Abstract
Increasing trend in antimicrobial resistance and failure of chemically synthesized antibiotics lead to discover alternative methods for the treatment of bacterial infections. Various medicinal plants are in use traditionally and their active compounds can be further applied for treatment of bacterial diseases. This study was designed to determine the antibacterial activity of Punica granatum (P. granatum L.) (pomegranate) peel extract against Enterobacteriaceae [Escherichia coli (E. coli), Salmonella Typhimurium (S. Typhimurium) and Shigella Dysenteriae (S. Dysenteriae)] and gram-positive bacterium [Staphylococcus aureus (Staph aureus)]. Methanolic extract of P. granatum L. peel was prepared by Soxhlet apparatus method. Total flavonoid and phenolic contents from the extract were determined by High Performance Liquid Chromatography (HPLC). The antibacterial activity of P. granatum L. peel extract was evaluated through agar well diffusion method. HPLC showed the range of phenolics (gallic acid, caffeic acid, benzoic acid, cinnamic acid) and flavonoid compounds. The chemical structures of flavonoid and phenolics found in the methanolic extract of P. granatum L. peel have been reported for the first time. The methanolic peel extract (50 ul) of yellow P. granatum L. showed 26, 10, 10 and 9mm zones of inhibition (ZOI) against S. aureus, S. Typhimurium, S. Dysenteriae and E. coli, respectively. The methanolic extract of red P. granatum L. (100 ul) showed 27, 8, 12 and 15 mm ZOI against Staph. aureus, S. Typhimurium, S. Dysenteriae and E. coli, respectively. Highest ZOI was observed against Staph. aureus. Many of the bacteria studied in the present work may cause serious gastrointestinal infections, which can lead to hemorrhagic diarrhea in children. These infections can be life-threatening to young children and the elderly. There is an incentive to find alternative control measures, such as plant and herbal extracts, especially in lesser-developed countries where traditional antibiotics may not be readily available.
Keywords:
P. granatum L.; antimicrobial activity; Enterobacteriaceae; Staphylococcus aureus; HPLC
Resumo
A tendência crescente na resistência antimicrobiana e na falha dos antibióticos sintetizados quimicamente leva à descoberta de métodos alternativos para o tratamento de infecções bacterianas. Várias plantas medicinais estão em uso tradicionalmente e seus compostos ativos podem ser posteriormente aplicados para o tratamento de doenças bacterianas. Este estudo foi desenhado para determinar a atividade antibacteriana do extrato de casca de Punica granatum (P. granatum L.) (romã) contra Enterobacteriaceae [Escherichia coli (E. coli), Salmonella Typhimurium (S. Typhimurium) e Shigella Dysenteriae (S. Dysenteriae) ] e bactéria gram-positiva [Staphylococcus aureus (Staph aureus)]. O extrato metanólico da casca de P. granatum L. foi preparado pelo método do aparelho de Soxhlet. O conteúdo total de flavonoides e fenólicos do extrato foi determinado por cromatografia líquida de alta eficiência (HPLC). A atividade antibacteriana do extrato da casca de P. granatum L. foi avaliada através do método de difusão em ágar. HPLC mostrou a gama de compostos fenólicos (ácido gálico, ácido cafeico, ácido benzoico, ácido cinâmico) e flavonoides. As estruturas químicas de flavonoides e fenólicos encontradas no extrato metanólico da casca de P. granatum L. foram relatadas pela primeira vez. O extrato metanólico da casca (50 ul) de P. granatum L. amarelo apresentou zonas de inibição (ZOI) de 26, 10, 10 e 9mm contra S. aureus, S. Typhimurium, S. Dysenteriae e E. coli, respectivamente. O extrato metanólico de P. granatum L. vermelho (100 ul) apresentou 27, 8, 12 e 15 mm IOI contra Staph. aureus, S. Typhimurium, S. Dysenteriae e E. coli, respectivamente. O ZOI mais alto foi observado contra Staph. aureus. Muitas das bactérias estudadas no presente trabalho podem causar infecções gastrointestinais graves, que podem levar à diarreia hemorrágica em crianças. Essas infecções podem ser fatais para crianças pequenas e idosos. Há um incentivo para encontrar medidas de controle alternativas, como extratos de plantas e ervas, especialmente em países menos desenvolvidos, onde os antibióticos tradicionais podem não estar prontamente disponíveis.
Palavras-chave:
P. granatum L.; atividade antimicrobiana; Enterobacteriaceae; Staphylococcus aureus; HPLC
1. Introduction
Excessive use of antibiotics is the leading cause of resistance in pathogenic bacteria mainly in Staph. aureus, E. coli O157, Salmonella and Pseudomonas. In recent years, the identification of food containing nutrients and biologically active components has been gaining immense attention. The active components in plants (phytochemicals) are helpful components against pathogenic microorganisms in replacement of antibiotics (Devi et al., 2011DEVI, A., SINGH, V. and BHATT, A.B., 2011. Comparative antibacterial study of different extracts of Pomegranate and its wild variety. International Journal of Pharmaceutical Sciences and Research, vol. 2, pp. 2647-2650.; Ahmad et al., 2020AHMAD, Y., ZAHRA, R., ALI, M.I., RIAZ, M.H., KHAN, R., KHAN, K., KHAN, M.T., KHAN, M.F., ULLAH, S., HASSAN, F., ALI, A. and ZEB, M.T., 2020. Molecular screening of resistant and virulent genes in Salmonella Enteritidis and Salmonella Typhimurium from Poultry in Khyber Pakhtunkhwa. Pakistan Veterinary Journal, vol. 40, pp. 343-349.).
