Abstract
Plectranthus barbatus Andrews (Lamiaceae) is widely distributed in the world and has a range of popular therapeutic indications. This work aimed to evaluate the phytochemical characterization of two leaf extracts of P. barbatus, and their antimicrobial, antineoplastic and immunomodulatory potential. After collection, herborization and obtainment of the P. barbatus aqueous extract (PBA) and acetone:water 7:3 P. barbatus organic extract (PBO), the phytochemical characterization was performed by high-performance liquid chromatography (HPLC). The antimicrobial activity was performed to determine the minimum inhibitory concentration (MIC) against eight bacterial strains using the microdilution test and the fungus Trichophyton rubrum by disc diffusion assay and microdilution test. Cytotoxicity was assessed by MTT and trypan blue methods in normal peripheral blood mononuclear cells (PBMCs) at concentrations ranged between 0.1 to 100 µg.mL-1 and in neoplastic cell lines Toledo, K562, DU-145 and PANC-1 at 1, 10 and 100 µg.mL-1 . Immunomodulatory activity, was evaluated by sandwich ELISA of proinflammatory cytokines at BALB/c mice splenocytes cultures supernatant. Both extracts presented flavonoids, cinnamic derivatives, steroids and ellagic acid. PBO showed bacteriostatic activity against Acinetobacter baumannii (MIC = 250 µg.mL-1) clinical isolate and PBA fungistatic activity against Trichophyton rubrum (MIC = 800 µg.mL-1). The extracts did not exhibit toxicity to PBMCs and neoplastic cells (IC50 > 100 µg.mL-1). Additionally, PBO at 100 µg.mL-1 significantly inhibited IFN-γ and IL-17A cytokines (p = 0.03). Plectranthus barbatus is a potential candidate for therapeutic use due to its low toxicity in healthy human cells and exhibits biological activities of medical interest as bacteriostatic, fungistatic and immunomodulatory.
Keywords:
antimicrobial; boldo; cancer; immunomodulation; medicinal plant
Resumo
Plectranthus barbatus Andrews (Lamiaceae) é amplamente distribuída no mundo e com uma série de indicações terapêuticas populares. Este trabalho teve como objetivo avaliar a caracterização fitoquímica de dois extratos da folha de P. barbatus e seu potencial antimicrobiano, antineoplásico e imunomodulador. Após coleta, herborização e obtenção do extrato aquoso (PBA) e acetona: água 7: 3 (orgânico) (PBO) de P. barbatus, a caracterização fitoquímica foi realizada por cromatografia líquida de alta eficiência (CLAE). A atividade antimicrobiana foi realizada para determinar a concentração inibitória mínima (CIM) contra oito cepas bacterianas usando o teste de microdiluição e o fungo Trichophyton rubrum por ensaio de difusão em disco e teste de microdiluição. A citotoxicidade foi avaliada por métodos MTT e azul de tripan em células normais mononucleares do sangue periférico (CMSP) em concentrações variadas entre 0,1 a 100 µg.mL-1 e nas linhagens celulares neoplásicas Toledo, K562, DU-145 e PANC-1 em 1, 10 e 100 µg.mL-1 . A atividade imunomoduladora foi avaliada por ELISA sanduíche de citocinas pró-inflamatórias em sobrenadante de culturas de esplenócitos de camundongos BALB/c. Ambos os extratos apresentaram flavonoides, derivados cinâmicos, esteróides e ácido elágico. O PBO mostrou atividade bacteriostática contra Acinetobacter baumannii (CIM = 250 µg.mL-1) e atividade fungistática do PBA contra Trichophyton rubrum (CIM = 800 µg.mL-1). Os extratos não apresentaram toxicidade para CMSP e células neoplásicas (IC50 > 100 µg.mL-1). Além disso, o PBO a 100 µg.mL-1 inibiu significativamente as citocinas IFN-γ e IL-17A (p = 0,03). Plectranthus barbatus é um candidato potencial para uso terapêutico devido à sua baixa toxicidade em células humanas saudáveis e exibe atividade de interesse médico como bacteriostática, fungistática e imunomoduladora.
Palavras-chave:
antimicrobiana; boldo; câncer; imunomodulação; planta medicinal
1. Introduction
In general, several species of plants are used for the treatment and prevention of diseases and are therefore considered as a source of new molecules with diverse biological activities (Cardoso et al., 2014CARDOSO, G.H.S., DANTAS, E.B.S., SOUSA, F.R.C. and PERON, A.P., 2014. Cytotoxicity of aqueous extracts of Rosmarinus officinalis L. (Labiatae) in plant test system. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 74, no. 4, pp. 886-889. http://dx.doi.org/10.1590/1519-6984.07313. PMid:25627599.
http://dx.doi.org/10.1590/1519-6984.0731...
).
Plectranthus barbatus Andrew (Lamiaceae) is generally distributed in Africa, Asia, Australia and South America, more specifically at tropical and subtropical areas of these countries (Lukhoba et al., 2006LUKHOBA, C.W., SIMMONDS, M.S. and PATON, A.J., 2006. Plectranthus: a review of ethnobotanical uses. Journal of Ethnopharmacology, vol. 103, no. 1, pp. 1-24. http://dx.doi.org/10.1016/j.jep.2005.09.011. PMid:16289602.
http://dx.doi.org/10.1016/j.jep.2005.09....
; Mothana et al., 2014MOTHANA, R., AL-SAID, M., AL-MUSAYEIB, N., GAMAL, A., AL-MASSARANI, S., AL-REHAILY, A., ABDULKADER, M. and MAES, L., 2014. In vitro antiprotozoal activity of abietane diterpenoids isolated from Plectranthus barbatus. International Journal of Molecular Sciences, vol. 15, no. 5, pp. 8360-8371. http://dx.doi.org/10.3390/ijms15058360. PMid:24823881.
http://dx.doi.org/10.3390/ijms15058360...
). Plectranthus genus comprehend more than 300 species and P. barbatus is one of the major specie commonly used in Brazilian traditional medicine (Alasbahi and Melzig, 2010ALASBAHI, R.H. and MELZIG, M.F., 2010. Plectranthus barbatus: a review of phytochemistry, ethnobotanical uses and pharmacology – Part 1. Planta Medica, vol. 76, no. 7, pp. 653-661. http://dx.doi.org/10.1055/s-0029-1240898. PMid:20178070.
http://dx.doi.org/10.1055/s-0029-1240898...
; Brito et al., 2018BRITO, E., GOMES, E., FALÉ, P.L., BORGES, C., PACHECO, R., TEIXEIRA, V., MACHUQUEIRO, M., ASCENSÃO, L. and SERRALHEIRO, M.L.M., 2018. Bioactivities of decoctions from Plectranthus species related to their traditional use on the treatment of digestive problems and alcohol intoxication. Journal of Ethnopharmacology, vol. 220, pp. 147-154. http://dx.doi.org/10.1016/j.jep.2018.04.006. PMid:29626671.
http://dx.doi.org/10.1016/j.jep.2018.04....
). In Brazil it is known as false boldo, Brazilian boldo, boldo do reino, alumã, malva santa, malva amarga, sete dores, boldo do jardim and folha de oxalá (Lorenzi and Matos, 2002LORENZI, H. and MATOS, F.J.A., 2002. Plantas medicinais no Brasil: nativas e exóticas cultivadas. Nova Odessa: Instituto Plantarum. 206 p.).
P. barbatus leaves is used in Brazilian traditional medicine in the form of infusion or decoction to treat a variety of diseases including intestinal disorders, heart diseases, liver illness and respiratory disorders. This plant species is also used to relieve inflammatory processes and to treat some nervous system diseases (Lukhoba et al., 2006LUKHOBA, C.W., SIMMONDS, M.S. and PATON, A.J., 2006. Plectranthus: a review of ethnobotanical uses. Journal of Ethnopharmacology, vol. 103, no. 1, pp. 1-24. http://dx.doi.org/10.1016/j.jep.2005.09.011. PMid:16289602.
http://dx.doi.org/10.1016/j.jep.2005.09....
; Rice et al., 2011RICE, L.J., BRITS, G.J., POTGIETER, C.J. and VAN STADEN, J., 2011. Plectranthus: a plant for the future? South African Journal of Botany, vol. 77, no. 4, pp. 947-959. http://dx.doi.org/10.1016/j.sajb.2011.07.001.
http://dx.doi.org/10.1016/j.sajb.2011.07...
).
The main constituent of P. barbatus is forskolin, one of more than 67 diterpenoids isolated from the species and whose range of pharmacological properties could explain the different traditional uses of the species. However, this compound has a water-insoluble nature that limits its clinical utility (Alasbahi and Melzig, 2010ALASBAHI, R.H. and MELZIG, M.F., 2010. Plectranthus barbatus: a review of phytochemistry, ethnobotanical uses and pharmacology – Part 1. Planta Medica, vol. 76, no. 7, pp. 653-661. http://dx.doi.org/10.1055/s-0029-1240898. PMid:20178070.
http://dx.doi.org/10.1055/s-0029-1240898...
). Other phytochemicals such as the phenolic derivatives nepetoidins A and B (Grayer et al., 2003GRAYER, R.J., ECKERT, M.R., VEITCH, N.C., KITE, G.C., MARIN, P.D., KOKUBUN, T., SIMMONDS, M.S. and PATON, A.J., 2003. The chemotaxonomic significance of two bioactive caffeic acid esters, nepetoidins A and B, in the Lamiaceae. Phytochemistry, vol. 64, no. 2, pp. 519-528. http://dx.doi.org/10.1016/S0031-9422(03)00192-4. PMid:12943769.
http://dx.doi.org/10.1016/S0031-9422(03)...
) and rosmarinic acid (Falé et al., 2011FALÉ, P.L., MADEIRA, P.J., FLORÊNCIO, M.H., ASCENSÃO, L. and SERRALHEIRO, M.L., 2011. Function of Plectranthus barbatus herbal tea as neuronal acetylcholinesterase inhibitor. Food & Function, vol. 2, no. 2, pp. 130-136. http://dx.doi.org/10.1039/C0FO00070A. PMid:21779558.
http://dx.doi.org/10.1039/C0FO00070A...
