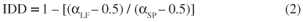Abstracts
Whether the functional structure of ecological communities is deterministic or historically contingent is still quite controversial. However, recent experimental tests did not find effects of species composition variation on trait convergence and therefore the environmental constraints should play the major role on community convergence into functional groups. Seasonal cerrados are characterized by a sharp seasonality, in which the water shortage defines the community functioning. Hyperseasonal cerrados experience additionally waterlogging in the rainy season. Here, we asked whether waterlogging modifies species convergences into life-forms in a hyperseasonal cerrado. We studied a hyperseasonal cerrado, comparing it with a nearby seasonal cerrado, never waterlogged, in Emas National Park, central Brazil. In each area, we sampled all vascular plants by placing 40 plots of 1 m² plots in four surveys. We analyzed the species convergences into life-forms in both cerrados using the Raunkiaer's life-form spectrum and the index of divergence from species to life-form diversity (IDD). The overall life-form spectra and IDDs were not different, indicating that waterlogging did not affect the composition of functional groups in the hyperseasonal cerrado. However, there was a seasonal variation in IDD values only in the hyperseasonal cerrado. As long as we did not find a seasonal variation in life-form diversity, the seasonal variation of convergence into life-forms in the hyperseasonal cerrado was a consequence of the seasonal variation of species diversity. Because of high functional redundancy of cerrado plants, waterlogging promoted a floristic replacement without major changes in functional groups. Thus, waterlogging in the hyperseasonal cerrado promoted seasonal changes in species convergence into life-forms by reducing species diversity.
hyperseasonal cerrado; IDD; life-form; species diversity; trait convergence
Se a estrutura ecológica das comunidades é determinística ou historicamente dependente é ainda um tema controverso. Entretanto, testes experimentais recentes não encontraram efeitos da variação da composição de espécies na convergência de traços funcionais e, portanto, as restrições ambientais devem ter um papel principal na convergência das comunidades em grupos funcionais. Cerrados estacionais são caracterizados por uma estacionalidade pronunciada, em que a seca define o funcionamento da comunidade. Cerrados hiperestacionais experimentam adicionalmente um alagamento na estação chuvosa. Aqui, perguntamo-nos se o alagamento modifica a convergência de espécies em formas de vida em um cerrado hiperestacional. Para tanto, estudamos um cerrado hiperestacional, comparando-o com um cerrado estacional, nunca alagado, no Parque Nacional das Emas, GO. Em cada cerrado, usamos 40 parcelas de 1 m² e amostramos todas as plantas vasculares. Analisamos a convergência de espécies em formas de vida usando o espectro biológico de Raunkiaer e o índice de divergência entre a diversidade de espécies e de formas de vida (IDD). Os espectros gerais e os IDDs não diferiram, indicando que o alagamento não afetou a composição dos grupos funcionais no cerrado hiperestacional. Entretanto, houve uma variação estacional nos valores de IDD somente no cerrado hiperestacional. Como não observamos variações estacionais na diversidade de formas de vida, a variação da convergência no cerrado hiperestacional foi uma conseqüência da variação estacional da diversidade de espécies. Devido à elevada redundância funcional das plantas do cerrado, o alagamento promoveu substituições florísticas sem maiores mudanças nos grupos funcionais. Portanto, o alagamento promoveu mudanças estacionais na convergência de espécies em formas de vida, reduzindo a diversidade de espécies.
cerrado hiperestacional; convergência funcional; diversidade de espécies; formas de vida; IDD
ECOLOGY
Species convergence into life-forms in a hyperseasonal cerrado in central Brazil
Convergência de espécies em formas de vida em um cerrado hiperestacional no Brasil central
Silva, IA.* * e-mail: igor6cordas@yahoo.com.br ; Batalha, MA.
