Abstract
Understanding the relation between the environmental stress factors and the hypothalamus-pituitary-thyroid (HPT) axis efficiency can reduce the susceptibility to thyroid diseases. In our study, thyroid dysfunction was induced in female rats by administration of 40 mg Na F/kg.bd.wt/day for a month.
Co-administration of the water extract of Arca noae (300 mg/kg. bw) was tested as a treatment for Na F induced thyroid dysfunction. A group of rats injected Arca noae extract only (300 mg/kg.bd.wt) was performed to observe the impact of the extract on the (HPT) axis in addition to the normal control group.
Results showed that there was a significant decrease in serum triglycerides, total protein and albumin levels in the fluoride supplemented group in addition to abnormal levels of TSH, (T4) and (T3) compared to the control group. In the treated group there was an improvement in the proteins level and lipid profile but pseudo-corrected serum (T4) and (T3) levels were observed in addition to a continuous increase in TSH level. Histological findings confirmed the harmful effect of fluoride on both the non treated and the treated groups.
Consequently, fluoride supplementation must be considered as a harmful stress that may affect permanently the HPT axis.
Keywords:
fluoride; HPT axis; Arca noae extract
Resumo
Compreender a relação entre os fatores de estresse ambiental e o eixo hipotálamo-hipófise-tireoide (HPT) pode reduzir a suscetibilidade a doenças da tireoide. Em nosso estudo, a disfunção tireoidiana foi induzida em ratos fêmeas pela administração de 40 mg Na F/kg.bw/dia durante um mês.
A administração concomitante do extrato aquoso de Arca noae (300 mg/kg.Pc) foi testada como tratamento para a disfunção tireoidiana induzida por Na F. Um grupo de ratos injetados apenas com extrato de Arca noae (300 mg/kg. Pc) foi pré-formado com o intuito de observar o impacto do extrato no eixo (HPT), além do grupo controle normal.
Os resultados mostraram que houve uma diminuição significativa nos níveis séricos de triglicerídeos, proteína total e albumina no grupo suplementado com fluoreto, além de níveis anormais de TSH, (T4) e (T3) em comparação ao grupo controle. No grupo tratado, houve uma melhora no nível de proteínas e perfil lipídico. Os níveis séricos pseudocorrigidos (T4) e (T3) foram observados, além de um aumento contínuo no nível de TSH. Os achados histológicos confirmaram o efeito prejudicial do flúor nos grupos não tratado e tratado.
Consequentemente, a suplementação de flúor é considerada um estresse prejudicial que pode afetar permanentemente o eixo HPT.
Palavras-chave:
fluoreto; eixo HPT; extrato de Arca noae
1. Introduction
For many decades, man believes that marine organisms and sea food consumption is beneficial for the improvement of his thyroid gland functions and his general health. But is this the case now among all the present environmental pollutions?. Nowadays, environmental pollution stress is increasing at a worrying rate worldwide and can affect greatly and alter body hormones production especially the thyroid hormones.
The thyroid gland is known as the master regulator of the metabolism in humans and animals. Thyroid hormones control metabolic processes that are essential for normal growth and development in addition to monitoring body weight and energy expenditure (Cheng et al., 2010CHENG, S.Y., LEONARD, J.L. and DAVIS, P.J., 2010. Molecular aspects of thyroid hormone actions. Endocrine Reviews, vol. 31, no. 2, pp. 139-170. http://dx.doi.org/10.1210/er.2009-0007. PMid:20051527.
http://dx.doi.org/10.1210/er.2009-0007...
; Iwen et al., 2013IWEN, K.A., SCHRODER, E. and BRABANT, G., 2013. Thyroid hormone and the metabolic syndrome. European Thyroid Journal, vol. 2, no. 2, pp. 83-92. http://dx.doi.org/10.1159/000351249. PMid:24783045.
http://dx.doi.org/10.1159/000351249...
).