) were also found in P. barbatus composition (Silva et al., 2016SILVA, C.F.G., MENDES, M.P., ALMEIDA, V.V., MICHELS, R.N., SAKANAKA, L.S. and TONIN, L.T.D., 2016. Parâmetros de qualidade físico-químicos e avaliação da atividade antioxidante de folhas de Plectranthus barbatus Andr. (Lamiaceae) submetidas a diferentes processos de secagem. Revista Brasileira de Plantas Medicinais, vol. 18, no. 1, pp. 48-56. http://dx.doi.org/10.1590/1983-084X/15_021.
http://dx.doi.org/10.1590/1983-084X/15_0...
). Phenolic plant-derived compounds comprise the composition of several classes of molecules such as simple phenolics, phenolic acids, coumarins, flavonoids, hydrolysable and condensed tannins, stilbenes, lignans and lignins (Silva et al., 2019SILVA, F.R.G., MATIAS, T.M.S., SOUZA, L.I.O., MATOS-ROCHA, T.J., FONSECA, S.A., MOUSINHO, K.C. and SANTOS, A.F., 2019. Phytochemical screening and in vitro antibacterial, antifungal, antioxidant and antitumor activities of the red propolis Alagoas. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 79, no. 3, pp. 452-459. http://dx.doi.org/10.1590/1519-6984.182959. PMid:30379200.
http://dx.doi.org/10.1590/1519-6984.1829...
) and, some of these have already been identified in P. barbatus extracts (Borges et al., 2020BORGES, A. S., MINOZZO, B. R., SANTOS, H., ARDISSON, J.S., RODRIGUES, R.P., ROMÃO, W., BORGES, W.S., Gonçalves, R.C.R., BELTRAME, F.R. and KITAGAWA, R. R. (2020). Plectranthus barbatus Andrews as anti-Helicobacter pylori agent with activity against adenocarcinoma gastric cells. Industrial Crops & Products vol. 146, pp; 1-12. https://doi.org/10.1016/j.indcrop.2020.112207.
https://doi.org/10.1016/j.indcrop.2020.1...
; Ezeonwumelu et al., 2019EZEONWUMELU, J.O.C., KAWOOYA, G.N., OKORUWA, A.G., DARE, S.S., EBOSIE, J.C., AKUNNE, A.A., TANAYEN, J.K. and UDECHUKWU, B.E., 2019. Phytochemical screening, toxicity, analgesic and anti-pyretic studies of aqueous leaf extract of Plectranthus barbatus [Andrews. Engl.] in rats. Pharmacology & Pharmacy, vol. 10, no. 4, pp. 205-221. http://dx.doi.org/10.4236/pp.2019.104018.
http://dx.doi.org/10.4236/pp.2019.104018...
; Rodrigues et al., 2016RODRIGUES, T. S., GUIMARÃES, S.F., BRAGA, T.V., BASTOS, J.C.S.A. and RODRIGUES-DAS-DÔRES, R.G., 2016. Determination of content of phenolic compounds and flavonoids in leaves extracts of Plectranthus sp. (“Boldos”), potential antioxidant and antibacterial action. Academia Journal of Medicinal Plants, vol. 4, no. 10, pp. 062-068. https://doi.org/10.15413/ajmp.2016.0111.
https://doi.org/10.15413/ajmp.2016.0111...
).
Given its importance and widespread use in folk medicine, the present study aimed to characterize phytochemically the aqueous and organic extracts of P. barbatus and further evaluate their antimicrobial, cytotoxic activity, and immunomodulatory potential.
2. Material and Methods
2.1. Plant material and extracts preparation
Plectranthus barbatus Andrew (Lamiaceae) was collected at the Centro de Treinamento do Instituto de Pesquisa Agropecuária (CETREINO/IPA), located in Carpina, Pernambuco, Brazil (07° 51′ 03′′ S 35° 15′ 17′′ W), under controlled growing conditions. After collection, a voucher specimen was identified by Dra. Rita de Cássia Pereira and deposited at the IPA Herbarium (#91410).
Subsequently, the leaves were dried in circulating air oven (82/480, Lucadema®) at 40°C during 48 hours and the dried material was pulverized in Willye-type knife mill (TE-680, Tecnal®). The extracts were obtained by 10% (w/v) turboextraction (Metvisa®) using water or the acetone: water mixture (7:3, v/v). The acetone: water extract, after filtration, was concentrated under reduced pressure (RV10 Basic, IKA®). Then, the remained extracts were frozen (-80 °C, for 3 days) and lyophilized (at -45 °C; L101, Liotop®), yielding the P. barbatus aqueous extract (PBA) and the P. barbatus organic extract (PBO).
2.2. Thin Layer Chromatography (TLC)
A final concentration of 1 mg.mL-1 was obtained dissolving the extracts in methanol and vortex for complete dissolution. The samples and standards (1 mg.mL-1) were applied, 20 and 5 µl, respectively, on silica gel 60 - F254 (Macherey-Nagel®) chromatographic plates, with a semi-automatic applicator (Camag®) and software (WinCats®). After saturation with the mobile phase (Supplementary Material - Table S1 Supplementary Material Supplementary Material accompanies this paper. Table S1. Mobile phase chromogenic agents and standards used to phytochemical characterization of Plectranthus barbatus extracts. Figure S1. Thin Layer Chromatography (TLC) analysis. Cinnamic derivatives (A); condensed tannins (B) hydrolysable tannins (C) terpenes/steroids (D). This material is available as part of the online article from http://www.scielo.br/bjb ) for about 30 minutes at room temperature (25 ± 2 °C) the chromatographic plates were developed in a twin trough glass chamber (20 cm x 10 cm, Camag®).
After elution, the plates were dried at room temperature and observed under ultraviolet light of 254 and 365 nm and visible light. Then, the plates were derivatized with specific reagents for each metabolite (Supplementary Material - Table S1) and the bands visualized on the samples were compared to the corresponding standards.
2.3. High Performance Liquid Chromatography (HPLC)
Twenty-five mg of PBA and PBO were transferred to a 25 mL volumetric flask and added 15 mL of ultrapure water. The samples were taken to the ultrasonic bath (Ultracleaner, Unique®) for 15 minutes for complete dissolution. The obtained solution was filtered through 0.45 µm syringe filters (CHROMAFIL PVDF, Macherey-Nagel®️) to vials. The caffeic acid and rutin standards (Sigma-Aldrich®) were prepared in ethanol: water (50%, v/v) (HPLC grade, Tedia®), filtered and analysed. The analysis was conducted in a chromatograph (Ultimate 3000, ThermoScientific®) equipped with photodiode array detector. A pre-column (3.9 mm) (Phenomenex®), 250 mm long and 4.6 mm internal diameter column (NST®), packed with silica chemically attached to the octadecylsilane group (5 µm), maintained at 24 °C. The mobile phase consisted of water (trifluoracetic acid 0.05%) as solvent A and methanol (trifluoracetic acid 0.05%) as solvent B, with flow maintained at 0.8 mL/minute. Both were degassed in an ultrasonic bath and filtered through a 0.45 µm pore membrane. The injection volume used was 20 µL. The wavelength of the analyzes was set at 280 nm after scanning. The separation was conducted using the following linear gradient: 0-10 min, 10-25% B; 10-20 min, 25-40% B; 20-25 min, 40-75% B; 25-28 min, 75-10% B; 28-30 min, 75-10% B. All assays were done in triplicate.
2.4. Antibacterial activity
Clinical isolates of Acinetobacter baumannii, Klebsiella pneumoniae, extended-spectrum beta-lactamase (ESBL), and carbapenemase-producing Klebsiella pneumoniae (KPC) were obtained from the Hospital das Clínicas of the Federal University of Pernambuco and kept at the Laboratory of Immunopathology Keizo Asami (LIKA / UFPE). These clinical isolates were phenotypically identified according to the guidelines of the Clinical and Laboratory Standard Institute (CLSI, 2016CLINICAL AND LABORATORY STANDARDS INSTITUTE – CLSI, 2016. Unusual suspects – resistance concerns and susceptibility testing among less common, but Noteworthy BacteriaTech.Rep. USA: CLSI.). Reference strains were obtained from the American Type Culture Collection (ATCC), such as methicillin-resistant Staphylococcus aureus (MRSA) ATCC 33591, methicillin-sensitive Staphylococcus aureus (MSSA) ATCC 29213, Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 29665 and Pseudomonas aeruginosa ATCC 27853.
The antibacterial activity of P. barbatus extracts was evaluated by the microdilution method according to the CLSI guidelines. Initially, the 96-well microdilution plates were filled with Müller-Hinton broth (MHB). Then the extracts previously diluted in 0.5% dimethylsulfoxide (DMSO) were distributed through serial dilution in order to obtain concentrations ranging from 1 to 500 µg.mL-1. As reference drugs Vancomycin and Ciprofloxacin, at 0.0075 to 3.84 µg.mL-1, were used for Gram-positive and Gram-negative bacteria.
The bacterial suspensions were further adjusted to 0.5 of the McFarland scale and diluted to obtain 105 CFU/mL in each well. The microplates were incubated at 35 ± 2°C for 24 hours and the minimum inhibitory concentration (MIC) was determined using 20 µL resazurin (Sigma).
Briefly, minimum bactericidal concentration (MBC) was determined on plates containing Müller-Hinton agar (MHA) and considered as the lowest concentration of extracts associated with absence of bacterial growth. Tests were done in triplicate.
2.5. Antifungal activity
Three cultures of Trichophyton rubrum preserved under mineral oil (Sherf, 1943SHERF, A.F., 1943. A method for maintaining Phytomonas sepedonica in culture for long periods without transfer. Phytopathology, vol. 33, pp. 330-332.) were used in duplicate, maintained and supplied by the Collection of Cultures - Micoteca URM, Department of Mycology, Center of Biosciences, Federal University of Pernambuco. The preserved cultures were reactivated, transferring a culture fragment to glycosated broth and then replaced to Sabouraud agar, maintained at room temperature (RT = 30°C ± 2°C).
The antifungal activity of the extracts against T. rubrum cultures was evaluated by means of the disc diffusion test according to the methodology modified by Ichikawa et al. (1971)ICHIKAWA, T., DATE, M., ISHIKURA, T. and OZAKI, A., 1971. Improvement of Kasugamycin – producing strain by the agar piece method and the prototroph method. Folia Microbiologica, vol. 16, no. 3, pp. 218-224. http://dx.doi.org/10.1007/BF02884210. PMid:4935426.
http://dx.doi.org/10.1007/BF02884210...