Due to bacterial resistance against antibiotics, herbal medicines can be used for the treatment of many diseases. Medicinal plants are natural products for the treatment of diseases without any adverse side effects (Moghaddam et al., 2013MOGHADDAM, G., SHARIFZADEH, M., HASSANZADEH, G., KHANAVI, M. and HAJIMAHMOODI, M., 2013. Anti-ulcerogenic activity of the pomegranate peel (Punica granatum) methanol extract. Food and Nutrition Sciences, vol. 4, no. 10, pp. 43-48. http://dx.doi.org/10.4236/fns.2013.410A008.
http://dx.doi.org/10.4236/fns.2013.410A0...
; Javed et al., 2021JAVED, F., SHARIF, M.K. and ABBAS, N., 2021. Hypoglycemic effect of flaxseed lignan and β glucan in postmenopausal diabetic patients. Agrobiological Records, vol. 5, pp. 32-40. http://dx.doi.org/10.47278/journal.abr/2020.031.
http://dx.doi.org/10.47278/journal.abr/2...
; Majeed et al., 2021MAJEED, Y., SHAUKAT, M.B., ABBASI, K.Y. and AHMAD, M.A., 2021. Indigenous plants of Pakistan for the treatment of Diabetes: a review. Agrobiological Records, vol. 4, pp. 44-63. http://dx.doi.org/10.47278/journal.abr/2020.028.
http://dx.doi.org/10.47278/journal.abr/2...
; Rafay et al., 2021RAFAY, M., GHAFFAR, M.U., ABID, M., MALIK, Z. and MADNEE, M., 2021. Phytochemicals analysis and antimicrobial activities ofEchinops echinatusfrom Cholistan Desert, Pakistan. Agrobiological Records, vol. 5, pp. 21-27. http://dx.doi.org/10.47278/journal.abr/2021.001.
http://dx.doi.org/10.47278/journal.abr/2...
).
Different plant extracts have been reported for their antimicrobial activities including some of the important appended here i.e., Aframomum melegueta (Doherty et al., 2011DOHERTY, V.F., OLANIRAN, O.O. and KANIFE, U.C., 2011. Antimicrobial activities of Aframomum melegueta (Alligator Pepper). Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 2, pp. 126-131.), aqueous and hydroalcoholic extracts ofAcanthospermum australe(Loefl.) Kuntze against diarrhea-inducing bacteria (Mallmann et al., 2018MALLMANN, R., ETHUR, E.M., BIANCHETTI, P., FALEIRO, D., HOEHNE, L. and GOETTERT, M.I., 2018. Effectiveness of aqueous and hydroalcoholic extracts ofAcanthospermum australe(Loefl.) Kuntze against diarrhea-inducing bacteria. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 78, no. 4, pp. 619-624. http://dx.doi.org/10.1590/1519-6984.167376. PMid:29319752.
http://dx.doi.org/10.1590/1519-6984.1673...
), antibacterial and antiproliferative activities of the fresh leaf essential oil of Psidium guajava L. (Silva et al., 2019SILVA, E.A.J., ESTEVAM, E.B.B., SILVA, T.S., NICOLELLA, H.D., FURTADO, R.A., ALVES, C.C.F., SOUCHIE, E.L., MARTINS, C.H.G., TAVARES, D.C., BARBOSA, L.C.A. and MIRANDA, M.L.D., 2019. Antibacterial and antiproliferative activities of the fresh leaf essential oil of Psidium guajava L. (Myrtaceae). Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 79, no. 4, pp. 697-702. http://dx.doi.org/10.1590/1519-6984.189089. PMid:30462815.
http://dx.doi.org/10.1590/1519-6984.1890...
) and antimicrobial activity ofCampomanesia aureaagainst three strains ofListeria monocytogenes (Pacheco et al., 2020PACHECO, L.A., ETHUR, E.M., SHEIBEL, T., BUHL, B., WEBER, A.C., KAUFFMANN, C., MARCHI, M.I., FREITAS, E.M. and HOEHNE, L., 2020. Chemical characterization and antimicrobial activity ofCampomanesia aureaagainst three strains of Listeria monocytogenes.Brazilian Journal of Biology, vol. 81, pp. 69-76.).
Punica granatum (P. granatum L.) locally named as pomegranate is a plant fruit that belongs to the punicaceae (family). It is an important horticultural fruit crop. Polyphenols, important minerals and saccharides are present in edible part of fruit. Various types of phytochemicals are present in the P. granatum L. fruit, tree and seed. The polyphenols are the major class of phytochemicals. Polyphenols have multiple hydroxyl groups on the phenolic rings. Polyphenols have hydrolysable tannins, condensed tannins and flavonoids. The hydrolysable tannin includes ellagitannins and gallotannins (Elalamy et al., 2020ELALAMY, R.A., TARTOR, Y.H., AMMAR, A.M., ELDESOUKY, I.E. and ESAWY, A.E.I., 2020. Molecular characterization of extensively drug-resistant Pasteurella multocida isolated from apparently healthy and diseased chickens in Egypt. Pakistan Veterinary Journal, vol. 40, pp. 319-324.). The condensed tannins include pro-anthocyanidins. Flavonols, anthocyanin and flavanols are included in the flavonoids (Abbas et al., 2020ABBAS, R.Z., ZAMAN, M.A., SINDHU, Z.U.D., SHARIF, M., RAFIQUE, A., SAEED, Z., REHMAN, T.U., SIDDIQUE, F., ZAHEER, T., KHAN, M.K., AKRAM, M.S., CHATTHA, A.J., FATIMA, U., MUNIR, T. and AHMAD, M., 2020. Anthelmintic effects and toxicity analysis of herbal dewormer against the infection of Haemonchus contortus and Fasciola hepatica in goat. Pakistan Veterinary Journal, vol. 40, no. 04, pp. 455-460. http://dx.doi.org/10.29261/pakvetj/2020.083.
http://dx.doi.org/10.29261/pakvetj/2020....