Laboratório de Ecologia Vegetal, Departamento de Botânica, Universidade Federal de São Carlos UFSCar, CP 676, CEP 13565-905, São Carlos, SP, Brazil
ABSTRACT
Whether the functional structure of ecological communities is deterministic or historically contingent is still quite controversial. However, recent experimental tests did not find effects of species composition variation on trait convergence and therefore the environmental constraints should play the major role on community convergence into functional groups. Seasonal cerrados are characterized by a sharp seasonality, in which the water shortage defines the community functioning. Hyperseasonal cerrados experience additionally waterlogging in the rainy season. Here, we asked whether waterlogging modifies species convergences into life-forms in a hyperseasonal cerrado. We studied a hyperseasonal cerrado, comparing it with a nearby seasonal cerrado, never waterlogged, in Emas National Park, central Brazil. In each area, we sampled all vascular plants by placing 40 plots of 1 m2 plots in four surveys. We analyzed the species convergences into life-forms in both cerrados using the Raunkiaer's life-form spectrum and the index of divergence from species to life-form diversity (IDD). The overall life-form spectra and IDDs were not different, indicating that waterlogging did not affect the composition of functional groups in the hyperseasonal cerrado. However, there was a seasonal variation in IDD values only in the hyperseasonal cerrado. As long as we did not find a seasonal variation in life-form diversity, the seasonal variation of convergence into life-forms in the hyperseasonal cerrado was a consequence of the seasonal variation of species diversity. Because of high functional redundancy of cerrado plants, waterlogging promoted a floristic replacement without major changes in functional groups. Thus, waterlogging in the hyperseasonal cerrado promoted seasonal changes in species convergence into life-forms by reducing species diversity.
Keywords: hyperseasonal cerrado, IDD, life-form, species diversity, trait convergence.
RESUMO
Se a estrutura ecológica das comunidades é determinística ou historicamente dependente é ainda um tema controverso. Entretanto, testes experimentais recentes não encontraram efeitos da variação da composição de espécies na convergência de traços funcionais e, portanto, as restrições ambientais devem ter um papel principal na convergência das comunidades em grupos funcionais. Cerrados estacionais são caracterizados por uma estacionalidade pronunciada, em que a seca define o funcionamento da comunidade. Cerrados hiperestacionais experimentam adicionalmente um alagamento na estação chuvosa. Aqui, perguntamo-nos se o alagamento modifica a convergência de espécies em formas de vida em um cerrado hiperestacional. Para tanto, estudamos um cerrado hiperestacional, comparando-o com um cerrado estacional, nunca alagado, no Parque Nacional das Emas, GO. Em cada cerrado, usamos 40 parcelas de 1 m2 e amostramos todas as plantas vasculares. Analisamos a convergência de espécies em formas de vida usando o espectro biológico de Raunkiaer e o índice de divergência entre a diversidade de espécies e de formas de vida (IDD). Os espectros gerais e os IDDs não diferiram, indicando que o alagamento não afetou a composição dos grupos funcionais no cerrado hiperestacional. Entretanto, houve uma variação estacional nos valores de IDD somente no cerrado hiperestacional. Como não observamos variações estacionais na diversidade de formas de vida, a variação da convergência no cerrado hiperestacional foi uma conseqüência da variação estacional da diversidade de espécies. Devido à elevada redundância funcional das plantas do cerrado, o alagamento promoveu substituições florísticas sem maiores mudanças nos grupos funcionais. Portanto, o alagamento promoveu mudanças estacionais na convergência de espécies em formas de vida, reduzindo a diversidade de espécies.
Palavras-chave: cerrado hiperestacional, convergência funcional, diversidade de espécies, formas de vida, IDD.
1. Introduction
Whether the functional group structure of ecological communities is deterministic or historically contingent is still quite controversial (Chase, 2003). The deterministic view suggests that communities converge towards a common structure determined by environmental conditions, irrespective of the history of community assembly (Fukami et al., 2005). The alternative view suggests that stochastic forces producing variation in the sequence and timing of species arrivals can cause divergence in community structure among localities, even under identical environmental conditions and regional species pool (Fukami et al., 2005). A reconcilable view, however, suggests that whether communities converge or diverge depends on the level of community organization considered. According to this, community assembly is deterministic in the general composition of functional groups, but historically contingent in the composition of species (Temperton et al., 2004; Fukami et al., 2005). Despite the possible relative influence of environment and history on assemblage composition (Gentry, 1988; Kinzig et al., 1999; Chase, 2003), recent experimental tests did not find effects of species composition variation on trait-group composition (Fukami et al., 2005). As a result, environmental constraints would play the major role on community convergence into functional groups (Fukami et al., 2005).
