolbul.2010.05.013 - SUGUIO, K., 1973. Introdução a sedimentologia São Paulo, Edgard Blucher/EDUSP, 317 p.
- THOMPSON, B.W., RIDDLE, M.J. and STARK, J.S., 2003. Cost-efficient methods for marine pollution monitoring at Casey Station, East Antarctica: the choice of sieve mesh-size and taxonomic resolution. Marine Pollution Bulletin, vol. 46, no. 2, pp. 232-243. http://dx.doi.org/10.1016/S0025-326X(02)00366-1 PMid:12586119.
» http://dx.doi.org/10.1016/S0025-326X(02)00366-1 - UNDERWOOD, A.J., 1991. Beyond BACI: experimental designs for detecting human environmental impacts on temporal variations in natural populations. Australian Journal of Marine and Freshwater Research, vol. 42, no. 5, pp. 569-587. http://dx.doi.org/10.1071/MF9910569
» http://dx.doi.org/10.1071/MF9910569 - UNDERWOOD, A.J., 1992. Beyond BACI: the detection of environmental impact on populations in the real, but variable, world. Journal of Experimental Marine Biology and Ecology, vol. 161, no. 2, pp. 145-178. http://dx.doi.org/10.1016/0022-0981(92)90094-Q
» http://dx.doi.org/10.1016/0022-0981(92)90094-Q - UNDERWOOD, A.J., 1994. On beyond BACI: sampling designs that might reliably detect environmental disturbances. Ecological Applications, vol. 4, no. 1, pp. 3-15. http://dx.doi.org/10.2307/1942110
» http://dx.doi.org/10.2307/1942110 - VALENTIN, J.L., TENENBAUM, D.R., BONECKER, A.C.T., BONECKER, S.L.C., NOGUEIRA, C.R. and VILLAC, M.C., 1999. O sistema planctônico da Baía de Guanabara: síntese do conhecimento. In: S.H.G. SILVA and H.P. LAVRADO, eds. Ecologia de Ambientes Costeiros do Estado do Rio de Janeiro Rio de Janeiro: PPGE-UFRJ, pp. 35-59. Série Oecologia Australis.
- VALIELA, I., 1995. Marine ecological processes 2nd ed. New York: Springer-Verlag, 686 p. http://dx.doi.org/10.1007/978-1-4757-4125-4
» http://dx.doi.org/10.1007/978-1-4757-4125-4 - VENTURA, E.C., GAELZER, L.R., ZANETTE, J., MARQUES, M.R.F. and BAINY, A.C.D., 2002. Biochemichal indicators of contaminant exposure in spotted pigfish (Orthopristis ruber) caught at three bays of Rio de Janeiro coast. Marine Environmental Research, vol. 54, no. 3-5, pp. 775-779. http://dx.doi.org/10.1016/S0141-1136(02)00137-X PMid:12408649.
» http://dx.doi.org/10.1016/S0141-1136(02)00137-X - VILLAC, M.C., MAYR, L.M., TENENBAUM, D.R. and PARANHOS, R., 1991. Sampling strategies proposed to monitor Guanabara Bay, RJ, Brazil. In: O.T. MAGOON, H.V. CONVERSE, V. TIPPIE, L.T. TOBIN and D. CLARCK, eds. Coastal Zone’91 New York: American Society of Civil Engineers, pp. 1168-1182.
- WARWICK, R.M., 1988. The level of taxonomic discrimination required to detect pollution effects on marine benthic communities. Marine Pollution Bulletin, vol. 19, no. 6, pp. 259-268. http://dx.doi.org/10.1016/0025-326X(88)90596-6
» http://dx.doi.org/10.1016/0025-326X(88)90596-6 - XAVIER DE BRITO, A.P., BRÜNING, I.M.R. and MOREIRA, I., 2002. Chlorinated pesticides in mussels from Guanabara Bay, Rio de Janeiro, Brazil. Marine Pollution Bulletin, vol. 44, no. 1, pp. 79-81. http://dx.doi.org/10.1016/S0025-326X(01)00222-3 PMid:11885566.
» http://dx.doi.org/10.1016/S0025-326X(01)00222-3
Publication Dates
-
Publication in this collection
11 Dec 2020 -
Date of issue
Jul-Sep 2021
History
-
Received
07 Nov 2019 -
Accepted
03 Feb 2020