. Cultures with 10 days of growth in Sabouraud Agar (g.L-1: dextrose 40, peptone 10 and agar 18) were incubated at 25°C in test tubes and suspensions were prepared in sterilized distilled water with tween and adjusted, for spectrophotometer, to obtain a transmittance of 78-80%. Subsequently, 2 mL of the standard suspension were added to previously sterilized Petri dishes, 15 mL of the Sabouraud agar medium being added at a temperature of approximately 45°C. After solidification, a sterile 5 mm diameter disc was placed and moistened with extracts containing 0.0016 mg dissolved in 1 mL DMSO. Plates were maintained at 28°C ± 2°C for 48 hours. After incubation, the presence or absence of halos was verified. The inhibition halos were measured in millimeters and expressed as arithmetic mean. As a control to check the solvent effect, a disk with DMSO was inoculated under the same conditions.
This method followed the conditions described by Clinical and Laboratory Standard Institute (CLSI, 2008CLINICAL AND LABORATORY STANDARDS INSTITUTE – CLSI, 2008. CLSI document M27A3. Reference method for broth dilution antifungal susceptibility testing of yeast: approved standard – third edition. USA: CLSI.). Fungus isolates were peeled in potato Dextrose Agar (BDA) medium in test tubes and incubated for six to seven days at 30ºC until sufficient conidia were present. Sequentially, 3 mL of 0.85% sterile saline solution added with tween 20 was added to the fungal colonies. The suspension was homogenized with a pipette and subsequently the resulting mixture was transferred to sterile test tubes. After five minutes of standing, the supernatant was transferred to another sterilized test tube and shaken for 15 seconds in vortex. The density of the suspension was adjusted in the spectrophotometer to obtain transmittance of 65-70% at wavelength 530 nm. The suspensions were then diluted (1:50) in RPMI 1640 to give a final concentration of 0.4 x 104 to 5 x 104 CFU/mL.
In 96-well flat-bottomed microtiter plates, 100 µL of RPMI 1640 culture medium was distributed in all wells. At the following dilution, 100 µL of the extract, at a concentration of 1600 to 3.125 µg.mL-1, was used as growth and sterilization control, respectively. Subsequently, 100 µL of the standardized inoculum was added to the wells of columns 1 to 11, and the microplate was incubated for 48 hours at 35°C. Minimum inhibitory concentration (MIC) was defined as the lowest concentration of antifungal capable of inhibiting 50% to 90% growth.
2.6. Antineoplastic activity
Cytotoxicity was evaluated initially against normal cells as described by Rêgo et al. (2014)RÊGO, M.J.B.D.M., GALDINO-PITTA, M.R., PEREIRA, D.T.M., DA SILVA, J.C., RABELLO, M.M., ALVES DE LIMA, M.C., HERNANDES, M.Z., DA ROCHA PITTA, I., GALDINO, S.L. and DA ROCHA PITTA, M.G., 2014. Synthesis, in vitro anticancer activity and in silico study of new disubstituted thiazolidinedione derivatives. Medicinal Chemistry Research, vol. 23, no. 6, pp. 3220-3226. http://dx.doi.org/10.1007/s00044-013-0902-z.
http://dx.doi.org/10.1007/s00044-013-090...
. The trials were only started after approval by the Ethics Committee on Research Involving Human Beings of the Health Science Center of UFPE, under the CAAE: 46976315.9.0000.5208. All volunteers gave their informed consent.
Four clinically healthy individuals were included in the selection. Peripheral blood mononuclear cells (PBMCs) contained in blood collected in heparin tubes as anticoagulants were isolated by centrifugation with Ficoll Paque ™ Plus (GE Healthcare Bio-Sciences). After, they were cultured in RPMI 1640 medium (Gibco) supplemented with L-Glutamine, 10% Fetal Bovine Serum (Lonza), 10 mM HEPES (Gibco) and 200 (4- (2-hydroxyethyl) -1-piperazineethanesulfonic acid) U/mL Penicillin/Streptomycin (Gibco). PBMCs were used only when they showed viability equal to or greater than 98% after counting in Neubauer chamber with trypan blue.
The PBMCs were grown at 5% CO2 and 37°C atmosphere in the amount of 5.5x105 cells/well in quadruplicate for 48 h. After that time, MTT (20 µg.mL-1) was added for 3 h in the oven and, finally, 20% sodium Dodecyl sulfate (SDS) for dissolution of the crystals. After 24 h, the plates were read in Spectrophotometer at 570 nm. The extracts were tested at 0.1 to 100 µg.mL-1. To evaluate the cytotoxicity by trypan blue assay, after incubation for 48 hours of PBA and PBO extracts at 1, 10 and 100 µg.mL-1, a 20 µL aliquot of the cell suspension was diluted in 20 µL of trypan blue. The cells were observed for their morphological changes and counted in Neubauer's chamber. After counting, the following mathematical formula 1 was applied, which gives the result of the cytotoxicity of the extracts against the PBMCs from the cellular viability values of each condition:
As a negative control, the condition with only cell was used for the extracts diluted in water (PBA), and the condition of cell with 0.1% DMSO for the extracts diluted in DMSO (PBO).
The same methodologies were performed for the cytotoxicity assay in neoplastic cells: TOLEDO (Non-Hodgkin B Cell Lymphoma), K562 (Chronic Myeloid Leukemia), DU-145 (Prostate Cancer) and PANC-1 (Pancreatic Cancer) at 1, 10 and 100 µg.mL-1 of PBA and PBO.
2.7. Immunomodulatory potential
To evaluate the cytokine modulation, we conducted the methodology described by Carvalho et al. (2016)CARVALHO, L.V.N., CORDEIRO, M.F., E LINS, T.U.L., SAMPAIO, M.C.P.D., DE MELLO, G.S.V., DA COSTA, V.C.M., MARQUES, L.L.M., KLEIN, T., DE MELLO, J.C.P., CAVALCANTI, I.M.F., PITTA, I.R., GALDINO DA ROCHA PITTA, M. and RÊGO, M.J.B.M., 2016. Evaluation of antibacterial, antineoplastic, and immunomodulatory activity of Paullinia cupana Seeds crude extract and ethyl-acetate fraction. Evidence-Based Complementary and Alternative Medicine, vol. 2016, pp. 1-7. http://dx.doi.org/10.1155/2016/1203274. PMid:28053639.
http://dx.doi.org/10.1155/2016/1203274...
. Six male BALB/c mice with 45 days of life were sacrificed in a CO2 chamber, according to the Guidelines of the Ethics Committee for the Use of Experimental Animals of the UFPE (Process nº 23076.041556/2015-62).
Briefly, the spleens were aseptically extracted and placed in a Petri dish containing RPMI-1640 (Gibco) to obtain splenocytes. The obtained cell suspension was filtered on 40 µm nylon (BD Falcon ™) and transferred to Falcon tubes. Spleen concentrates were washed and lysed with 1x RBC lysis buffer (eBiosciences) and subsequently resuspended in RPMI-1640 medium (Sigma) supplemented with 10% fetal bovine serum, 10 mM HEPES (4- (2-hydroxyethyl) -1-piperazinoethanesulfonic acid) (Gibco) and 200 U/mL penicillin/streptomycin (Gibco). The evaluation of PBA and PBO cytotoxicity was performed by incubation (10 and 100 µg.mL-1) for 48 h at 5% CO2 and 37°C. After 48 h, MTT was added as described in item 2.5.
Splenocytes were then cultured in 24 well plates (2x106/mL in each well) in RPMI-1640 medium (Gibco) supplemented with 10% fetal bovine serum (Gibco), 10 mM HEPES (Gibco) and penicillin and 200 U streptomycin/mL (Gibco). As a stimulus, was used Concanavalin A (ConA) at 100 ng.mL-1. As the reference drug, methylprednisolone was added at 100 µM. The PBA and PBO extracts were introduced at 10 and 100 µg.mL-1 and incubated at 37°C and 5% CO2 for 48 h.
After incubation, 1 mL of culture supernatant was collected from each well, stored at -30°C until use. Cytokine determination was performed by sandwich ELISA (mouse), following the manufacturer's information. The minimum detection limits for IFN-γ and IL-17A analysis was 15.62 pg.mL-1.
2.8. Statistics
To assess the normal distribution of variables, the Kolmogorov-Smirnov test was used. The variables that did not present normal distribution were analyzed through the Wilcoxon non-parametric test and presented as maximum and minimum median. Analyzes were performed using GraphPad Prism® software version 6 and considered significant values when p < 0.05.
3. Results
According to the analysis by TLC, spots of yellow and orange color indicating the presence of flavonoids and blue-green indicating the presence of cinnamic derivatives (A); no condensed tannins (B) and hydrolysable tannins (C) were found in any of the extracts; finally, bands of blue color were observed in the plate obtained for terpenes/steroids (D), indicating the presence of compounds of the class (Figure S1).
The chromatograms from HPLC analysis of PBA and PBO extracts can be visualized in Figure 1. In both chromatographic profiles, the presence of cinnamic derivatives was detected, with a retention time (tR) of 19.83 (peak 1, corresponding to the presence of caffeic acid) and 25.5 min (peak 2) approximately in both extracts PBA and PBO. In addition, the others peaks (3-6) have characteristic of the flavonoids metabolites observed in both extracts, but with greater area value evidenced in the PBO extract, with tR at 26.25, 26.55, 26.92, 27.29 and 27.41 min. Caffeic acid and rutin standards were injected, and the presence of caffeic acid in the samples was confirmed by the same retention time and scanning spectrum of the obtained peaks. In addition, although no peak corresponds to the rutin (flavonoid), the spectra of peaks 3 to 7 show a characteristic of the flavonoid class with three absorption maxima.
Chromatograms of Plectranthus barbatus aqueous extract (PBA) and acetone: water (PBO). Detection at 280 nm.
PBA did not show antibacterial activity against clinical isolates. PBO, in turn, had a bacteriostatic effect against all eight isolates tested, including strains with resistance profile (MIC = 500 µg.mL-1) as ESBL, KPC and MRSA. In addition, PBO had bacteriostatic activity against the Acinetobacter baumannii clinical isolate (MIC = 250 µg.mL-1) as shown in Table 1.