Ricotta et al. (2002) proposed an index of divergence from species to functional group diversity that is useful to measure species convergence of a given community into ecologically relevant groups irrespective of their taxonomic classification. The index of intrinsic diversity divergence (IDD) is based on the notion of intrinsic diversity ordering in which community A is intrinsically more diverse than community B if and only if community A has its diversity profile everywhere above that of community B (for details see Patil and Tailie, 1979; 1982). The IDD is obtained by comparing the area under the species diversity profile with the area under the corresponding functional group diversity profile (Ricotta et al., 2002). The IDD may range from zero to one and, when one uses species life-form, IDD is also a structural measure of communities (Ricotta et al., 2002). Consequently, communities that are structurally more homogeneous should present higher IDD values than less homogeneous communities (Ricotta et al., 2002).
Raunkiaer (1934) proposed a system to classify plant life-forms based on the position and protection degree of the regeneration buds. In his classification, there are five major classes, arranged according to increased protection of the regeneration buds: phanerophytes, chamaephytes, hemicryptophytes, cryptophytes, and therophytes. Accordingly, harsh growing conditions should favour species with periodic reduction to a remnant shoot system that either lies flat on the ground (hemicryptophytes) or is embedded in the soil (cryptophytes) (Cornelissen et al., 2003). Therophytes are expected to prevail where growing conditions become so extreme that the probability of survival to the second year becomes very small (Bazzaz and Morse, 1991). Although sometimes strongly criticized (e.g., Sarmiento and Monasterio, 1983), Raunkiaer's system is the simplest and, in many ways, the most satisfying classification of plants into life-forms (Begon et al., 1996). His system has gained wide acceptance in the sense that publications report life-form categories as a matter of routine (Westoby, 1998) and, thus, is suitable for the scope of our study.
Seasonal savannas are characterized by a sharp seasonality, in which the pronounced rainless season defines the community functioning (Sarmiento, 1983). Phanerophytes, chamaephytes, and hemicryptophytes are generally well represented in life-form spectra of Brazilian seasonal savannas (see Batalha and Martins, 2002 for references). Hyperseasonal savannas are characterized by the alternation of two contrasting stresses during each annual cycle, one induced by drought; the other, by waterlogging in some months in the rainy season (Sarmiento et al., 2004). The consequences of waterlogging to savanna species can be fatal, because, as aerobic respiration ceases, levels of energy-rich adenylates drop rapidly, causing a dramatic decline in ion uptake and transport (Crawford, 1982; Koslowski, 1997). It is known that waterlogging determines the species abundance in hyperseasonal savannas (Sarmiento, 1983; Sarmiento, 1996; Batalha et al., 2005; Cianciaruso et al., 2005), but does it alter species convergences towards life-forms with more protected regeneration buds?
We related the first occurrence of a hyperseasonal cerrado in Emas National Park (ENP), central Brazil, flooded by the accumulation of rain water, with fewer species and less diverse than the seasonal cerrado (Batalha et al., 2005; Cianciaruso et al., 2005). We expected that the life-form spectra and IDDs could highlight the effects of waterlogging upon the convergence of cerrado species into life-forms, considering that both hyperseasonal and seasonal cerrados have the same physiognomy (Batalha et al., 2005), are on similar soils (Amorin and Batalha, 2006), and under the same climate and fire regimes (Ramos-Neto and Pivello, 2000). Our aim was to study the life-form spectrum and IDD of the hyperseasonal cerrado, comparing it to a nearby seasonal cerrado. We addressed the following questions: What are the prevailing life-forms in the hyperseasonal cerrado? Is the life-form spectrum of the hyperseasonal cerrado different from the life-form spectrum of the seasonal cerrado? Is the IDD of the hyperseasonal cerrado different from the IDD of the seasonal cerrado, that is, is the convergence of species into life-forms different between the two vegetation forms? Do the IDDs of both cerrados vary throughout the year, accompanying the climatic seasonality and environmental constraints?