In healthy cases, the hypothalamus-pituitary-thyroid (HPT) axis functions efficiently and synergistically for controlling the normal blood levels of thyroid hormones. On the level of the hypothalamus, the thyroid releasing hormone (TRH) is released which stimulates the pituitary to secrete thyroid-stimulating hormone (thyrotropin or TSH). TSH in turn drives the thyroid gland to release the pro-hormone thyroxine (T4) and 3,5,3’-tri-iodothyronine (T3) into the circulation. Also in peripheral tissues, (T4) is converted to (T3) and reverse T3 (rT3) which is thought to be metabolically inactive. (T4) and (T3) in turn exert a negative feedback control on the TRH and TSH hormones level secreted by the hypothalamus and the pituitary respectively (Pałkowska-Goździk et al., 2017PAŁKOWSKA-GOŹDZIK, E., LACHOWICZ, K. and ROSOŁOWSKA-HUSZCZ, D., 2017. Effects of dietary protein on thyroid axis activity. Nutrients, vol. 10, no. 5, pp. 1-15. PMid:29271877.).
Thyroid disorders represent one of the important endocrine alterations that cause harmful effects on the total body health. Four common thyroid disorder cases can exist; Hypothyrodism (decrease in the thyroid hormones blood level), hyperthyroidism (increase in thyroid hormones blood level), thyroid nodule (swelling in certain part of the gland which can be benign or malignant) and thyroid cancer (Sharma et al., 2014SHARMA, R., BHARTI, S. and KUMAR, K.V.S.H., 2014. Diet and thyroid – myths and facts. J Med Nutr Nutraceut., vol. 3, no. 2, pp. 60-65.). These cases take much time to appear and to show significant changeable hormonal levels in blood.
The thyroid can accumulate harmful elements, heavy metals and halogens from the surrounding media that replace the useful ones as iodine and selenium which are naturally drawn to the thyroid cells and leads to its dysfunction (Zoeller, 2007ZOELLER, R.T., 2007. Environmental Chemicals Impacting the Thyroid: targets and Consequences. Thyroid, vol. 17, no. 9, pp. 811-817. http://dx.doi.org/10.1089/thy.2007.0107. PMid:17956155.
http://dx.doi.org/10.1089/thy.2007.0107...
).
Ingestion of excess fluoride is considered an important environmental risk factor that lowers the iodine level and replaces the iodine molecules in the thyroid which may cause the interference with the thyroid hormones production (Cinar and Selcuk, 2005CINAR, A. and SELCUK, M., 2005. Effects of chronic fluorosis on thyroxin, tri-iodothyronine, and protein-bound iodine in cows. Fluoride, vol. 38, pp. 65-68.; Wang et al., 2009WANG, H., YANG, Z., ZHOU, B., GAO, H., YAN, X. and WANG, J., 2009. Fluoride-induced thyroid dysfunction in rats: roles of dietary protein and calcium level. Toxicology and Industrial Health, vol. 25, no. 1, pp. 49-57. http://dx.doi.org/10.1177/0748233709102720. PMid:19318504.
http://dx.doi.org/10.1177/07482337091027...
). Fluoride can enter the body silently everyday through numerous ways such as consuming plants in soils that are irrigated with fluoridated water, smoking some tobacco species, preparing food in teflon pans in addition to using some mouthwashs and toothpasts or some medicinal drugs that contain fluoride such as Flurouracil (Abdul Rahman and Fetouh, 2013ABDUL RAHMAN, M.M. and FETOUH, F.A., 2013. Effect of sodium fluoride on the thyroid follicular cells and the amelioration by calcium supplementation in albino rats: A light and an electron microscopic study. The Journal of American Science, vol. 9, no. 10, pp. 107-114.; Waugh et al., 2017WAUGH, D.T., GODFREY, M., LIMEBACK, H. and POTTER, W., 2017. Black tea source, production, and consumption: assessment of health risks of fluoride intake in New Zealand. Journal of Environmental and Public Health, vol. 2017, Article ID 5120504. http://dx.doi.org/10.1155/2017/5120504. PMid:28713433.
http://dx.doi.org/10.1155/2017/5120504...