Antibacterial activity of aqueous extracts (PBA) and acetone: water (PBO) of Plectranthus barbatus against pathogenic bacteria.
After analysis of disc-diffusion assay were identified a halo inhibition about 6mm with PBA treatment. However, the halo inhibition activity of PBO was not evidenced. Due to Trichophyton rubrum growth inhibition by PBA we conducted the microdilution test and it was evidenced that PBA shows fungistatic activity (MIC = 800 µg.mL-1) against this microorganism.
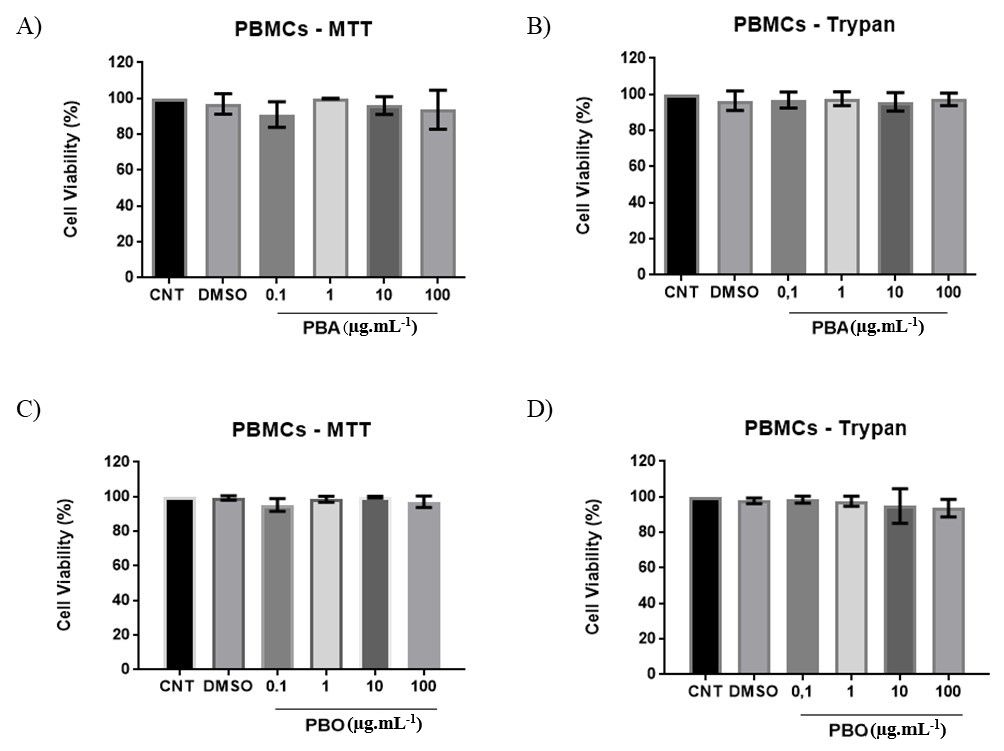
The results of MTT and trypan blue viability tests showed that PBA (see Figures 22B) and PBO (see Figures 22D) did not exhibit cytotoxicity against PBMCs. However, for neoplastic lineages, PBA and PBO exhibited different cytotoxicity depending on the tested concentration. PBO at 100 µg.mL-1 appear to be more cytotoxic for all neoplastic tested lineages. Despite this we did not found IC50 values to no one evaluated neoplastic cells (Figure 3).
Evaluation of cytotoxic activity of Plectranthus barbatus extracts against untransformed human cells in A (MTT) and B (Trypan Blue) are shown aqueous extract – PBA profile; C (MTT) and D (Trypan Blue) are present acetone: water – PBO profile at four different concentrations (0.1, 1, 10, and 100 µg.mL-1). CNT: Untreated control.
Plectranthus barbatus extracts influence against neoplastic cells lineages. TOLEDO, K562, DU-145 and PANC-1 neoplastic cell lines at 1, 10 and 100 µg.mL-1 of PBA and PBO with MTT (A, C, E, G) and Trypan Blue (B, D, F, H) assays.
Regarding the evaluation of extracts in reduction of cytokine levels, PBA [188.63 pg.mL-1 (70.79 pg.mL-1 - 610.37 pg.mL-1)] did not inhibit IL-17A cytokine production at tested concentrations when compared to ConA (stimulated control) [128.63 pg.mL-1 (55.79 pg.mL-1 - 387.76 pg.mL-1)]. However, the greater result was found after PBO treatment, decreasing IL-17A levels, notably at 100 µg.mL-1 (15.62 pg.mL-1 (15.62 pg.mL-1 - 45.79 pg.mL-1)) (see Figure 4A) (p=0.03).
Cytokine titration in culture supernatant of BALB/c splenocytes treated with Plectranthus barbatus (aqueous: PBA; acetone: water: PBO) extracts at 10 and 100 µg.mL-1. (A) levels of IL-17A; (B) IFN-γ levels. CELL = untreated control; CONA = Concanavalin A; MP = methylprednisolone at 100 μM; (*) p = 0.03; (●) represents outliers.
Additionally, the treatment with PBO in relation to ConA [13554.1 pg.mL-1 (2342.96 pg.mL-1 - 35280.12 pg.mL-1)], significative decreased IFN-γ levels at all evaluated concentrations as we describe below. PBA 10 µg.mL-1 was not significant but PBA 100 µg.mL-1 [5765.64 pg.mL-1 (62.5 pg.mL-1 - 12846.41 pg.mL-1)], PBO 10 µg.mL-1 [2023.33 pg.mL-1(62.5 pg.mL-1 - 8577.18 pg.mL-1)] and PBO 100 µg.mL-1 [39.06 pg.mL-1 (15.62 pg.mL-1 - 1884.87 pg.mL-1)] showed significant values (p = 0.03) (see Figure 4B).
4. Discussion
P. barbatus, especially from India, Brazil, Kenya and China has been an attractive plant for development of chemical and pharmacological studies to discovery novel biologically active constituents. P. barbatus main constituents isolated from different parts of this species are diterpenoids, and these phytochemicals are responsible for many described biological activities (Alasbahi and Melzig, 2010ALASBAHI, R.H. and MELZIG, M.F., 2010. Plectranthus barbatus: a review of phytochemistry, ethnobotanical uses and pharmacology – Part 1. Planta Medica, vol. 76, no. 7, pp. 653-661. http://dx.doi.org/10.1055/s-0029-1240898. PMid:20178070.
http://dx.doi.org/10.1055/s-0029-1240898...
). However, the presence of phenolic compounds in P. barbatus extracts has been reported in several studies (Brito et al., 2018BRITO, E., GOMES, E., FALÉ, P.L., BORGES, C., PACHECO, R., TEIXEIRA, V., MACHUQUEIRO, M., ASCENSÃO, L. and SERRALHEIRO, M.L.M., 2018. Bioactivities of decoctions from Plectranthus species related to their traditional use on the treatment of digestive problems and alcohol intoxication. Journal of Ethnopharmacology, vol. 220, pp. 147-154. http://dx.doi.org/10.1016/j.jep.2018.04.006. PMid:29626671.
http://dx.doi.org/10.1016/j.jep.2018.04....
, Ganash & Qanash, 2018GANASH, M. and QANASH, S., 2018. Phenolic acids and biological activities of Coleus forskohlii and Plectranthus barbatus as traditional medicinal plants. International Journal of Pharmacology, vol. 14, no. 6, pp. 856-865. http://dx.doi.org/10.3923/ijp.2018.856.865.
http://dx.doi.org/10.3923/ijp.2018.856.8...
, Shaheen et al., 2017SHAHEEN, U., KHALIK, K.A., ABDELHADY, M.I.S., HOWLADAR, S., ALARJAH, M. and ABOUREHAB, M.A.S., 2017. HPLC Profile of Phenolic Constituents, Essential Oil Analysis and Antioxidant Activity of Six Plectranthus Species Growing in Saudi Arabia. Journal of Chemical and Pharmaceutical Research, vol. 9, no. 4, pp. 345-354., Silva et al., 2016SILVA, C.F.G., MENDES, M.P., ALMEIDA, V.V., MICHELS, R.N., SAKANAKA, L.S. and TONIN, L.T.D., 2016. Parâmetros de qualidade físico-químicos e avaliação da atividade antioxidante de folhas de Plectranthus barbatus Andr. (Lamiaceae) submetidas a diferentes processos de secagem. Revista Brasileira de Plantas Medicinais, vol. 18, no. 1, pp. 48-56. http://dx.doi.org/10.1590/1983-084X/15_021.
http://dx.doi.org/10.1590/1983-084X/15_0...
, Rodrigues et al., 2016RODRIGUES, T. S., GUIMARÃES, S.F., BRAGA, T.V., BASTOS, J.C.S.A. and RODRIGUES-DAS-DÔRES, R.G., 2016. Determination of content of phenolic compounds and flavonoids in leaves extracts of Plectranthus sp. (“Boldos”), potential antioxidant and antibacterial action. Academia Journal of Medicinal Plants, vol. 4, no. 10, pp. 062-068. https://doi.org/10.15413/ajmp.2016.0111.
https://doi.org/10.15413/ajmp.2016.0111...
). Thus, in order to elucidate the biological activities of P. barbatus leaves extracts that contains phenolic compounds, here we conducted the evaluation of antimicrobial, antineoplastic and immunomodulatory potential of these extracts.
The phytochemical characterization of our extracts indicated the presence of flavonoids, cinnamic derivatives, steroids and ellagic acid in both extracts. Caffeic acid was evidenced in both extracts, however rosmarinic acid is the major evidenced compound in P. barbatus extracts (Brito et al., 2018BRITO, E., GOMES, E., FALÉ, P.L., BORGES, C., PACHECO, R., TEIXEIRA, V., MACHUQUEIRO, M., ASCENSÃO, L. and SERRALHEIRO, M.L.M., 2018. Bioactivities of decoctions from Plectranthus species related to their traditional use on the treatment of digestive problems and alcohol intoxication. Journal of Ethnopharmacology, vol. 220, pp. 147-154. http://dx.doi.org/10.1016/j.jep.2018.04.006. PMid:29626671.
http://dx.doi.org/10.1016/j.jep.2018.04....