2. Material and Methods
We carried out this study in Emas National Park (ENP), one of the largest and most important reserves in the cerrado region. It comprises 132,941 ha and is located in the Brazilian Central Plateau, southwestern Goiás State (17° 49'-18° 28' S and 52° 39'-53° 10' W). We sampled the hyperseasonal cerrado (approximately, 18° 18' 07" S and 52° 57' 56" W) and a nearby seasonal cerrado (approximately, 18° 17' 34" S and 52° 58' 12" W) in four surveys during 2003. In each vegetation form, we placed randomly ten plots (1 m2) in each one of the four surveys carried out: February 2003, in mid rainy season, when the hyperseasonal cerrado was waterlogged; May 2003, in late rainy season; August 2003, in the dry season; and November 2003, in early rainy season. For a detailed description of the studied areas and sampling methods, see Batalha et al. (2005), Cianciaruso et al. (2005), and Amorin and Batalha (2006).
We assigned the life-form of each species with an identification key (Mueller-Dombois and Ellenberg, 1974), considering only Raunkiaer's major classes phanerophytes, chamaephytes, hemicryptophytes, cryptophytes, and therophytes and including lianas in the "phanerophyte" class and geophytes in the "cryptophyte" class, as originally proposed by Raunkiaer (1934). We used data from all surveys to construct the life-form spectra and to compute the overall IDDs of cerrados (n = 40). For seasonal comparisons of IDDs, we used the data from each survey (n = 10). To check whether the life-form spectrum of the hyperseasonal cerrado was significantly different from the spectrum of the seasonal cerrado, we applied a heterogeneity chi-square analysis (Zar 1999). With this analysis, we determined whether the two life-form spectra came from the same data population (Zar 1999).
To determine the IDD of each cerrado, first we computed the intrinsic diversity profiles for species (αSP) and the intrinsic diversity profiles for life-forms (αLF), which are related, respectively, to the area under the species diversity profile and the life-form diversity profile. We constructed the diversity profiles following the procedures of Patil and Tailie (1979): we arranged the relative abundances in descending order and rearranged them into a cumulative relative pattern; then, we obtained the diversity profiles by joining the points given by the cumulative relative species and life-form abundances and by the cumulative species number, being (0; 0) the first plotted points. We determined the area under a given profile as:
in which:
I = [(2σ i x pi) 1] / N and i (1 < i < N) was the rank of the relative abundances pi arranged in descending order (Ricotta et al., 2002). We calculated the index of intrinsic divergence (IDD) of each cerrado as:
We applied a randomization test (Manly, 1997), with 5,000 permutations, to check whether the IDDs of the hyperseasonal and seasonal cerrados were significantly different (α = 0.05), using a computer routine we wrote in C++. The randomization test was applied as follows: first, we calculated the difference D1 between the IDDs of both cerrados (D1 = | IDD seasonal cerrado IDD hyperseasonal cerrado |); then, we randomly resampled the species of both cerrados and determined the difference D between the IDDs thus obtained a step repeated 5,000 times, providing us with a distribution of D values; finally, we calculated the proportion of all D values that were higher than or equal to D1. We used the same procedure to test for differences in the IDDs among the seasons in each vegetation form.
3. Results
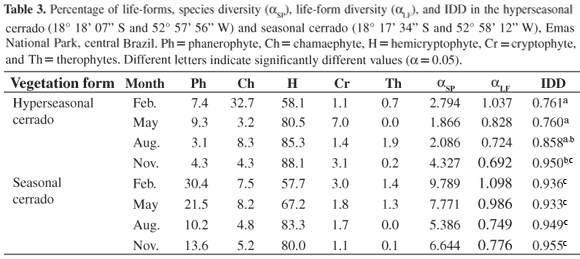
In the hyperseasonal cerrado, we sampled 63 species (Table 1), of which nine were phanerophytes (14.3%); nine, chamaephytes (14.3%); 37, hemicryptophytes (58.7%); three, cryptophytes (4.8%); and five, therophytes (7.9%). In the seasonal cerrado, we sampled 107 species (Table 2), of which 28 were phanerophytes (26.2%); 19, chamaephytes (17.7%); 54, hemicryptophytes (50.5%); three, cryptophytes (2.8%); and three, therophytes (2.8%). These life-form spectra were not significantly different (χ2 = 4.351, df = 4, P = 0.361). The overall IDDs of the hyperseasonal and seasonal cerrados were, respectively, 0.950 (αSP = 5.682 and αLF = 0.759) and 0.966 (αSP = 11.889 and αLF = 0.883), values not significantly different (P = 0.363; Figure 1).