).
Acute stress and critical illness are accused of affecting the thyroid axis within a few hours after their onset. They cause a drop in (T3) and a rise in (rT3) circulating levels accompanied by an observed increase in (T4) and TSH circulating levels (Mebis and Van den Berghe, 2009MEBIS, L. and VAN DEN BERGHE, G., 2009. The hypothalamus-pituitary-thyroid axis in critical illness. The Netherlands Journal of Medicine, vol. 67, no. 10, pp. 332-340. PMid:19915227.; Economidou et al., 2011ECONOMIDOU, F., DOUKA, E., TZANELA, M., NANAS, S. and KOTANIDOU, A., 2011. Thyroid function during critical illness. Hormones (Athens, Greece), vol. 10, no. 2, pp. 117-124. http://dx.doi.org/10.14310/horm.2002.1301. PMid:21724536.
http://dx.doi.org/10.14310/horm.2002.130...
) which represents a different case with different signs from the previously mentioned thyroid disorders.
Marine organisms with their high content in proteins, polyunsaturated fatty acids, antioxidants, micronutrients and iodine are recommended to improve and strengthen the thyroid and the hypothalamus-pituitary-thyroid (HPT) axis efficiency against many diseases (Choi and Kim, 2014CHOI, W.J. and KIM, J., 2014. Dietary factors and the risk of thyroid cancer: a review. Clinical Nutrition Research, vol. 3, no. 2, pp. 75-88. http://dx.doi.org/10.7762/cnr.2014.3.2.75. PMid:25136535.
http://dx.doi.org/10.7762/cnr.2014.3.2.7...
; Sarkar and Pal, 2014SARKAR, C. and PAL, S., 2014. Ameliorative effect of Resveratrol against fluoride-induced alteration of thyroid function in male wistar rats. Biological Trace Element Research, vol. 162, no. 1-3, pp. 278-287. http://dx.doi.org/10.1007/s12011-014-0108-3. PMid:25164033.
http://dx.doi.org/10.1007/s12011-014-010...
).
Arca noae is a bivalve mollusk and mostly distributed along the coasts of the Mediterranean sea and the Atlantic ocean besides the Black seas and West Indies (Dupcic Radic et al., 2014DUPCIC RADIC, I., CARIĆ, M., NAJDEK, M., JASPRICA, N., BOLOTIN, J., PEHARDA, M. and BRATOŠ CETINIĆ, A., 2014. Biochemical and fatty acid composition of Arca noae (Bivalvia: Arcidae) from the Mali Ston Bay, Adriatic Sea. Mediterranean Marine Science, vol. 15, no. 3, pp. 520-531. http://dx.doi.org/10.12681/mms.436.
http://dx.doi.org/10.12681/mms.436...
). Studying the distribution and the density of the population ecology of any important mollusk species may help in monitoring fisher men activity. These ecosystems tend to be highly productive and heterogeneous and, therefore, represent an excellent opportunity for studies on the population structure (Maia et al., 2018MAIA, A.M.L.R., MEDEIROS, E. and HENRY-SILVA, G.G., 2018. Distribution and density of the bivalve Anomalocardia brasiliana in the estuarine region of Northeastern Brazil. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 78, no. 1, pp. 32-40. http://dx.doi.org/10.1590/1519-6984.02316. PMid:28678977.
http://dx.doi.org/10.1590/1519-6984.0231...
).
Arca noae is considered as a marine invertebrate that is abundant in Egypt coastal governorates such as Alexandria, Port-Said and also exists in some lakes such as El-Timsah lake (Saad El-Din and Gad El-Hak, 2017SAAD EL-DIN, M.I. and GAD EL-HAK, H.N., 2017. Impact of heavy metals contamination on spring abundance of aquatic macro-invertebrates inhabiting lake Timsah, Egypt. Journal of Water Security, vol. 3, no. 1, pp. 1-6. http://dx.doi.org/10.15544/jws.2017.003.
http://dx.doi.org/10.15544/jws.2017.003...