). Rosmarinic acid is an ester of caffeic acid and 3,4dihydroxyphenyllactic acid and it is known that this compound presents several biological activities such as antimicrobial, anti-inflammatory, antioxidant, anti-viral and adstringent. As presented in literature, the rosmarinic acid is formed by tow molecules of caffeic acid, joined by an ester bond (Gamaro et al., 2011GAMARO, G.D., SUYENAGA, E., BORSOI, M., LERMEN, J., PEREIRA, P. and ARDENGHI, P., 2011. Effect of rosmarinic and caffeic acids on inflammatory and nociception process in rats. International Scholarly Research Network ISRN Pharmacology, vol. 2011, pp. 1-6. http://dx.doi.org/10.5402/2011/451682. PMid:22084714.
http://dx.doi.org/10.5402/2011/451682...
). Both molecules belong to the same class of compounds, the phenylpropanoids, therefore it can be related to the biological activities here observed. In addition, the differences of chemical contents in our extracts and the related literature about extracts of P. barbatus leaves is probably be associated with the phenological cycle, where the temperature, light and humidity environmental when the plant was collected can influence the amount of the different substances found on herbal material (Raudone et al., 2017RAUDONE, L., ZYMONE, K., RAUDONIS, R., VAINORIENE, R., MOTIEKAITYTE, V. and JANULIS, V., 2017. Phenological changes in triterpenic and phenolic composition of Thymus L. Species. Industrial Crops and Products, vol. 109, pp. 445-451. http://dx.doi.org/10.1016/j.indcrop.2017.08.054.
http://dx.doi.org/10.1016/j.indcrop.2017...
).
Then we evaluated the antibacterial activity and observed that the organic extract PBO presented bacteriostatic activity against all bacterial isolates, especially against Acinetobacter baumannii. A study conducted by Rodrigues et al. (2016)RODRIGUES, T. S., GUIMARÃES, S.F., BRAGA, T.V., BASTOS, J.C.S.A. and RODRIGUES-DAS-DÔRES, R.G., 2016. Determination of content of phenolic compounds and flavonoids in leaves extracts of Plectranthus sp. (“Boldos”), potential antioxidant and antibacterial action. Academia Journal of Medicinal Plants, vol. 4, no. 10, pp. 062-068. https://doi.org/10.15413/ajmp.2016.0111.
https://doi.org/10.15413/ajmp.2016.0111...
identified the antibacterial activity in extract of the aerial parts of P. barbatus against S. aureus however they evaluated this activity through the growth inhibition zone and the extract showed 9.03 ± 0.103 mm of inhibition. In addition, these authors correlated this observation with the presence of phenolic compounds in the extract of P. barbatus once that the probable mechanism of action of phenolic compounds is related on bacterial cytoplasmic membrane. These molecules alter the structure and function of cytoplasmic membrane coagulating the cell content and interfering with active transport (Almeida, 2007ALMEIDA, A.A.P., 2007. Atividade antimicrobiana de extratos e de compostos fenólicos nitrogenados de café: avaliação in vitro e em modelo alimentar. Belo Horizonte: Faculdade de Farmácia, Universidade Federal de Minas Gerais, 137 p. Tese de Doutorado em Ciência de Alimentos.). Ahmad et al. (2015)AHMAD, A., KALEEM, M., AHMED, Z. and SHAFIQ, H., 2015. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral Infections-a review. Food Research International, vol. 77, pp. 221-235. http://dx.doi.org/10.1016/j.foodres.2015.06.021.
http://dx.doi.org/10.1016/j.foodres.2015...
also explain that the phenolic compounds can acts interacting with proteins, generating redox imbalance and acting on the cell wall.
In addition, Acinetobacter baumannii is gram-negative bacteria that is related with several nosocomial infections. Other microorganisms, as E. coli and S. aureus that causes these infections were objects of study regarding the discovery of new therapeutic options. Matu and Van Staden (2003)MATU, E.N. and VAN STADEN, J., 2003. Antibacterial and anti-inflammatory activities of some plants used for medicinal purposes in Kenya. Journal of Ethnopharmacology, vol. 87, no. 1, pp. 35-41. http://dx.doi.org/10.1016/S0378-8741(03)00107-7. PMid:12787952.
http://dx.doi.org/10.1016/S0378-8741(03)...
used the aqueous, hexanic and methanolic extracts of P. barbatus leaves to identify their actions against gram-positive bacteria (Bacillus subtilis, Micrococcus luteus, Staphylococcus aureus) and gram-negative (E. coli and K. pneumoniae) bacteria. At their study it was identified that the methanolic extract showed activity against all Gram-positive isolates. However, Santos Veríssimo et al. (2014)SANTOS VERÍSSIMO, R., LINS, T., ASSIS BASTOS, M., ALBUQUERQUE SARMENTO, P., ALVINO, V., SILVA ARAUJO, M., LOPES SILVA, A. and ARAÚJO-JÚNIOR, J.2014. Antimicrobial activity of Plectranthus barbatus (Lamiacea). BMC Proceedings, vol. 8, suppl. 4, pp. 264. http://dx.doi.org/10.1186/1753-6561-8-S4-P264.
http://dx.doi.org/10.1186/1753-6561-8-S4...
identified the antibacterial activity of the crude ethanol extract of P. barbatus leaves against E. coli showing a MIC = 6250 µg.mL-1 and also against Staphylococcus aureus (MIC = 3120 µg.mL-1). Araújo et al. (2014)ARAÚJO, S.G., ALVES, L.F., PINTO, M.E., OLIVEIRA, G.T., SIQUEIRA, E.P., RIBEIRO, R.I., FERREIRA, J.M. and LIMA, L.A., 2014. Volatile compounds of Lamiaceae exhibit a synergistic antibacterial activity with streptomycin. Brazilian Journal of Microbiology, vol. 45, no. 4, pp. 1341-1347. http://dx.doi.org/10.1590/S1517-83822014000400026. PMid:25763039.
http://dx.doi.org/10.1590/S1517-83822014...
also evaluated the antibacterial action of the leaf extract of P. barbatus, which presented bacteriostatic activity against E. coli (MIC = 250 µg.mL-1, MBC > 2000 µg.mL-1) and P. aeruginosa (MIC = 250 µg.mL-1, MBC = 2000 µg.mL-1). Our data corroborate with these authors regarding the greater activity of the organic extract compared to the aqueous one in relation to pathogenic bacteria.
Regarding the activity of extract against multidrug-resistant strains, Assis et al. (2018)ASSIS, F.V., SIQUEIRA, F.L., GONÇALVES, I.E., LACERDA, R.P., NASCIMENTO, R.A., ARAÚJO, S.G., ANDRADE, J.T., HERRERA, K.M.S., LIMA, L.A.R.S. and FERREIRA, J.M.S., 2018. Antibacterial activity of Lamiaceae plant extracts in clinical isolates of multidrug-resistant bacteria. Anais da Academia Brasileira de Ciências, vol. 90, no. 2, pp. 1665-1670. http://dx.doi.org/10.1590/0001-3765201820160870. PMid:29668795.
http://dx.doi.org/10.1590/0001-376520182...
evaluated the ethanol extract of three Lamiaceae species against Acinetobacter baumannii, Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa clinical isolates with multidrug-resistant profile. They identified that the P. barbatus extract was the most effectiveness, exhibiting MIC of 2000, 1000, 1000 and 500 µg.mL-1 respectively, however, they did not identify the extract composition. Furthermore, our extract that presents phenolic compounds showed bacteriostatic activity against KPC, ESBL and MRSA, and some studies indicate that these molecules showed activity against multidrug-resistant strains (Kępa et al., 2018KĘPA, M., MIKLASIŃSKA-MAJDANIK, M., WOJTYCZKA, R.D., IDZIK, D., KORZENIOWSKI, K., SMOLEŃ-DZIRBA, J. and WĄSIK, T.J., 2018. Antimicrobial potential of caffeic acid against Staphylococcus aureus clinical strains. BioMed Research International, vol. 2018, pp. 1-8. http://dx.doi.org/10.1155/2018/7413504. PMid:30105241.
http://dx.doi.org/10.1155/2018/7413504...
; Aldulaimi, 2017ALDULAIMI, O.A., 2017. General overview of phenolics from plant to laboratory, good antibacterials or Not. Pharmacognosy Reviews, vol. 11, no. 22, pp. 123-127. http://dx.doi.org/10.4103/phrev.phrev_43_16. PMid:28989246.
http://dx.doi.org/10.4103/phrev.phrev_43...
; Luís et al., 2013LUÍS, Â., SILVA, F., SOUSA, S., DUARTE, A.P. and DOMINGUES, F., 2013. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling, vol. 30, no. 1, pp. 69-79. http://dx.doi.org/10.1080/08927014.2013.845878. PMid:24228999.
http://dx.doi.org/10.1080/08927014.2013....
). Thus, phenolic compounds are considered promising weapons in the arsenal of natural antimicrobials acting against a wide spectrum of microorganisms, including multidrug-resistant bacteria, that cause nosocomial infections (Miklasínska-Majdanik et al., 2018).
Though PBA did not exhibit bacteriostatic activity, this extract was fungistatic against Trichophyton rubrum. The study about antifungal activity of P. barbatus is scarce, however Runyoro et al. (2006)RUNYORO, D.K.B., MATEE, M.I.N., NGASSAPA, O.D., JOSEPH, C.C. and MBWAMBO, Z.H., 2006. Screening of Tanzanian medicinal plants for anti-Candida activity. BMC Complementary and Alternative Medicine, vol. 6, no. 11, pp. 1-10. https://doi.org/10.1186/1472-6882-6-11. PMID: 16571139.
https://doi.org/10.1186/1472-6882-6-11...
studied the root extract of this plant against Candida albicans and it was effective on in vitro treatment of this microorganism. Araújo et al. (2019)ARAÚJO, S.G., LIMA, W.G., PINTO, M.E.A., MORAIS, M.I., DE SÁ, N.P., JOHANN, S., ROSA, C.A. and LIMA, L.A.R.S., 2019. Pharmacological prospection in-vitro of Lamiaceae species against human pathogenic fungi associated to invasive infections. Biocatalysis and Agricultural Biotechnology, vol. 21, pp. 101345. https://doi.org/10.1016/j.bcab.2019.101345.
https://doi.org/10.1016/j.bcab.2019.1013...
identified the antifungal activity of ethanol extract of P. barbatus leaves against species involved in invasive fungal disease and showed the greatest MIC values against Candida glabrata (31.2 µg.mL-1), Saccharomyces cerevisiae, Rhodotorula mucilaginosa, Candida krusei, Candida parapsilosis and Candida gattii (all with MIC = 62.5 µg.mL-1), associating these findings with the presence of steroids/triterpenes, flavonoids, and alkaloid.