When calculated for each season of the year, the only significant differences were those between February and November (P = 0.016) and between May and November (P = 0.022) in the hyperseasonal cerrado (Table 3). In the hyperseasonal cerrado, the waterlogging decreased the IDD (Figure 2), and the seasonal variation of αSP was higher than of αLF (Figure 3).
4. Discussion
In the life-form spectra of both cerrados, the most represented class was the hemicryptophytes. Hemicryptophytes and phanerophytes are generally well represented in cerrado life-form spectra (Batalha and Martins, 2002). Phanerophytes, however, were poorly represented in the cerrados we sampled and this may be a consequence of their open physiognomies. Although Raunkiaer's system was not originally proposed for physiognomic comparisons, Raunkiaer (1934) himself advocated that the life-form spectrum of a given site reflects the vegetation physiognomy. Thus, since the importance of trees and shrubs increases from open to closed cerrado physiognomies, hemicryptophytes should indeed prevail and phanerophytes should be almost absent in grassland cerrados (Batalha and Martins, 2002).
Even if therophytes are well represented in Venezuelan and African savannas (Batalha and Martins, 2002), we found an underproportion of them in both cerrados. The occurrence of therophytes in plant communities is related to the harshness of growing conditions (Bazzaz and Morse, 1991; Cornelissen et al., 2003). Unfavorable growing conditions for part of the year, however, do not prevent perennials from appearing, since they favor selection of other strategies, such as dormant structures that enable hemicryptophytes to survive conditions detrimental to herbaceous growth (Bazzaz and Morse, 1991). Therefore, even the environmental constraints resulting from drought and waterlogging in the hyperseasonal cerrado should not be extreme enough to favor the strategy of therophytes.
The analysis of life-form spectra indicated that there should be a high functional redundancy among cerrado species. Life-form synthesizes various morphological traits related to responses of plants to climate, soil resource, competitive strength, and disturbances (Schulze, 1982; Cornelissen et al., 2003). Nevertheless, the life-form spectrum of the hyperseasonal cerrado, when compared to those of the seasonal cerrado, was not altered by waterlogging, even though the number of species decreased almost by half. Where the environment situations are spatiotemporally heterogeneous, as in savannas, species are adapted to dynamism and evolve strategies to respond to shifting opportunities (Fjeldsa and Lovett, 1997). Hence, the many responses of savanna species to environmental perturbations should explain the persistence of savanna communities within a broad range of environmental variation (Silva, 1996), from an excessive drought to a temporary waterlogging (Sarmiento, 1983).
The overall IDD comparison also pointed out that the cerrado species should be very functionally redundant. At the trait-group level of community organization, the environmental rules seem to determine the species convergence (Temperton et al., 2004; Fukami et al., 2005) against the neutral trait-group assembly driven by random sampling from regional species pools (Hubbell, 2001). Since the overall IDDs were not significantly different from a random resampling, we may conclude that the species convergences into life-forms in hyperseasonal and seasonal cerrados are, in general, similar. The high IDD values of the hyperseasonal and seasonal cerrados indicated that both communities are structurally very homogeneous. The dominance of only one life-form, hemicryptophytes, in the spectra of both cerrados justifies the high structural homogeneity of their grassland physiognomy.
On the other hand, the seasonal analyses corroborated that environmental constraints play the major role on community convergence into functional groups (Fukami et al., 2005). Comparing the IDDs throughout the year, we did not observe significant differences among the seasons in the seasonal cerrado. Although the water shortage in the dry season reduced the species diversity, drought did not affect species convergence in the seasonal cerrado, and the IDD values were constant throughout the year. On the contrary, we noticed a significant IDD variation throughout the year in the hyperseasonal cerrado. The IDD in November, in early rainy season, was higher than those in February, when the hyperseasonal cerrado was waterlogged, and in May, in late rainy season. When we considered the floras of both cerrados as a whole, we found no difference between the IDDs, but when we analyzed them throughout the year, we found seasonality only in the hyperseasonal cerrado. As a consequence, waterlogging shall modify cerrado species convergence into life-forms.