). Its cheap cost and its easiness in consuming it raw without the need of cooking makes Arca noae a suitable and nutritious meal for the low- income people. There are few studies that discuss the nutritional value of Arca noae present in the Mediterranean coasts. A recent Tunisian study revealed that Arca noae is useful and beneficable to human health (Ghribi et al., 2018GHRIBI, F., BOUSSOUFA, D., AOUINI, F., BEJAOUI, S., CHETOUI, I., RABEH, I. and EL CAFSI, M., 2018. Seasonal variation of biochemical composition of Noah’s ark shells (Arca noae L. 1758) in a Tunisian coastal lagoon in relation to its reproductive cycle and environmental conditions. Aquatic Living Resources, vol. 31, pp. 14-15. http://dx.doi.org/10.1051/alr/2018002.
http://dx.doi.org/10.1051/alr/2018002...
). The aim of our study is suggesting Arca noae water extract as a co-administrated treatment for improving fluoride induced thyroid dysfunction case in female rats.
This was achieved by measuring serum TSH, (T4), (T3) and IL-6 in addition to serum total cholesterol (TC), triglycerides (TG), total protein content and albumin level in the treated and the non treated groups compared to the control one. Histological examinations for thyroid gland was preformed to compare the thyroid status in all the groups.
2. Material and Methods
2.1. Materials
2.1.1. Animals
Forty adult female Wistar albino rats weighing about 100g were obtained from the animal house unit in the National Research Centre, Giza, Egypt. The animals were housed under standard laboratory conditions (12 h light and 12 h dark) in a room of controlled temperature (24°C) during the experimental period. The rats were provided ad libitum with tap water and fed with standard commercial rat chow. All the studies were conducted in accordance with the Animal Ethical Committee of the National Research Center, Dokki, Giza, Egypt under the ethics number (18157).
2.1.2. Chemicals and kits
All chemicals used in the experiments were of analytical grade. Sodium fluoride (Na F) was obtained from Sigma.
Kits used for the quantitative determination of TC,TG and albumin were purchased from Erba Lachema Diagnostika Company, Czech Republic. Thyroid hormone detection kits were purchased from Abia Diagnostic Company, Gmbh, Berlin. IL-6 kit was purchased from Wuhan Fine Biotech. Co Ltd, China.
Arca noae snails were obtained from the Egyptian market in winter of 2018. The snails were rinsed in tap water to get rid of any sediments then the soft tissue was separated from the external snail, washed and stored in -20 till making the homogenate.
2.2. Experimental methods
2.2.1. Dose preparation
Na F was dissolved in distilled water and was administered in a dose of 40 mg/kg. bw/day orally by gavages (Luo et al., 2017LUO, Q., CUI, H., DENG, H., KUANG, P., LIU, H., LU, Y., FANG, J., ZUO, Z., DENG, J., LI, Y., WANG, X. and ZHAO, L., 2017. Sodium fluoride induces renal inflammatory responses by activating NF-κB signaling pathway and reducing anti-inflammatory cytokine expression in mice. Oncotarget, vol. 8, no. 46, pp. 80192-80207. http://dx.doi.org/10.18632/oncotarget.19006. PMid:29113295.
http://dx.doi.org/10.18632/oncotarget.19...
).
Arca noae water extract
Water extract of Arca noae dose was chosen according to applying one-tenth of the maximum safe dose detected in the acute toxicity study.