The tested extracts in our study showed no toxicity to PBMCs. We observed that the toxicity of the organic extract at 100 µg.mL-1 was highlighted in the four neoplastic cell lines but did not present IC50 values lower than 100 µg.mL-1. Saeed et al. (2016)SAEED, M.E.M., MEYER, M., HUSSEIN, A. and EFFERTH, T., 2016. Cytotoxicity of South-African medicinal plants towards sensitive and multidrug-resistant cancer cells. Journal of Ethnopharmacology, vol. 186, pp. 209-223. http://dx.doi.org/10.1016/j.jep.2016.04.005. PMid:27058630.
http://dx.doi.org/10.1016/j.jep.2016.04....
evaluated the cytotoxicity of the methanolic extract of P. barbatus leaves against two neoplastic leukemia lines, CCRF-CEM, as sensitive lineage and a multiresistant drug cell line (CEM / ADR5000), through resazurin assay. The tested concentration of the extract (10 µg.mL-1) presented cytotoxic activity against the two cells lines, with IC50 = 5.7 ± 1.29 µg.mL-1 (CCRF-CEM) and IC50 = 7.93 ± 0.64 µg.mL-1 (CEM / ADR5000). Although they correlated these results to the presence of forskolin and coleusin factor, they did not isolate them, but mentioned that these molecules showed cytotoxicity to murine melanoma cells (Agarwal and Parks Junior, 1983AGARWAL, K.C. and PARKS JUNIOR, R.E., 1983. Forskolin: a potential antimetastatic agent. International Journal of Cancer, vol. 32, no. 6, pp. 801-804. http://dx.doi.org/10.1002/ijc.2910320622. PMid:6686215.
http://dx.doi.org/10.1002/ijc.2910320622...
) and osteosarcoma (Geng et al., 2014GENG, S., SUN, B., LU, R. and WANG, J., 2014. Coleusin factor, a novel anticancer diterpenoid, inhibits osteosarcoma growth by inducing bone morphogenetic protein-2-dependent differentiation. Molecular Cancer Therapeutics, vol. 13, no. 6, pp. 1431-1441. http://dx.doi.org/10.1158/1535-7163.MCT-13-0934. PMid:24723453.
http://dx.doi.org/10.1158/1535-7163.MCT-...
). In addition, Borges et al. (2020)BORGES, A. S., MINOZZO, B. R., SANTOS, H., ARDISSON, J.S., RODRIGUES, R.P., ROMÃO, W., BORGES, W.S., Gonçalves, R.C.R., BELTRAME, F.R. and KITAGAWA, R. R. (2020). Plectranthus barbatus Andrews as anti-Helicobacter pylori agent with activity against adenocarcinoma gastric cells. Industrial Crops & Products vol. 146, pp; 1-12. https://doi.org/10.1016/j.indcrop.2020.112207.
https://doi.org/10.1016/j.indcrop.2020.1...
identified an antineoplastic activity (IC50 = 33.89 ± 0.32 µg.mL-1) of ethyl acetate fraction (EAF) of P. barbatus leaves, but the identified constituents of EAF were plectrin, hydrolyzed abieatane, barbatusin, 3β-hydroxy-3-deoxibarbatusin, cyclobutatusin, luteolin, rosmarinic acid and coleoside B which we did not detect in our extracts.
Regarding the action of caffeic acid, although there are several studies with the antineoplastic activity of this substance (Kampa et al., 2004KAMPA, M., ALEXAKI, V.I., NOTAS, G., NIFLI, A.P., NISTIKAKI, A., HATZOGLOU, A., BAKOGEORGOU, E., KOUIMTZOGLOU, E., BLEKAS, G., BOSKOU, D., GRAVANIS, A. and CASTANAS, E., 2004. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Research, vol. 6, no. 2, pp. 63-74. http://dx.doi.org/10.1186/bcr752. PMid:14979919.
http://dx.doi.org/10.1186/bcr752...
; Jaganathan and Mandal, 2009JAGANATHAN, S.K. and MANDAL, M., 2009. Antiproliferative Effects of Honey and of Its Polyphenols: A Review. Journal of Biomedicine & Biotechnology, vol. 2009, pp. 1-13. http://dx.doi.org/10.1155/2009/830616. PMid:19636435.
http://dx.doi.org/10.1155/2009/830616...
; Feng et al., 2010FENG, L., JIA, X., JIANG, J., ZHU, M., CHEN, Y., TAN, X.-B. and SHI, F., 2010. Combination of Active Components Enhances the Efficacy of Prunella in Prevention and Treatment of Lung Cancer. Molecules (Basel, Switzerland), vol. 15, no. 11, pp. 7893-7906. http://dx.doi.org/10.3390/molecules15117893. PMid:21060297.
http://dx.doi.org/10.3390/molecules15117...
; Puangpraphant et al., 2011PUANGPRAPHANT, S., BERHOW, M.A., VERMILLION, K., POTTS, G. and GONZALEZ DE MEJIA, E., 2011. Dicaffeoylquinic acids in Yerba mate (Ilex paraguariensis St. Hilaire) inhibit NF-kB nucleus translocation in macrophages and induce apoptosis by activating caspases-8 and-3 in human colon cancer cells. Molecular Nutrition & Food Research, vol. 55, no. 10, pp. 1509-1522. http://dx.doi.org/10.1002/mnfr.201100128. PMid:21656672.
http://dx.doi.org/10.1002/mnfr.201100128...
; Weng and Yen, 2012WENG, C.J. and YEN, G., 2012. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treatment Reviews, vol. 38, no. 1, pp. 76-87. http://dx.doi.org/10.1016/j.ctrv.2011.03.001. PMid:21481535.
http://dx.doi.org/10.1016/j.ctrv.2011.03...
), in our study we were unable to identify a significant activity. These findings are probably associated with the interference of several molecules present in the extract, which may have inhibited the antineoplastic action of this constituent (Devlin, 1997DEVLIN, J.P., 1997. High throughput screening: the discovery of bioactive substances. Marcel Dekker. 704 p. http://dx.doi.org/10.1201/9781482269802.
http://dx.doi.org/10.1201/9781482269802...
).
We also evaluate P. barbatus influence on cytokine modulation and found that PBO at 100 µg.mL-1 significantly inhibited IFN-γ and IL-17A cytokines. In accordance with these results, Kapewangolo et al. (2013)KAPEWANGOLO, P., HUSSEIN, A.A. and MEYER, D., 2013. Inhibition of HIV-1 enzymes, antioxidant and anti-inflammatory activities of Plectranthus barbatus. Journal of Ethnopharmacology, vol. 149, no. 1, pp. 184-190. http://dx.doi.org/10.1016/j.jep.2013.06.019. PMid:23811046.
http://dx.doi.org/10.1016/j.jep.2013.06....
evaluated the influence of ethyl acetate fraction of P. barbatus leaves on secretion inhibition of proinflammatory cytokines by PBMCs and verified that this extract significatively inhibited the secretion of IL-2, IL-6, IL-10, TNF and IL-17A at 25 and 50 µg.mL-1. Matu and Van Staden (2003)MATU, E.N. and VAN STADEN, J., 2003. Antibacterial and anti-inflammatory activities of some plants used for medicinal purposes in Kenya. Journal of Ethnopharmacology, vol. 87, no. 1, pp. 35-41. http://dx.doi.org/10.1016/S0378-8741(03)00107-7. PMid:12787952.
http://dx.doi.org/10.1016/S0378-8741(03)...
performed an anti-inflammatory activity test of the aqueous, hexanic and methanolic extracts of P. barbatus leaves at 500 μg.L-1 in a COX-1 inhibition assay and found a percentage of methanolic extract inhibition (88 ± 3.5%) higher than the aqueous extract (7.0 ± 1.5%).
Besides the observation that P. barbatus extracts have immunomodulatory activity evidenced in some studies, as mentioned, our findings also corroborate with Gamaro et al. (2011)GAMARO, G.D., SUYENAGA, E., BORSOI, M., LERMEN, J., PEREIRA, P. and ARDENGHI, P., 2011. Effect of rosmarinic and caffeic acids on inflammatory and nociception process in rats. International Scholarly Research Network ISRN Pharmacology, vol. 2011, pp. 1-6. http://dx.doi.org/10.5402/2011/451682. PMid:22084714.
http://dx.doi.org/10.5402/2011/451682...
work due to the presence of caffeic acid in both extracts. At their study, where the authors demonstrated the immunomodulatory activity of caffeic acid and rosmarinic acid through carrageenan-induced pleurisy model and tail-flick assay in rats, the caffeic acid presented the most pronounced anti-inflammatory effect compared to rosmarinic acid. Thus, these results reinforce that extracts of P. barbatus have an anti-inflammatory potential, especially when is detect the phenolic compounds.
After the identification of the main constituents and biological activities of P. barbatus aqueous and organic extracts here observed, we reinforce the importance of researches related of phenolic compounds biological activities in order to elucidate novel therapeutic approaches for a variety of diseases. Moreover, we encourage the identification of the possible mechanisms of action of these substances with important pharmacological effects and medical interest.
5. Conclusion
Due to the fact of its low toxicity in human healthy cells and exhibits a variety of biological activities, including bacteriostatic, fungistatic and immunomodulatory activity, Plectranthus barbatus with phenolic compounds is a potential candidate for use as herbal medicine of medical interest.
Supplementary Material
Supplementary Material accompanies this paper.
Table S1. Mobile phase chromogenic agents and standards used to phytochemical characterization of Plectranthus barbatus extracts.
Figure S1. Thin Layer Chromatography (TLC) analysis. Cinnamic derivatives (A); condensed tannins (B) hydrolysable tannins (C) terpenes/steroids (D).