The seasonal comparisons also indicated that species abundance affects the convergence into functional groups, even though species composition variation does not (Fukami et al., 2005). The IDD measures the decrease from species to life-form diversity (Ricotta et al., 2002). Since life-form diversity was maintained throughout the year, structural changes in hyperseasonal cerrado were due to changes in species diversity. The IDDs of the hyperseasonal cerrado in February and May were lower, because the waterlogging reduced species diversity (Cianciaruso et al., 2005). Conversely, the IDD in November was higher, because the favorable moisture soil level of the early rainy season increased species diversity (Cianciaruso et al., 2005). Accordingly, annual variation of the hyperseasonal cerrado structure followed the seasonality and its environmental constraints, being a consequence of the seasonal changes of species diversity rather than of life-form diversity.
Savannas should be more stable in functional than in floristic terms (Sarmiento, 1996). This was corroborated by the non-significant difference between the life-form spectra and overall IDDs of the hyperseasonal and seasonal cerrados, as well as by the maintenance of life-form diversity throughout the year. As redundant species within each life-form are not entirely equivalent, they have different tolerances to environmental constraints (Sarmiento, 1996). Consequently, changing conditions may result in a certain degree of floristic replacement, without major changes in functional groups. Thus, due to the high functional redundancy of cerrado plants, waterlogging in the hyperseasonal cerrado promotes seasonal changes in species convergence into life-forms by reducing species diversity.
Acknowledgements We are grateful to FAPESP, for financial support; to CAPES, for the scholarship granted to the first author; to Ibama, for research permission; to the Emas National Park staff, for logistical assistance; to A.W. Valenti, for computational support on the randomization test; to São Paulo State Botanical Institute, UNB and IBGE herbaria staff; to P.K. Amorim, C.A. Casali, M.V. Cianciaruso, A.V.F. Jardim, H.F. Lima, R.A. Miotto, F. Oliveira, O.L. Silva, and M.W. Valenti, for help in field; and to the taxonomists T.S. Filgueiras, R.C. Oliveira, and M.A. Silva, for their assistance in the identification of species.
Received September 21, 2006
Accepted November 30, 2006
Distributed May 31, 2008
- AMORIN, PK. and BATALHA, MA., 2006. Soil characteristics of a hyperseasonal cerrado compared to a seasonal cerrado and a floodplain grassland: implications for plant community structure. Braz J Biol= Rev Bras Biol, vol. 66, no. 2b, p. 661-670.
- BATALHA, MA., CIANCIARUSO, MV., SILVA, IA. and DELITTI, WCB., 2005. Hyperseasonal cerrado, a new Brazilian vegetation form. Braz. J. Biol.=Rev. Bras. Biol, vol. 65, no. 4, p. 735-738.
- BATALHA, MA. and MARTINS, FR., 2002. Life-form spectra of Brazilian cerrado sites. Flora, vol. 197, no. 6, p. 452-460.
- BAZZAZ, FA. and MORSE SR., 1991. Annual plants: potential responses to multiple stresses. In MOONEY, HA., WINNER, WE. and PELL, EJ. (eds). Response of plants to multiple stresses London: Academic.
- BEGON, M., HARPER, JL. and TOWNSEND, CR., 1996. Ecology: individuals, populations and communities Oxford: Blackwell. 945 p.
- CHASE, JM., 2003. Community assembly: when should history matter? Oecologia, vol. 136, no. 4, p. 489-498.
- CIANCIARUSO, MV., BATALHA, MA. and SILVA, IA., 2005. Seasonal variation of a hyperseasonal cerrado in Emas National Park, central Brazil. Flora, vol. 200, no. 4, p. 345-353.
- CORNELISSEN, JHC., LAVOREL, S., GARNIER, E., DÍAZ, S., BUCHMANN, N., GURVICH, DE., REICH, PB., STEEGE, H., MORGAN, HD., Van-Der-HEIJDEN, MGA., PAUSAS, JG. and POORTER, H., 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J. Bot., vol. 51, no. 4, p. 335-380.
- CRAWFORD, RM., 1982. Physiological response to flooding. In LANGE, OL., NOBEL, PS., OSMOND, CB. and ZIEGLER, H. (eds.). Physiological plant ecology II. Encyclopedia of plant physiology, vol. 12B, Berlin: Springer.
- FJELDSA, J. and LOVETT, J., 1997. Biodiversity and environmental stability. Biodiversity Conserv, vol. 6, no. 3, p. 315-323.