).
The components of P. granatum L. plant includes juice, leaves, seed oil, peel, rind (pericarp), barks, roots and flowers. The constituents of P. granatum L. include ascorbic acid, anthocyanin, amino acids, glucose, caffeic acid, gallic acid, catechin, ellagic acid, minerals and iron. P. granatum L. leaves contain tannins (punicafolin and the punicalin) and flavones glycosides (include apgenin and the luteolin). The P. granatum L. seed oil has ellagic acid, sterols, fatty acids and punicic acid. The components of P. granatum L. peel and rind include gallic acid, phenolic punicalagins, rutin, flavonols (flavonones, flavones and anthocyanidins), quercetin, and fatty acids (catechin). P. granatum L. barks and roots have piperidine alkaloids and Ellagitannins (punicalagin and punicalin). Gallic acid, triterpenoid, ursolic acid, and other unidentified components are included in flower part of the P. granatum L. plant. According to chemical composition (per 100 grams), P. granatum L. has 1.6% protein, 14.5% carbohydrates, 0.7% minerals, 0.1% fat, 5.1% fiber and 78% moisture. The iron concentration of P. granatum L. is 0.3 mg. The concentration of calcium in P. granatum L. is 10 mg and concentration of phosphorus is 70 mg. Vitamin C (16.0 mg) is present in P. granatum L. plant. Vitamin B is present in trace amount. The caloric value of P. granatum L. is in 65 mg (Dahham et al., 2010DAHHAM, S.S., Ali, M.N., TABASSUM, H. and KHAN, M., 2010. Studies on antibacterial and antifungal activity of pomegranate (Punica Granatum L.). American Journal of Agriculture and Environmental Sciences, vol. 9, pp. 273-281.).
All parts of the P. granatum L. plant including trunk skin, seed, root and flower are used in medicine. P. granatum L. flowers are used in the treatment of diarrhea. P. granatum L. has bioactive components that can be used for the treatment of the dental diseases, cardiovascular diseases, cancer, diarrhea , skin infections, diabetes mellitus, hemorrhoids and other bacterial and fungal infections (Hajifattahi et al., 2016HAJIFATTAHI, F., MORAVEJ-SALEHI, E., TAHERI, M., MAHBOUBI, A. and KAMALINEJAD, M., 2016. Antibacterial effect of Hydroalcoholic extract of Punica granatum Linn. petal on common oral microorganisms. International Journal of Biomaterials, vol. 2016, pp. 8098943.).
P. granatum L. peel extract has polyphenols that inhibit the oxidation process, microbial growth, eliminate free radicals and decrease the risk of cancer and cardiovascular diseases. P. granatum L. peel extract is also rich in vitamin C. It is useful as the raw material for pharmaceutical industry because of its antimicrobial properties. Gallic acid, punicallins and ellagic acid extracted from the P. granatum L. revealed good inhibitory effect against pathogenic microorganisms (Opara et al., 2009OPARA, L.U., AL-ANI, M.R. and AL-SHUAIBI, Y.S., 2009. Physico-chemical properties, vitamin C content, and antimicrobial properties of pomegranate fruit (Punica granatum L.). Food and Bioprocess Technology, vol. 2, no. 3, pp. 315-321. http://dx.doi.org/10.1007/s11947-008-0095-5.
http://dx.doi.org/10.1007/s11947-008-009...
). Pomegranate has exhibited bactericidal activity against food and waterborne pathogenic bacteria includingSalmonellaTyphi (S. Typhi) (Perez and Anesini, 1994PÉREZ, C. and ANESINI, C., 1994. In-vitroantibacterial activity of Argentine folk medicinal plants againstSalmonella Typhi. Journal of Ethnopharmacology, vol. 44, no. 1, pp. 41-46. http://dx.doi.org/10.1016/0378-8741(94)90097-3. PMid:7990503.
http://dx.doi.org/10.1016/0378-8741(94)9...
; Rani and Khullar, 2004RANI, P. and KHULLAR, N., 2004. Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistantSalmonella Typhi. Phytotherapy Research, vol. 18, no. 8, pp. 670-673. http://dx.doi.org/10.1002/ptr.1522. PMid:15476301.
http://dx.doi.org/10.1002/ptr.1522...
),Vibrio cholerae(Guevara et al., 1994GUEVARA, J.M., CHUMPITAZ, J. and VALENCIA, E., 1994. Thein-vitroaction of plants onVibrio cholerae. Revista de Gastroenterologia del Peru, vol. 14, no. 1, pp. 27-31. PMid:8018898.; Mathabe et al., 2006MATHABE, M.C., NIKOLOVA, R.V., LALL, N. and NYAZEMA, N.Z., 2006. Antibacterial activities of medicinal plants used for the treatment of diarrhoea in Limpopo Province, South Africa. Journal of Ethnopharmacology, vol. 105, no. 1-2, pp. 286-293. http://dx.doi.org/10.1016/j.jep.2006.01.029. PMid:16545928.
http://dx.doi.org/10.1016/j.jep.2006.01....
),Yersinia enterocolitica,Shigellaspp. (Alanis et al., 2005ALANÍS, A.D., CALZADA, F., CERVANTES, J.A., TORRES, J. and CEBALLOS, G.M., 2005. Antibacterial properties of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. Journal of Ethnopharmacology, vol. 100, no. 1-2, pp. 153-157. http://dx.doi.org/10.1016/j.jep.2005.02.022. PMid:16005589.
http://dx.doi.org/10.1016/j.jep.2005.02....