This material is available as part of the online article from http://www.scielo.br/bjb
Acknowledgements
The authors thank Dr. Rita de Cássia Pereira from the Herbarium of the Agricultural Research Institute (IPA, Recife, PE, Brasil) for plant species identification, and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
References
- AGARWAL, K.C. and PARKS JUNIOR, R.E., 1983. Forskolin: a potential antimetastatic agent. International Journal of Cancer, vol. 32, no. 6, pp. 801-804. http://dx.doi.org/10.1002/ijc.2910320622 PMid:6686215.
» http://dx.doi.org/10.1002/ijc.2910320622 - AHMAD, A., KALEEM, M., AHMED, Z. and SHAFIQ, H., 2015. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral Infections-a review. Food Research International, vol. 77, pp. 221-235. http://dx.doi.org/10.1016/j.foodres.2015.06.021
» http://dx.doi.org/10.1016/j.foodres.2015.06.021 - ALASBAHI, R.H. and MELZIG, M.F., 2010. Plectranthus barbatus: a review of phytochemistry, ethnobotanical uses and pharmacology – Part 1. Planta Medica, vol. 76, no. 7, pp. 653-661. http://dx.doi.org/10.1055/s-0029-1240898 PMid:20178070.
» http://dx.doi.org/10.1055/s-0029-1240898 - ALDULAIMI, O.A., 2017. General overview of phenolics from plant to laboratory, good antibacterials or Not. Pharmacognosy Reviews, vol. 11, no. 22, pp. 123-127. http://dx.doi.org/10.4103/phrev.phrev_43_16 PMid:28989246.
» http://dx.doi.org/10.4103/phrev.phrev_43_16 - ALMEIDA, A.A.P., 2007. Atividade antimicrobiana de extratos e de compostos fenólicos nitrogenados de café: avaliação in vitro e em modelo alimentar Belo Horizonte: Faculdade de Farmácia, Universidade Federal de Minas Gerais, 137 p. Tese de Doutorado em Ciência de Alimentos.
- ARAÚJO, S.G., ALVES, L.F., PINTO, M.E., OLIVEIRA, G.T., SIQUEIRA, E.P., RIBEIRO, R.I., FERREIRA, J.M. and LIMA, L.A., 2014. Volatile compounds of Lamiaceae exhibit a synergistic antibacterial activity with streptomycin. Brazilian Journal of Microbiology, vol. 45, no. 4, pp. 1341-1347. http://dx.doi.org/10.1590/S1517-83822014000400026 PMid:25763039.
» http://dx.doi.org/10.1590/S1517-83822014000400026 - ARAÚJO, S.G., LIMA, W.G., PINTO, M.E.A., MORAIS, M.I., DE SÁ, N.P., JOHANN, S., ROSA, C.A. and LIMA, L.A.R.S., 2019. Pharmacological prospection in-vitro of Lamiaceae species against human pathogenic fungi associated to invasive infections. Biocatalysis and Agricultural Biotechnology, vol. 21, pp. 101345. https://doi.org/10.1016/j.bcab.2019.101345
» https://doi.org/10.1016/j.bcab.2019.101345 - ASSIS, F.V., SIQUEIRA, F.L., GONÇALVES, I.E., LACERDA, R.P., NASCIMENTO, R.A., ARAÚJO, S.G., ANDRADE, J.T., HERRERA, K.M.S., LIMA, L.A.R.S. and FERREIRA, J.M.S., 2018. Antibacterial activity of Lamiaceae plant extracts in clinical isolates of multidrug-resistant bacteria. Anais da Academia Brasileira de Ciências, vol. 90, no. 2, pp. 1665-1670. http://dx.doi.org/10.1590/0001-3765201820160870 PMid:29668795.
» http://dx.doi.org/10.1590/0001-3765201820160870 - BORGES, A. S., MINOZZO, B. R., SANTOS, H., ARDISSON, J.S., RODRIGUES, R.P., ROMÃO, W., BORGES, W.S., Gonçalves, R.C.R., BELTRAME, F.R. and KITAGAWA, R. R. (2020). Plectranthus barbatus Andrews as anti-Helicobacter pylori agent with activity against adenocarcinoma gastric cells. Industrial Crops & Products vol. 146, pp; 1-12. https://doi.org/10.1016/j.indcrop.2020.112207
» https://doi.org/10.1016/j.indcrop.2020.112207 - BRITO, E., GOMES, E., FALÉ, P.L., BORGES, C., PACHECO, R., TEIXEIRA, V., MACHUQUEIRO, M., ASCENSÃO, L. and SERRALHEIRO, M.L.M., 2018. Bioactivities of decoctions from Plectranthus species related to their traditional use on the treatment of digestive problems and alcohol intoxication. Journal of Ethnopharmacology, vol. 220, pp. 147-154. http://dx.doi.org/10.1016/j.jep.2018.04.006 PMid:29626671.
» http://dx.doi.org/10.1016/j.jep.2018.04.006 - CARDOSO, G.H.S., DANTAS, E.B.S., SOUSA, F.R.C. and PERON, A.P., 2014. Cytotoxicity of aqueous extracts of Rosmarinus officinalis L. (Labiatae) in plant test system. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 74, no. 4, pp. 886-889. http://dx.doi.org/10.1590/1519-6984.07313 PMid:25627599.
» http://dx.doi.org/10.1590/1519-6984.07313 - CARVALHO, L.V.N., CORDEIRO, M.F., E LINS, T.U.L., SAMPAIO, M.C.P.D., DE MELLO, G.S.V., DA COSTA, V.C.M., MARQUES, L.L.M., KLEIN, T., DE MELLO, J.C.P., CAVALCANTI, I.M.F., PITTA, I.R., GALDINO DA ROCHA PITTA, M. and RÊGO, M.J.B.M., 2016. Evaluation of antibacterial, antineoplastic, and immunomodulatory activity of Paullinia cupana Seeds crude extract and ethyl-acetate fraction. Evidence-Based Complementary and Alternative Medicine, vol. 2016, pp. 1-7. http://dx.doi.org/10.1155/2016/1203274 PMid:28053639.
» http://dx.doi.org/10.1155/2016/1203274 - CLINICAL AND LABORATORY STANDARDS INSTITUTE – CLSI, 2008. CLSI document M27A3. Reference method for broth dilution antifungal susceptibility testing of yeast: approved standard – third edition USA: CLSI.
- CLINICAL AND LABORATORY STANDARDS INSTITUTE – CLSI, 2016. Unusual suspects – resistance concerns and susceptibility testing among less common, but Noteworthy BacteriaTech.Rep USA: CLSI.
- DEVLIN, J.P., 1997. High throughput screening: the discovery of bioactive substances Marcel Dekker. 704 p. http://dx.doi.org/10.1201/9781482269802
» http://dx.doi.org/10.1201/9781482269802 - EZEONWUMELU, J.O.C., KAWOOYA, G.N., OKORUWA, A.G., DARE, S.S., EBOSIE, J.C., AKUNNE, A.A., TANAYEN, J.K. and UDECHUKWU, B.E., 2019. Phytochemical screening, toxicity, analgesic and anti-pyretic studies of aqueous leaf extract of Plectranthus barbatus [Andrews. Engl.] in rats. Pharmacology & Pharmacy, vol. 10, no. 4, pp. 205-221. http://dx.doi.org/10.4236/pp.2019.104018
» http://dx.doi.org/10.4236/pp.2019.104018 - FALÉ, P.L., MADEIRA, P.J., FLORÊNCIO, M.H., ASCENSÃO, L. and SERRALHEIRO, M.L., 2011. Function of Plectranthus barbatus herbal tea as neuronal acetylcholinesterase inhibitor. Food & Function, vol. 2, no. 2, pp. 130-136. http://dx.doi.org/10.1039/C0FO00070A PMid:21779558.
» http://dx.doi.org/10.1039/C0FO00070A - FENG, L., JIA, X., JIANG, J., ZHU, M., CHEN, Y., TAN, X.-B. and SHI, F., 2010. Combination of Active Components Enhances the Efficacy of Prunella in Prevention and Treatment of Lung Cancer. Molecules (Basel, Switzerland), vol. 15, no. 11, pp. 7893-7906. http://dx.doi.org/10.3390/molecules15117893 PMid:21060297.
» http://dx.doi.org/10.3390/molecules15117893 - GAMARO, G.D., SUYENAGA, E., BORSOI, M., LERMEN, J., PEREIRA, P. and ARDENGHI, P., 2011. Effect of rosmarinic and caffeic acids on inflammatory and nociception process in rats. International Scholarly Research Network ISRN Pharmacology, vol. 2011, pp. 1-6. http://dx.doi.org/10.5402/2011/451682 PMid:22084714.
» http://dx.doi.org/10.5402/2011/451682 - GANASH, M. and QANASH, S., 2018. Phenolic acids and biological activities of Coleus forskohlii and Plectranthus barbatus as traditional medicinal plants. International Journal of Pharmacology, vol. 14, no. 6, pp. 856-865. http://dx.doi.org/10.3923/ijp.2018.856.865
» http://dx.doi.org/10.3923/ijp.2018.856.865 - GENG, S., SUN, B., LU, R. and WANG, J., 2014. Coleusin factor, a novel anticancer diterpenoid, inhibits osteosarcoma growth by inducing bone morphogenetic protein-2-dependent differentiation. Molecular Cancer Therapeutics, vol. 13, no. 6, pp. 1431-1441. http://dx.doi.org/10.1158/1535-7163.MCT-13-0934 PMid:24723453.
» http://dx.doi.org/10.1158/1535-7163.MCT-13-0934 - GRAYER, R.J., ECKERT, M.R., VEITCH, N.C., KITE, G.C., MARIN, P.D., KOKUBUN, T., SIMMONDS, M.S. and PATON, A.J., 2003. The chemotaxonomic significance of two bioactive caffeic acid esters, nepetoidins A and B, in the Lamiaceae. Phytochemistry, vol. 64, no. 2, pp. 519-528. http://dx.doi.org/10.1016/S0031-9422(03)00192-4 PMid:12943769.
» http://dx.doi.org/10.1016/S0031-9422(03)00192-4 - ICHIKAWA, T., DATE, M., ISHIKURA, T. and OZAKI, A., 1971. Improvement of Kasugamycin – producing strain by the agar piece method and the prototroph method. Folia Microbiologica, vol. 16, no. 3, pp. 218-224. http://dx.doi.org/10.1007/BF02884210 PMid:4935426.