Water extract of Arca noae was prepared by homogenizing the soft tissue in sterile water by using an electric homogenizer with a teflon rod. -The homogenate was then centrifuged at 4000 r.p.m for 10 min. The supernatant was stored in -20 ° C till used for the intraperitonial injection to rats at a dose of 300 mg/kg.bd.wt/day (Song et al., 2008SONG, L., LI, T., YU, R., YAN, C., REN, S. and ZHAO, Y., 2008. Antioxidant activities of hydrolysates of Arca subcrenata prepared with three proteases. Marine Drugs, vol. 6, no. 4, pp. 607-619. http://dx.doi.org/10.3390/md6040607. PMid:19172198.
http://dx.doi.org/10.3390/md6040607...
).
2.2.2. Experimental design
2.2.2.1. Acute toxicity studies
LD50 value of single different doses of the extract intraperitonially injected were performed up till the dose of 3000 mg/kg bd. wt. Observing the rat mortality and behavior were estimated for two weeks. Acute toxicity studies were preformed according to the method described by Hu et al. (2012)HU, X., SONG, L., HUANG, L., ZHENG, Q. and YU, R., 2012. Antitumor Effect of a polypeptide fraction from Arca subcrenata in vitro and in vivo. Marine Drugs, vol. 10, no. 12, pp. 2782-2794. http://dx.doi.org/10.3390/md10122782. PMid:23342393.
http://dx.doi.org/10.3390/md10122782...
.
2.2.2.2. Groups & biochemical studies
-
After one week of acclimation, the animals were divided into 4 groups (8 to 10 rats in each group);
-
The 1st group did not receive any supplementations and served as a negative control.
-
The 2nd group received Na F at a dose of 40 mg/kg bd.wt/ day orally by gavages for 35 days and served as a positive control.
-
The 3rd group received water extract of Arca noae intraperitonially at a dose of 300 mg/kg bw/ day for three weeks.
-
The 4th group received Na F at a dose of 40 mg/kg. bd.wt/ day for two weeks followed by a co-administration of the water extract of Arca noae at a dose of 300 mg/kg bd.wt/ day intraperitonially for three weeks.
The rats were dissected and the blood was taken from the retro-orbital plexus of the eyes and centrifuged at 4000 r.p.m for 10 min obtaining the serum. Serum was stored at -20°C till subjected to the biochemical studies.
The thyroid gland was removed and cleaned then was rinsed in 10% formalin and was prepared for histological studies.
2.2.3. Biochemical analysis
2.2.3.1. Main constituents of Arca noae soft tissue and trace elements
Main constituents of Arca noae soft tissue was determined according to Egan et al. (1981)EGAN, H., KIRK, R.S. and SAWYER, R., 1981. Pearson’s chemical analysis of food. Edinburgh: Churchill Livingstone. while trace elements level was measured by the standard methods using atomic absorption spectrophotometer.
2.2.3.2. Serum lipid profile, total protein content and albumin
Total cholesterol and triglycerides were estimated as a quantitative enzymatic colorimetric method in serum according to Searcy (1969)SEARCY, R.L., 1969. Diagnostic biochemistry. New York: McGraw-Hill. and Cole et al. (1997)COLE, T.G., KLOTZSCH, S.G. and MCNAMARA, J., 1997. Measurment of triglyceride concentration. In: N. RIFAI, G.R. WARNICK and M.H. DOMINICZAK, eds. Handbook of lipoprotein testing. Washington: AACC Press, pp. 155. respectively. Serum total protein was measured quantitatively by a colorimetric method as described by Gornall et al. (1949)GORNALL, A.G., BARDAWILL, C.J. and DAVID, M.M., 1949. Determination of serum proteins by means of the biuret reaction. The Journal of Biological Chemistry, vol. 177, no. 2, pp. 751-766. PMid:18110453.. Albumin in serum was measured according to the method of Leonard et al. (1971)LEONARD, P.J., PERSAUD, J. and MOTWANI, R., 1971. The estimation of plasma albumin by BCG dye binding on the Technicon SMA 12/60 analyzer and a comparison with the HABA dye binding technique. Clinica Chimica Acta; International Journal of Clinical Chemistry, vol. 35, no. 2, pp. 409-412. http://dx.doi.org/10.1016/0009-8981(71)90214-2. PMid:5125326.
http://dx.doi.org/10.1016/0009-8981(71)9...