; Mathabe et al., 2006MATHABE, M.C., NIKOLOVA, R.V., LALL, N. and NYAZEMA, N.Z., 2006. Antibacterial activities of medicinal plants used for the treatment of diarrhoea in Limpopo Province, South Africa. Journal of Ethnopharmacology, vol. 105, no. 1-2, pp. 286-293. http://dx.doi.org/10.1016/j.jep.2006.01.029. PMid:16545928.
http://dx.doi.org/10.1016/j.jep.2006.01....
), andListeria monocytogenes(L. monocytogenes) (Lucas and Were, 2009LUCAS, D.L. and WERE, L.M., 2009. Anti-Listeria monocytogenes activity of heat-treated lyophilized pomegranate juice in media and in ground top round beef.Journal of Food Protection, vol. 72, pp. 2508-2516.; Shan et al., 2011SHAN, B., CAI, Y.Z., BROOKS, J.D. and CORKE, H., 2011. Potential application of spice and herb extracts as natural preservatives in cheese. Journal of Medicinal Food, vol. 14, no. 3, pp. 284-290. http://dx.doi.org/10.1089/jmf.2010.0009. PMid:21142945.
http://dx.doi.org/10.1089/jmf.2010.0009...
). Typhoid fever (causal agent,S. Typhi) is a life-threatening enteric infection that can be transmitted by consuming food or drinking water contaminated with feces from an infected person. It is more common in less industrialized countries. Extracts of pomegranate fruit pericarp were tested by agar well diffusion and found to be highly active when compared to a reference concentration-response curve for ampicillin. In one study, ethanolic pomegranate extracts exhibited greater antibacterial activity than the antibiotic chloramphenicol, but lower activity than trimethoprim (Alanis et al., 2005ALANÍS, A.D., CALZADA, F., CERVANTES, J.A., TORRES, J. and CEBALLOS, G.M., 2005. Antibacterial properties of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. Journal of Ethnopharmacology, vol. 100, no. 1-2, pp. 153-157. http://dx.doi.org/10.1016/j.jep.2005.02.022. PMid:16005589.
http://dx.doi.org/10.1016/j.jep.2005.02....
; Zhang et al., 2020ZHANG, K., LI, X., NA, C., ABBAS, A., ABBAS, R.Z. and ZAMAN, M.A., 2020. Anticoccidial effects of Camellia sinensis (green tea) extract and its effect on blood and serum chemistry of broiler chickens. Pakistan Veterinary Journal, vol. 40, pp. 77-80.).Shigella sonneishowed the highest susceptibility to the extracts.
This study includes the phytochemical analysis of P. granatum L. through HPLC and antibacterial effect against range of gram negative and gram-positive bacteria. The active compounds, present in P. granatum L. and their antibacterial activity was evaluated through HPLC and agar well diffusion method, respectively.
2. Materials and Methods
2.1. Plant sample
The fresh P. granatum L. plant fruits were purchased from the local market of Faisalabad, Punjab, Pakistan. The fruit was confirmed and verified from a botanist following the Bessey classification system. These collected fruit peels were dried and stored safely for further proceedings.
2.2. Test organisms
The test organisms were obtained from the culture bank of the Institute of Microbiology, University of Agriculture, Faisalabad Pakistan. The confirmed isolates of three-gram negative bacteria belong to the Enterobacteriaceae E. coli, S. Typhimurium and S. Dysenteriae and a gram positive bacteria Staph. aureus in a final culture strength of 0.5 (1500 × 107 cells /ml) were used. MacFarland standard inoculum was prepared in nutrient broth.
2.3. Preparation of the P. granatum L. extract
The 10 grams of dried peels of P. granatum L. were crushed in the electric blender machine to make fine powder. Methanolic extract of P. granatum L. peel was prepared through Soxhlet apparatus method in 500ml of methanol solvent by following the already reported method (Lin et al., 2021)LIN, X., RAFIQUE, A., FAYYAZ, T., BASHIR, W., LUQMAN, M., ZAHID, F.M. and ZHOU, K., 2021. Appraisal of cymbopogon citratus (Lemon grass) for antibacterial activity against uropathogens. Pakistan Veterinary Journal, vol. 41, pp. 122-126.. P. granatum L. powder was poured in the thimbles and plugged with cotton. The thimble and solvent (methanol) was placed in the Soxhlet apparatus. The extract was prepared after 8 hours of cyclic procedure of the apparatus. The cycles were repeated in the apparatus continuously until the solvent changed to colorless from colored. The extract was removed from apparatus and dried in the rotary evaporator. The extract was stored in the refrigerator at 4°C in the Falcon tubes.
2.4. Phytochemical analysis
High Performance Liquid Chromatography (HPLC) was performed for the phytochemical analysis of yellow and red pomegranate ethanolic extracts in Shim-Pack CLC-ODS (C-18) column, 25cm×4.6mm, 5µm in the mobile phase of Gradient: A(H2O:AA-94:6. pH=2.27), B(ACN 100%), flow rate was 1ml/min. The detection was done through UV-Vis Detector 280 nm SPD-10Av. Reverse Phase Gradient HPLC made by Shimadzu, Japan was used for analysis. The pump used in this system was LC-10AT. The diluted extracted solution is injected into the HPLC system in a specific volume of 10 µm (Yasmin et al., 2020YASMIN, S., NAWAZ, M., ANJUM, A.A., ASHRAF, K., BASRA, M.A.R., MEHMOOD, A., KHAN, I. and MALIK, F., 2020. Phytochemical analysis and in-vitro activity of essential oils of selected plants against Salmonella enteritidis and Salmonella gallinarum of poultry origin. Pakistan Veterinary Journal, vol. 40, no. 02, pp. 139-144. http://dx.doi.org/10.29261/pakvetj/2019.110.
http://dx.doi.org/10.29261/pakvetj/2019....