- FUKAMI, T., BEZEMER, TM., MORTIMER, SR. and Van-Der-PUTTEN, WH., 2005. Species divergence and trait convergence in experimental plant community assembly. Ecol. Lett, vol. 8, no. 12, p. 1283-1290.
- GENTRY, AH., 1988. Changes in plant community diversity and floristic composition on environmental and geographic gradients. Ann. Mo. Bot. Gard, vol. 75, no. 1, p. 1-34.
- HUBBELL, SP., 2001. The unified neutral theory of biodiversity and biogeography Princeton: Princeton University. 375 p.
- KINZIG, AP., LEVIN, SA., DUSHOFF, J. and PACALA, S., 1999. Limiting similarity, species packing, and system stability for hierarchical competition-colonization models. Am. Nat, vol. 153, no. 4, p. 371-383.
- KOSLOWSKI, TT., 1997. Responses of woody plants to flooding and salinity. Tree physiology monograph, no.1. Victoria: Heron Publishing. 29 p.
- MANLY, BFJ., 1997. Randomization, bootstrap and Monte Carlo methods in biology London: Chapman and Hall. 399 p.
- MUELLER-DOMBOIS, D. and ELLENBERG, H., 1974. Aims and methods of vegetation ecology New York: John Wiley and Sons. 547 p.
- PATIL, GP. and TAILIE, C., 1979. An overview of diversity. In GRASSLE, F., SMITH, WK., PATIL, GP., and TAILLIE, C. (eds.). Ecological diversity in theory and practice. Fairland: International Cooperative.
- PATIL, GP. and TAILIE, C., 1982, Diversity as a concept and its measurement. J. Am. Stat. Assoc, vol. 77, no. 379, p. 48-567.
- RAMOS-NETO, MB. and PIVELLO, VR., 2000. Lightning fires in a Brazilian savanna National Park: rethinking management strategies. Environ. Manage, vol. 26, no. 6, p. 675-684.
- RAUNKIAER, C., 1934. The life forms of plants and statistical geography Oxford: Claredon. 632 p.
- RICOTTA, C., AVENA, G. and CHIARUCCI, A., 2002. An index of divergence from species to life-form diversity based on the notion of intrinsic diversity ordering. Plant Ecol, vol. 165, no. 2, p. 217-222.
- SARMIENTO, G., 1983. The savannas of tropical America. In GOODALL, DW (ed.). Ecosystems of the world tropical savannas. Amsterdam: Elsevier.
- -, 1996. Biodiversity and water relations in tropical savannas. In SOLBRIG, OT., MEDINA, E. and SILVA, JF. (eds.). Biodiversity and savanna ecosystem processes: a global perspective. Berlin: Springer.
- SARMIENTO, G. and MONASTERIO, M., 1983. Life forms and phenology. In BOURLIÈRE, F. (ed.). Ecosystems of the world: tropical savannas Amsterdam: Elsevier.
- SARMIENTO, G., PINILLOS, M., SILVA, MP. and ACEVEDO, D., 2004. Effects of soil water regime and grazing on vegetation diversity and production in a hyperseasonal savanna at the Apure Llanos, Venezuela. J. Trop. Ecology, vol. 20, no. 2, p. 209-220.
- SCHULZE, ED., 1982. Plant life forms and their carbon, water and nutrient relations. In LANGE, OL., NOBEL, PS., OSMOND, CB. and ZIEGLER, H. (eds.). Physiological plant ecology. II. Encyclopedia of plant physiology, vol. 12B. Berlin: Springer.
- SILVA, JF., 1996. Biodiversity and stability in tropical savannas. In SOLBRIG, OT., MEDINA, E. and SILVA, JF. (eds.). Biodiversity and savanna ecosystem processes: a global perspective. Berlin: Springer.
- TEMPERTON, VM., HOBBS, RJ., NUTTLE, T. and HALLE, S., 2004. Assembly rules and restoration ecology: bridging the gap between theory and practice Washington: Island. 439 p.
- WESTOBY, M., 1998. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil, vol. 199, no. 2, p. 213227
- ZAR, JH., 1999. Biostatistical analysis New Jersey: Prentice Hall. 663 p.
Publication Dates
-
Publication in this collection
21 July 2008 -
Date of issue
May 2008
History
-
Accepted
30 Nov 2006 -
Received
21 Sept 2006