. Quantitative determinations were performed by using the automated Erba XL-300.
2.2.3.3. Serum thyroid Hormones assay
Thyroid hormones were detected in serum quantitatively as an immunoassay by ELISA technique using the automated ELISA reader Expert Plus UV, biochrom., G 020151.
TSH was estimated by the method described by Fisher (1996)FISHER, D.A., 1996. Physiological variations in thyroid hormones: physiological and pathophysiological considerations. Clinical Chemistry, vol. 42, no. 1, pp. 135-139. http://dx.doi.org/10.1093/clinchem/42.1.135. PMid:8565215.
http://dx.doi.org/10.1093/clinchem/42.1....
. (T4) and (T3) were detected as shown by Nelson and Wilcox (1996)NELSON, J.C. and WILCOX, R.B., 1996. Analytical performance of free and total thyroxine assays. Clinical Chemistry, vol. 42, no. 1, pp. 146-154. http://dx.doi.org/10.1093/clinchem/42.1.146. PMid:8565218.
http://dx.doi.org/10.1093/clinchem/42.1....
and Ekins (1993)EKINS, R., 1993. Free hormone assays. Nuclear Medicine Communications, vol. 14, no. 8, pp. 676-688. http://dx.doi.org/10.1097/00006231-199308000-00008. PMid:8371893.
http://dx.doi.org/10.1097/00006231-19930...
respectively.
2.2.3.4. Serum IL-6 assay.
IL-6 was determined according to the manufacturer kit using the automated ELISA reader Stat-Fax -2100, Awareness Technology.
2.2.4. Histological study
Specimens of thyroid gland were taken in a tissue block composed of thyroid gland, trachea and surrounding connective tissue and fixed in 10% formalin and processed for paraffin sections of 4 micron thickness. The sections were stained with Hematoxylin and Eosin. The method was performed as described by Banchroft et al. (1996)BANCHROFT, J.D., STEVENS, A.A. and TURNER, D.R., 1996. Theory and practice of histological techniques. 4th ed. Churchil Livingstone: Elsevier..
2.2.5. Statistical analysis
All values were expressed as the mean ± SD. Significant differences between the groups were statistically analyzed using a one- way analysis of variance (ANOVA). A P value of 0.05 or less was considered statistically significant.
3. Results
3.1. Acute toxicity results
Intraperitoneal injection of different single doses of Arca noae water extract showed no toxicity signs detected when given to female rats up to the dose 3000 mg /kg.bd.wt. No mortality was recorded after twenty four hrs and during two weeks after extract injection. Accordingly the extract is considered safe and secure when supplemented to rats.
3.2. Biochemical results
3.2.1. Main constituents of Arca noae dry tissue
Table 1 showed that the main tissue contents of Arca noae are the proteins as they represent about 40% followed by the carbohydrates that represent about 24% from the dry weight. Small amounts of lipids and ash exist in the dry tissue represented by a value equal to 3% for each. The tissue also contains moisture that represents 30% from the whole soft tissue.
3.2.2. Detection of some trace elements and heavy metals in Arca noae water extract
Equal concentration levels were observed for copper and iron elements (0.1 mg/l). On the other hand, zinc concentration recorded double the conc. of both copper and iron (0.2 mg/l). Selenium showed high concentration (4.7 mg/l) recorded for an invertebrate, while magnesium displayed the most highest concentration among elements (26 mg/l). Neglected level for lead was observed (0.07mg/l) and also for cadmium as it recorded (0.02mg/l). Arsenic detected (7.26 mg/l), while trace aluminum levels were observed as shown in (Table 2).
Concentrations of some trace elements and heavy metals in Arca noae water extract at a dose 300 mg/kg. bd. wt /day.