).
2.5. Antibacterial assay
The antibacterial activity of P. granatum L.) peel extract was determined against three gram negative bacteria (E. coli, S. Typhimurium and S. Dysenteriae) and a gram positive bacteria Staph. aureus by following the agar well diffusion method used by Dahiya and Purkayastha, 2012 with little modifications. 50µl and 100µl of yellow and red pomegranate ethanolic extracts were tested with the positive (Ampicillin and piperacillin tazobactam) and negative control. Zones were measured after incubation at 37°C for 24 hours.
2.6. Minimum Inhibitory Concentration (MIC)
The MIC of P. granatum L. peel extract was determined by broth dilution method. Total 2ml volume contains nutrient broth, inoculum standardized through 0.5 MacFarland standard (1500×107 cells /ml). Each tube was incubated at 37°C for 24 hours and results were analyzed.
3. Results
3.1. Antibacterial activity
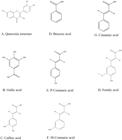
Chemical structures of flavonoid and phenolics found in the methanolic extract of Punica granatum L. peel is shown in Figure 1. P. granatum L. peel extracts (red and yellow) showed significant antibacterial activity against Staph. aureus, S. Typhimurium, S. Dysenteriae and E. coli. The extract produced greatest zones of inhibition against Staph. aureus as compared to bacteria including even positive control. The zones of inhibition (ZOI) of both red and yellow P. granatum L. extracts against different bacteria have been shown in Table 1 and Figure 2.
Chemical structures of flavonoid and phenolics found in the methanolic extract of Punica granatum L. peel.
ZOI of bacterial strains against different concentrations of P. grantum L. peel methanolic extracts.
Zones of inhibition of flavonoid and phenolics found in the methanolic extract of P. granatum L. peel against different public health bacteria.
3.2. Phytochemical analysis of P. granatum L. peel methanol extract
The HPLC chromatogram identified various phenolics and flavonoid compounds in methanolic extract of P. granatum L. peel. The most important phenolic compounds identified through HPLC in both red and yellow pomegranate are given in Table 2.
Concentration of different flavonoid and phenolics compounds in P. granatum L. methanolic extract determined through HPLC.
3.3. Minimum Inhibitory Concentration (MIC)
The MIC values of red and yellow pomegranate extract against Staph. aureus were 40 µl/ 2ml and 80 µl/2ml or 20 µl/ml and 40 µl/ml, respectively.
4. Discussion
Red and yellow P. grantum L. are already reported and showed comprehensively good results against different microbes. ZOI against E. coli and Staph. aureus determined by Khan and Hanee, 2011KHAN, J.A. and HANEE, S., 2011. Antibacterial properties of Punica granatum peels. International Journal of Applied Biology and Pharmaceutical Technology, vol. 2, pp. 23-27. were 25.5 and 22.5 mm, respectively. In current study, a significant difference was observed against Staph. aureus, between methanolic extract of both red and yellow P. granatum L. showed upto 30 mm ZOI in triplicates. It was more than positive control. So, it can be stated that methanolic extract of P. granatum L. peel can be effective against infections that are caused by Staph. aureus and it was more effective against gram positive bacteria than gram negative. In the study carried by Jaisinghani et al., 2018JAISINGHANI, R.N., MAKHWANA, S. and KANOJIA, A., 2018. Study on antibacterial and flavonoid content of ethanolic extract of Punica granatum (Pomegranate) Peel. Microbiology Research, vol. 9, pp. 7480-7484.. The inhibition zone was greater against S. Typhimurium than Staph. aureus and E. coli. The zones of inhibition against S. Typhimurium were around 28mm while against Staph. aureus and E. coli were 26 and 23mm, respectively. In current study, the highest zones of inhibition were against Staph. aureus.
Antibacterial activity of P. granatum L. peel extract in ethanol solvent against S. typhimurium showed minimum-inhibitory concentration (MIC) ranged from 62.5 to 1000x03BCgmL−1. The zones of inhibitions were 11, 14 and 15mm against S. Typhimurium. All the three strains showed inhibition zones (Choi et al., 2011CHOI, J.G., KANG, O.H., LEE, Y.S., CHAE, H.S., OH, Y.C., BRICE, O.O., KIM, M.S., SOHN, D.H., KIM, H.S., PARK, H., SHIN, D.W., RHO, J.R. and KWON, D.Y., 2011. In-vitro and in-vivo antibacterial activity of Punica granatum peel ethanol extract against Salmonella. Evidence-Based Complementary and Alternative Medicine, vol. 2011, pp. 690518. PMid:19687188.). In the current study, the average of zone of inhibition is 16 and 11 mm. There is much similarity in the diameter of inhibition zones against S. Typhimurium.
Chebaibi and Filali (2013)CHEBAIBI, A. and FILALI, F.R., 2013. Bactericidal activity and phytochemical screening of Moroccan Pomegranate (Punica granatum Linn.) peel aqueous extracts. Journal Medicinal Plants Research, vol. 7, pp. 887-891. reported that antimicrobial activity of P. granatum L. is due to the presence of active components. They isolated the components (phenols, tannins, flavonoids and steroids) but they did not explain the quantities of the active components. We isolated the same active components and with their quantity in ppm (parts per million).
P. granatum L. extract has no antibacterial effects against gram negative bacteria (Kanatt et al., 2010KANATT, S.R., CHANDER, R. and SHARMA, A., 2010. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. International Journal of Food Science and Technology, vol. 45, pp. 216-222.). They reported that there were no effects of P. granatum L. extract on E. coli and S. Typhimurium. In the gram-positive bacteria, P. granatum L. extract showed good antimicrobial activity even at the concentration of 0.01%. In the current study, the P. granatum L. extract shows good antimicrobial activity against both the gram positive and gram-negative bacteria.