» http://dx.doi.org/10.1007/BF02884210 - JAGANATHAN, S.K. and MANDAL, M., 2009. Antiproliferative Effects of Honey and of Its Polyphenols: A Review. Journal of Biomedicine & Biotechnology, vol. 2009, pp. 1-13. http://dx.doi.org/10.1155/2009/830616 PMid:19636435.
» http://dx.doi.org/10.1155/2009/830616 - KAMPA, M., ALEXAKI, V.I., NOTAS, G., NIFLI, A.P., NISTIKAKI, A., HATZOGLOU, A., BAKOGEORGOU, E., KOUIMTZOGLOU, E., BLEKAS, G., BOSKOU, D., GRAVANIS, A. and CASTANAS, E., 2004. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Research, vol. 6, no. 2, pp. 63-74. http://dx.doi.org/10.1186/bcr752 PMid:14979919.
» http://dx.doi.org/10.1186/bcr752 - KAPEWANGOLO, P., HUSSEIN, A.A. and MEYER, D., 2013. Inhibition of HIV-1 enzymes, antioxidant and anti-inflammatory activities of Plectranthus barbatus. Journal of Ethnopharmacology, vol. 149, no. 1, pp. 184-190. http://dx.doi.org/10.1016/j.jep.2013.06.019 PMid:23811046.
» http://dx.doi.org/10.1016/j.jep.2013.06.019 - KĘPA, M., MIKLASIŃSKA-MAJDANIK, M., WOJTYCZKA, R.D., IDZIK, D., KORZENIOWSKI, K., SMOLEŃ-DZIRBA, J. and WĄSIK, T.J., 2018. Antimicrobial potential of caffeic acid against Staphylococcus aureus clinical strains. BioMed Research International, vol. 2018, pp. 1-8. http://dx.doi.org/10.1155/2018/7413504 PMid:30105241.
» http://dx.doi.org/10.1155/2018/7413504 - LORENZI, H. and MATOS, F.J.A., 2002. Plantas medicinais no Brasil: nativas e exóticas cultivadas Nova Odessa: Instituto Plantarum. 206 p.
- LUÍS, Â., SILVA, F., SOUSA, S., DUARTE, A.P. and DOMINGUES, F., 2013. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling, vol. 30, no. 1, pp. 69-79. http://dx.doi.org/10.1080/08927014.2013.845878 PMid:24228999.
» http://dx.doi.org/10.1080/08927014.2013.845878 - LUKHOBA, C.W., SIMMONDS, M.S. and PATON, A.J., 2006. Plectranthus: a review of ethnobotanical uses. Journal of Ethnopharmacology, vol. 103, no. 1, pp. 1-24. http://dx.doi.org/10.1016/j.jep.2005.09.011 PMid:16289602.
» http://dx.doi.org/10.1016/j.jep.2005.09.011 - MATU, E.N. and VAN STADEN, J., 2003. Antibacterial and anti-inflammatory activities of some plants used for medicinal purposes in Kenya. Journal of Ethnopharmacology, vol. 87, no. 1, pp. 35-41. http://dx.doi.org/10.1016/S0378-8741(03)00107-7 PMid:12787952.
» http://dx.doi.org/10.1016/S0378-8741(03)00107-7 - MIKLASI’NSKA-MAJDANIK, M.K., KĘPA, M., WOJTYCZKA, R.D., IDZIK, D., and WĄSIK, T.J., 2018. Phenolic compounds diminish antibiotic resistance of Staphylococcus Aureus clinical strains. International Journal of Environmental Research and Public Health, vol. 15, no. 10, pp. 2321. https://doi.org/10.3390/ijerph15102321 PMID: 30360435.
» https://doi.org/10.3390/ijerph15102321 - MOTHANA, R., AL-SAID, M., AL-MUSAYEIB, N., GAMAL, A., AL-MASSARANI, S., AL-REHAILY, A., ABDULKADER, M. and MAES, L., 2014. In vitro antiprotozoal activity of abietane diterpenoids isolated from Plectranthus barbatus. International Journal of Molecular Sciences, vol. 15, no. 5, pp. 8360-8371. http://dx.doi.org/10.3390/ijms15058360 PMid:24823881.
» http://dx.doi.org/10.3390/ijms15058360 - PUANGPRAPHANT, S., BERHOW, M.A., VERMILLION, K., POTTS, G. and GONZALEZ DE MEJIA, E., 2011. Dicaffeoylquinic acids in Yerba mate (Ilex paraguariensis St. Hilaire) inhibit NF-kB nucleus translocation in macrophages and induce apoptosis by activating caspases-8 and-3 in human colon cancer cells. Molecular Nutrition & Food Research, vol. 55, no. 10, pp. 1509-1522. http://dx.doi.org/10.1002/mnfr.201100128 PMid:21656672.
» http://dx.doi.org/10.1002/mnfr.201100128 - RAUDONE, L., ZYMONE, K., RAUDONIS, R., VAINORIENE, R., MOTIEKAITYTE, V. and JANULIS, V., 2017. Phenological changes in triterpenic and phenolic composition of Thymus L. Species. Industrial Crops and Products, vol. 109, pp. 445-451. http://dx.doi.org/10.1016/j.indcrop.2017.08.054
» http://dx.doi.org/10.1016/j.indcrop.2017.08.054 - RÊGO, M.J.B.D.M., GALDINO-PITTA, M.R., PEREIRA, D.T.M., DA SILVA, J.C., RABELLO, M.M., ALVES DE LIMA, M.C., HERNANDES, M.Z., DA ROCHA PITTA, I., GALDINO, S.L. and DA ROCHA PITTA, M.G., 2014. Synthesis, in vitro anticancer activity and in silico study of new disubstituted thiazolidinedione derivatives. Medicinal Chemistry Research, vol. 23, no. 6, pp. 3220-3226. http://dx.doi.org/10.1007/s00044-013-0902-z
» http://dx.doi.org/10.1007/s00044-013-0902-z - RICE, L.J., BRITS, G.J., POTGIETER, C.J. and VAN STADEN, J., 2011. Plectranthus: a plant for the future? South African Journal of Botany, vol. 77, no. 4, pp. 947-959. http://dx.doi.org/10.1016/j.sajb.2011.07.001
» http://dx.doi.org/10.1016/j.sajb.2011.07.001 - RODRIGUES, T. S., GUIMARÃES, S.F., BRAGA, T.V., BASTOS, J.C.S.A. and RODRIGUES-DAS-DÔRES, R.G., 2016. Determination of content of phenolic compounds and flavonoids in leaves extracts of Plectranthus sp. (“Boldos”), potential antioxidant and antibacterial action. Academia Journal of Medicinal Plants, vol. 4, no. 10, pp. 062-068. https://doi.org/10.15413/ajmp.2016.0111
» https://doi.org/10.15413/ajmp.2016.0111 - RUNYORO, D.K.B., MATEE, M.I.N., NGASSAPA, O.D., JOSEPH, C.C. and MBWAMBO, Z.H., 2006. Screening of Tanzanian medicinal plants for anti-Candida activity. BMC Complementary and Alternative Medicine, vol. 6, no. 11, pp. 1-10. https://doi.org/10.1186/1472-6882-6-11 PMID: 16571139.
» https://doi.org/10.1186/1472-6882-6-11 - SAEED, M.E.M., MEYER, M., HUSSEIN, A. and EFFERTH, T., 2016. Cytotoxicity of South-African medicinal plants towards sensitive and multidrug-resistant cancer cells. Journal of Ethnopharmacology, vol. 186, pp. 209-223. http://dx.doi.org/10.1016/j.jep.2016.04.005 PMid:27058630.
» http://dx.doi.org/10.1016/j.jep.2016.04.005 - SANTOS VERÍSSIMO, R., LINS, T., ASSIS BASTOS, M., ALBUQUERQUE SARMENTO, P., ALVINO, V., SILVA ARAUJO, M., LOPES SILVA, A. and ARAÚJO-JÚNIOR, J.2014. Antimicrobial activity of Plectranthus barbatus (Lamiacea). BMC Proceedings, vol. 8, suppl. 4, pp. 264. http://dx.doi.org/10.1186/1753-6561-8-S4-P264
» http://dx.doi.org/10.1186/1753-6561-8-S4-P264 - SHAHEEN, U., KHALIK, K.A., ABDELHADY, M.I.S., HOWLADAR, S., ALARJAH, M. and ABOUREHAB, M.A.S., 2017. HPLC Profile of Phenolic Constituents, Essential Oil Analysis and Antioxidant Activity of Six Plectranthus Species Growing in Saudi Arabia. Journal of Chemical and Pharmaceutical Research, vol. 9, no. 4, pp. 345-354.
- SHERF, A.F., 1943. A method for maintaining Phytomonas sepedonica in culture for long periods without transfer. Phytopathology, vol. 33, pp. 330-332.
- SILVA, C.F.G., MENDES, M.P., ALMEIDA, V.V., MICHELS, R.N., SAKANAKA, L.S. and TONIN, L.T.D., 2016. Parâmetros de qualidade físico-químicos e avaliação da atividade antioxidante de folhas de Plectranthus barbatus Andr. (Lamiaceae) submetidas a diferentes processos de secagem. Revista Brasileira de Plantas Medicinais, vol. 18, no. 1, pp. 48-56. http://dx.doi.org/10.1590/1983-084X/15_021
» http://dx.doi.org/10.1590/1983-084X/15_021 - SILVA, F.R.G., MATIAS, T.M.S., SOUZA, L.I.O., MATOS-ROCHA, T.J., FONSECA, S.A., MOUSINHO, K.C. and SANTOS, A.F., 2019. Phytochemical screening and in vitro antibacterial, antifungal, antioxidant and antitumor activities of the red propolis Alagoas. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 79, no. 3, pp. 452-459. http://dx.doi.org/10.1590/1519-6984.182959 PMid:30379200.
» http://dx.doi.org/10.1590/1519-6984.182959 - WENG, C.J. and YEN, G., 2012. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treatment Reviews, vol. 38, no. 1, pp. 76-87. http://dx.doi.org/10.1016/j.ctrv.2011.03.001 PMid:21481535.
» http://dx.doi.org/10.1016/j.ctrv.2011.03.001
Publication Dates
-
Publication in this collection
29 Mar 2021 -
Date of issue
2022
History
-
Received
09 Apr 2020 -
Accepted
09 July 2020