3.2.3. Effect of Na F and / or Arca noae extract administration on body weight gain
A significant increase in bd wts was observed in all groups compared to their corresponding initial weights (Table 3). This increase was represented by percent changes equal 33.64, 33.53 and 30.08 for the groups supplemented with the Arca noae extract, treatment and negative control groups respectively.
Effect of sodium fluoride and /or Arca noae water extract administration on the body weight gain in female rats of different groups.
On the other hand, there was a significant retardation in gaining weight in the positive control group supplemented with Na F as the increase in weight was represented by 25.61% compared to its corresponding initial weight as shown in Table 3 .
With respect to the negative control group, a significant decrease in weight in the group supplemented Na F was represented by a value equal to -11.01%. A slight improvement in bd.wt gain was observed in the treatment group as there was a decrease in bd.wt by a value equal to -8.2%.
3.2.4. Effect of Na F and/or Arca noae extract administration on serum parameters
Significant decreasing levels in serum triglycerides, total protein and albumin were observed in the positive control (Na F supplemented) group which were accompanied with a non significant increase in cholesterol level compared to control group as shown in Table 4.
Effect of sodium fluoride and /or Arca noae water extract administration on serum lipid profile and proteins in female rats of different groups.
Non significant increasing levels of cholesterol, triglycerides and total proteins in addition to an unchanged values of serum albumin were detected in the Arca noae injected extract normal group compared to the control group.
With respect to the positive control group, the extract showed the same effect on the serum cholesterol level (64.8 mg/dl) which represented a non significant increase compared to the control group. On the other hand, the extract showed a strong impact in increasing serum triglycerides and significant impact on total protein levels. So we can deduce that the treatment with the extract can compensate the great loss in triglycerides and proteins that resulted from Na F supplementation besides keeping the cholesterol values around the normal levels.
In the treated group, the therapeutic effect of the administration of the extract was shown in the significant decreasing in cholesterol and the significant increasing in triglycerides serum levels compared to the positive control group. The extract also kept the total protein content and the albumin serum levels around normal levels compared to the positive control group as shown in table 4.
3.2.5. Effect of Na F and/or Arca noae extract administration on serum thyroid hormones
Table 5 showed that although there were non significant changes in serum TSH, (T4) and (T3) observed in the positive group supplemented Na F, the data may express the beginning of the initiation of the thyroid dysfunction case. Thyroid hormones disruption was greatly shown in the increasing serum levels of both the TSH and (T4) which were accompanied by decreasing levels of (T3) compared to the negative control group.
Effect of sodium fluoride and /or Arca noae water extract administration on serum thyroid hormones and IL-6 in female rats of different groups.
On the other hand, in the Arca noae administrated normal group, there was a detectable decrease in TSH serum levels as a result of the increase in (T4) serum levels which exerted a negative feedback mechanism on TSH levels. Unexpected low serum (T3) levels were expressed in this group compared to the negative control one.
With respect to the Na F supplemented group, the Arca noae injected normal group showed lower levels of TSH and nearly similar levels of (T4). On the other hand, there was a great drop in T3 serum level in the extract normal group compared to Na F supplemented group. These decreasing levels in TSH and (T3) may act as a therapeutic effect on the abnormal levels of thyroid hormones due to Na F supplementation.
An unimproved and increased TSH serum levels were detected in the treated group compared to the control group.
On the other hand, an improvement in (T4) and (T3) serum levels were detected in the treated group compared to control one. The co-administration of the extract affected positively the increase in (T4) and the decrease in (T3) serum levels that were detected in the Na F supplemented group.
IL-6 showed no significant difference in all groups compared to control.
3.3. Histological examinations
3.3.1. Group of rats kept as control
There was no histo-pathological alteration. The normal histological structure of the active follicles with cuboidal to columner lining epithelium with round narrow lumen and scanty colloid were recorded in Figure 1.