P. granatum L. is good antimicrobial agent against gram positive and gram-negative bacteria (Opara et al., 2009OPARA, L.U., AL-ANI, M.R. and AL-SHUAIBI, Y.S., 2009. Physico-chemical properties, vitamin C content, and antimicrobial properties of pomegranate fruit (Punica granatum L.). Food and Bioprocess Technology, vol. 2, no. 3, pp. 315-321. http://dx.doi.org/10.1007/s11947-008-0095-5.
http://dx.doi.org/10.1007/s11947-008-009...
). There is much similarity in our results of anti-microbial activity against the pathogenic bacteria (gram positive and gram negative).
Pomegranate has exhibited bactericidal activity against food and waterborne pathogenic bacteria includingSalmonellaTyphi (S. Typhi) (Perez and Anesini, 1994PÉREZ, C. and ANESINI, C., 1994. In-vitroantibacterial activity of Argentine folk medicinal plants againstSalmonella Typhi. Journal of Ethnopharmacology, vol. 44, no. 1, pp. 41-46. http://dx.doi.org/10.1016/0378-8741(94)90097-3. PMid:7990503.
http://dx.doi.org/10.1016/0378-8741(94)9...
; Rani and Khullar, 2004RANI, P. and KHULLAR, N., 2004. Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistantSalmonella Typhi. Phytotherapy Research, vol. 18, no. 8, pp. 670-673. http://dx.doi.org/10.1002/ptr.1522. PMid:15476301.
http://dx.doi.org/10.1002/ptr.1522...
),Vibrio cholerae(Guevara et al., 1994GUEVARA, J.M., CHUMPITAZ, J. and VALENCIA, E., 1994. Thein-vitroaction of plants onVibrio cholerae. Revista de Gastroenterologia del Peru, vol. 14, no. 1, pp. 27-31. PMid:8018898.; Mathabe et al., 2006MATHABE, M.C., NIKOLOVA, R.V., LALL, N. and NYAZEMA, N.Z., 2006. Antibacterial activities of medicinal plants used for the treatment of diarrhoea in Limpopo Province, South Africa. Journal of Ethnopharmacology, vol. 105, no. 1-2, pp. 286-293. http://dx.doi.org/10.1016/j.jep.2006.01.029. PMid:16545928.
http://dx.doi.org/10.1016/j.jep.2006.01....
),Yersinia enterocolitica,Shigellaspp. (Alanis et al., 2005ALANÍS, A.D., CALZADA, F., CERVANTES, J.A., TORRES, J. and CEBALLOS, G.M., 2005. Antibacterial properties of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. Journal of Ethnopharmacology, vol. 100, no. 1-2, pp. 153-157. http://dx.doi.org/10.1016/j.jep.2005.02.022. PMid:16005589.
http://dx.doi.org/10.1016/j.jep.2005.02....
; Mathabe et al., 2006MATHABE, M.C., NIKOLOVA, R.V., LALL, N. and NYAZEMA, N.Z., 2006. Antibacterial activities of medicinal plants used for the treatment of diarrhoea in Limpopo Province, South Africa. Journal of Ethnopharmacology, vol. 105, no. 1-2, pp. 286-293. http://dx.doi.org/10.1016/j.jep.2006.01.029. PMid:16545928.
http://dx.doi.org/10.1016/j.jep.2006.01....
), andListeria monocytogenes(L. monocytogenes) (Lucas and Were, 2009LUCAS, D.L. and WERE, L.M., 2009. Anti-Listeria monocytogenes activity of heat-treated lyophilized pomegranate juice in media and in ground top round beef.Journal of Food Protection, vol. 72, pp. 2508-2516.; Shan et al., 2011SHAN, B., CAI, Y.Z., BROOKS, J.D. and CORKE, H., 2011. Potential application of spice and herb extracts as natural preservatives in cheese. Journal of Medicinal Food, vol. 14, no. 3, pp. 284-290. http://dx.doi.org/10.1089/jmf.2010.0009. PMid:21142945.
http://dx.doi.org/10.1089/jmf.2010.0009...
). Typhoid fever (causal agent,S. Typhi) is a life-threatening enteric infection that can be transmitted by consuming food or drinking water contaminated with feces from an infected person particularly pediatric infections. The use of pomegranate extracts as therapeutic intervention is more common in less industrialized countries. Extracts of pomegranate fruit and peel are also tested by agar well diffusion and found to be highly active when compared to a reference concentration-response curve for ampicillin. In one study, ethanolic pomegranate extracts exhibited greater antibacterial activity than the antibiotic chloramphenicol, but lower activity than trimethoprim (Alanis et al., 2005ALANÍS, A.D., CALZADA, F., CERVANTES, J.A., TORRES, J. and CEBALLOS, G.M., 2005. Antibacterial properties of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. Journal of Ethnopharmacology, vol. 100, no. 1-2, pp. 153-157. http://dx.doi.org/10.1016/j.jep.2005.02.022. PMid:16005589.
http://dx.doi.org/10.1016/j.jep.2005.02....
).
5. Conclusion
From the study undertaken, it can be concluded that methanol extract of P. granatum L. peel has flavonoid and phenolic contents and showed antimicrobial activity particularly against Staph. aureus. Pomegranate extracts may be used therapeutically against public health bacteria and infections causing pediatric infections e.g., diarrhea and dysentery along with several other diseases.
References
- ABBAS, R.Z., ZAMAN, M.A., SINDHU, Z.U.D., SHARIF, M., RAFIQUE, A., SAEED, Z., REHMAN, T.U., SIDDIQUE, F., ZAHEER, T., KHAN, M.K., AKRAM, M.S., CHATTHA, A.J., FATIMA, U., MUNIR, T. and AHMAD, M., 2020. Anthelmintic effects and toxicity analysis of herbal dewormer against the infection of Haemonchus contortus and Fasciola hepatica in goat. Pakistan Veterinary Journal, vol. 40, no. 04, pp. 455-460. http://dx.doi.org/10.29261/pakvetj/2020.083
» http://dx.doi.org/10.29261/pakvetj/2020.083 - AHMAD, Y., ZAHRA, R., ALI, M.I., RIAZ, M.H., KHAN, R., KHAN, K., KHAN, M.T., KHAN, M.F., ULLAH, S., HASSAN, F., ALI, A. and ZEB, M.T., 2020. Molecular screening of resistant and virulent genes in Salmonella Enteritidis and Salmonella Typhimurium from Poultry in Khyber Pakhtunkhwa. Pakistan Veterinary Journal, vol. 40, pp. 343-349.
- ALANÍS, A.D., CALZADA, F., CERVANTES, J.A., TORRES, J. and CEBALLOS, G.M., 2005. Antibacterial properties of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. Journal of Ethnopharmacology, vol. 100, no. 1-2, pp. 153-157. http://dx.doi.org/10.1016/j.jep.2005.02.022 PMid:16005589.
» http://dx.doi.org/10.1016/j.jep.2005.02.022 - CHEBAIBI, A. and FILALI, F.R., 2013. Bactericidal activity and phytochemical screening of Moroccan Pomegranate (Punica granatum Linn) peel aqueous extracts. Journal Medicinal Plants Research, vol. 7, pp. 887-891.
- CHOI, J.G., KANG, O.H., LEE, Y.S., CHAE, H.S., OH, Y.C., BRICE, O.O., KIM, M.S., SOHN, D.H., KIM, H.S., PARK, H., SHIN, D.W., RHO, J.R. and KWON, D.Y., 2011. In-vitro and in-vivo antibacterial activity of Punica granatum peel ethanol extract against Salmonella. Evidence-Based Complementary and Alternative Medicine, vol. 2011, pp. 690518. PMid:19687188.
- DAHHAM, S.S., Ali, M.N., TABASSUM, H. and KHAN, M., 2010. Studies on antibacterial and antifungal activity of pomegranate (Punica Granatum L.). American Journal of Agriculture and Environmental Sciences, vol. 9, pp. 273-281.
- DEVI, A., SINGH, V. and BHATT, A.B., 2011. Comparative antibacterial study of different extracts of Pomegranate and its wild variety. International Journal of Pharmaceutical Sciences and Research, vol. 2, pp. 2647-2650.
- DOHERTY, V.F., OLANIRAN, O.O. and KANIFE, U.C., 2011. Antimicrobial activities of Aframomum melegueta (Alligator Pepper). Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 2, pp. 126-131.
- ELALAMY, R.A., TARTOR, Y.H., AMMAR, A.M., ELDESOUKY, I.E. and ESAWY, A.E.I., 2020. Molecular characterization of extensively drug-resistant Pasteurella multocida isolated from apparently healthy and diseased chickens in Egypt. Pakistan Veterinary Journal, vol. 40, pp. 319-324.
- GUEVARA, J.M., CHUMPITAZ, J. and VALENCIA, E., 1994. Thein-vitroaction of plants onVibrio cholerae. Revista de Gastroenterologia del Peru, vol. 14, no. 1, pp. 27-31. PMid:8018898.
- HAJIFATTAHI, F., MORAVEJ-SALEHI, E., TAHERI, M., MAHBOUBI, A. and KAMALINEJAD, M., 2016. Antibacterial effect of Hydroalcoholic extract of Punica granatum Linn petal on common oral microorganisms. International Journal of Biomaterials, vol. 2016, pp. 8098943.
- JAISINGHANI, R.N., MAKHWANA, S. and KANOJIA, A., 2018. Study on antibacterial and flavonoid content of ethanolic extract of Punica granatum (Pomegranate) Peel. Microbiology Research, vol. 9, pp. 7480-7484.
- JAVED, F., SHARIF, M.K. and ABBAS, N., 2021. Hypoglycemic effect of flaxseed lignan and β glucan in postmenopausal diabetic patients. Agrobiological Records, vol. 5, pp. 32-40. http://dx.doi.org/10.47278/journal.abr/2020.031
» http://dx.doi.org/10.47278/journal.abr/2020.031 - KANATT, S.R., CHANDER, R. and SHARMA, A., 2010. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. International Journal of Food Science and Technology, vol. 45, pp. 216-222.
- KHAN, J.A. and HANEE, S., 2011. Antibacterial properties of Punica granatum peels. International Journal of Applied Biology and Pharmaceutical Technology, vol. 2, pp. 23-27.
- LIN, X., RAFIQUE, A., FAYYAZ, T., BASHIR, W., LUQMAN, M., ZAHID, F.M. and ZHOU, K., 2021. Appraisal of cymbopogon citratus (Lemon grass) for antibacterial activity against uropathogens. Pakistan Veterinary Journal, vol. 41, pp. 122-126.
- LUCAS, D.L. and WERE, L.M., 2009. Anti-Listeria monocytogenes activity of heat-treated lyophilized pomegranate juice in media and in ground top round beef.Journal of Food Protection, vol. 72, pp. 2508-2516.
- MAJEED, Y., SHAUKAT, M.B., ABBASI, K.Y. and AHMAD, M.A., 2021. Indigenous plants of Pakistan for the treatment of Diabetes: a review. Agrobiological Records, vol. 4, pp. 44-63. http://dx.doi.org/10.47278/journal.abr/2020.028
» http://dx.doi.org/10.47278/journal.abr/2020.028 - MALLMANN, R., ETHUR, E.M., BIANCHETTI, P., FALEIRO, D., HOEHNE, L. and GOETTERT, M.I., 2018. Effectiveness of aqueous and hydroalcoholic extracts ofAcanthospermum australe(Loefl.) Kuntze against diarrhea-inducing bacteria. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 78, no. 4, pp. 619-624. http://dx.doi.org/10.1590/1519-6984.167376 PMid:29319752.
» http://dx.doi.org/10.1590/1519-6984.167376 - MATHABE, M.C., NIKOLOVA, R.V., LALL, N. and NYAZEMA, N.Z., 2006. Antibacterial activities of medicinal plants used for the treatment of diarrhoea in Limpopo Province, South Africa. Journal of Ethnopharmacology, vol. 105, no. 1-2, pp. 286-293. http://dx.doi.org/10.1016/j.jep.2006.01.029 PMid:16545928.
» http://dx.doi.org/10.1016/j.jep.2006.01.029 - MOGHADDAM, G., SHARIFZADEH, M., HASSANZADEH, G., KHANAVI, M. and HAJIMAHMOODI, M., 2013. Anti-ulcerogenic activity of the pomegranate peel (Punica granatum) methanol extract. Food and Nutrition Sciences, vol. 4, no. 10, pp. 43-48. http://dx.doi.org/10.4236/fns.2013.410A008
» http://dx.doi.org/10.4236/fns.2013.410A008 - OPARA, L.U., AL-ANI, M.R. and AL-SHUAIBI, Y.S., 2009. Physico-chemical properties, vitamin C content, and antimicrobial properties of pomegranate fruit (Punica granatum L.). Food and Bioprocess Technology, vol. 2, no. 3, pp. 315-321. http://dx.doi.org/10.1007/s11947-008-0095-5
» http://dx.doi.org/10.1007/s11947-008-0095-5 - PACHECO, L.A., ETHUR, E.M., SHEIBEL, T., BUHL, B., WEBER, A.C., KAUFFMANN, C., MARCHI, M.I., FREITAS, E.M. and HOEHNE, L., 2020. Chemical characterization and antimicrobial activity ofCampomanesia aureaagainst three strains of Listeria monocytogenes.Brazilian Journal of Biology, vol. 81, pp. 69-76.
- PÉREZ, C. and ANESINI, C., 1994. In-vitroantibacterial activity of Argentine folk medicinal plants againstSalmonella Typhi. Journal of Ethnopharmacology, vol. 44, no. 1, pp. 41-46. http://dx.doi.org/10.1016/0378-8741(94)90097-3 PMid:7990503.
» http://dx.doi.org/10.1016/0378-8741(94)90097-3 - RAFAY, M., GHAFFAR, M.U., ABID, M., MALIK, Z. and MADNEE, M., 2021. Phytochemicals analysis and antimicrobial activities ofEchinops echinatusfrom Cholistan Desert, Pakistan. Agrobiological Records, vol. 5, pp. 21-27. http://dx.doi.org/10.47278/journal.abr/2021.001
» http://dx.doi.org/10.47278/journal.abr/2021.001 - RANI, P. and KHULLAR, N., 2004. Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistantSalmonella Typhi. Phytotherapy Research, vol. 18, no. 8, pp. 670-673. http://dx.doi.org/10.1002/ptr.1522 PMid:15476301.
» http://dx.doi.org/10.1002/ptr.1522 - SHAN, B., CAI, Y.Z., BROOKS, J.D. and CORKE, H., 2011. Potential application of spice and herb extracts as natural preservatives in cheese. Journal of Medicinal Food, vol. 14, no. 3, pp. 284-290. http://dx.doi.org/10.1089/jmf.2010.0009 PMid:21142945.
» http://dx.doi.org/10.1089/jmf.2010.0009 - SILVA, E.A.J., ESTEVAM, E.B.B., SILVA, T.S., NICOLELLA, H.D., FURTADO, R.A., ALVES, C.C.F., SOUCHIE, E.L., MARTINS, C.H.G., TAVARES, D.C., BARBOSA, L.C.A. and MIRANDA, M.L.D., 2019. Antibacterial and antiproliferative activities of the fresh leaf essential oil of Psidium guajava L. (Myrtaceae). Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 79, no. 4, pp. 697-702. http://dx.doi.org/10.1590/1519-6984.189089 PMid:30462815.
» http://dx.doi.org/10.1590/1519-6984.189089 - YASMIN, S., NAWAZ, M., ANJUM, A.A., ASHRAF, K., BASRA, M.A.R., MEHMOOD, A., KHAN, I. and MALIK, F., 2020. Phytochemical analysis and in-vitro activity of essential oils of selected plants against Salmonella enteritidis and Salmonella gallinarum of poultry origin. Pakistan Veterinary Journal, vol. 40, no. 02, pp. 139-144. http://dx.doi.org/10.29261/pakvetj/2019.110
» http://dx.doi.org/10.29261/pakvetj/2019.110 - ZHANG, K., LI, X., NA, C., ABBAS, A., ABBAS, R.Z. and ZAMAN, M.A., 2020. Anticoccidial effects of Camellia sinensis (green tea) extract and its effect on blood and serum chemistry of broiler chickens. Pakistan Veterinary Journal, vol. 40, pp. 77-80.
Publication Dates
-
Publication in this collection
20 Aug 2021 -
Date of issue
2023
History
-
Received
09 Oct 2020 -
Accepted
07 Feb 2021