Control group (H&Ex80). The figure shows normal histological structure of the active follicles (black arrow) with columner lining epithelium (white arrow) and few colloid (C) in the lumen.
3.3.2. Group of rats supplemented sodium fluoride (NaF)
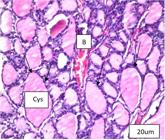
Cystic follicular dilatation with flattened lining epithelium and wide lumen with heavy colloid were observed. Congestion in blood vessels was also found as shown in Figure 2.
Positive control supplemented Na F (H&Ex 40). The figure shows follicular cystic dilatation (cys) with flattened lining epithelium (black arrow) and huge eosinophilic colloid with congestion in blood vessels (B).
3.3.3. Group of rats injected intraperitonially Arca noae extract only
Normal active follicles were observed as shown in Figure 3.
Arca noae injected normal group (H&Ex 80). The figure shows normal active follicles (black arrow).
3.3.4. Group of rats co-administrated Arca noae extract with Na F (treated group)
The follicular lumen showed cystic dilatation with desquamation of the lining epithelium; the epithelium was ruptured and the nuclii were scattered in the colloid and there was a congestion in the stromal blood vessels representing a deteriorated case of inactive thyroid gland as shown in Figures 44b.
(a) Treated group (H&E x 40); (b) Treated group (H &Ex 80). The figure 4a shows cystic dilatation with desquamation of the lining epithelium (E) and (black arrow). The figure 4b shows the magnification of Figure 4a to identify the inactive cystic follicles, the epithelium was ruptured (E) and the nuclii were scattered (N) in the colloid and there was a congestion in the stromal blood vessels (B) representing a deteriorated case of inactive thyroid gland.
4. Discussion
Although many studies discussed the harmful effect of the fluoride on the thyroid gland, many people are still ignoring this fact by doing their daily bad habits continuously such as smoking, drinking much tea, using teflon pans in cooking and adding fluoride to drinking water.
In our study, the results showed that fluoride not only harms the thyroid gland but it harms the (HPT) axis also. When observing the results in Tables 3, 4 and 5, we can notice that Na F supplementation significantly affected the body weight and serum parameters such as TC, TG, total protein content and albumin compared to the control group. The study also detected the non significant disturbance, in serum thyroid hormones which represented the beginning of the initiation of the thyroid dysfunction case. These data are in accordance with the data observed by Patil and Dhurvey (2015)PATIL V.V. and DHURVEY V.T., 2015. Exposure to sodium fluoride affects thyroid follicular cells in albino rats. International Journal of Plant, Animal and Environmental Sciences, vol. 5, no. 1, pp. 56-61. who reported the interaction between Na F and the thyroid follicular cells leading to its distortion. Also Wang et al. (2009)WANG, H., YANG, Z., ZHOU, B., GAO, H., YAN, X. and WANG, J., 2009. Fluoride-induced thyroid dysfunction in rats: roles of dietary protein and calcium level. Toxicology and Industrial Health, vol. 25, no. 1, pp. 49-57. http://dx.doi.org/10.1177/0748233709102720. PMid:19318504.
http://dx.doi.org/10.1177/07482337091027...
observed a decrease in rats body weight and a decrease in both serum (T4) and (T3) after ingestion of fluoride ions for a month.
Detecting a decrease in serum albumin levels as that observed in case of Na F supplemented group can lead to a decrease in transferring both (T4) and (T3) to body organs as denoted by Choksi et al. (2003)CHOKSI N.Y., JAHNKE G.D., HILAIRE C. ST. and SHELBY M., 2003. Role of thyroid hormones in human and laboratory animal reproductive health. Birth Defects Research, vol. 68(Pt B), pp. 479-491., Gamit et al. (2017)GAMIT, A.M., KHUBCHANDANI, A.S., GAMIT, M.R., PARMAR, U., ADARSH, A. and GAADHE, P., 2017. A study of serum total protein, serum albumin and thyroid hormones in protein energy malnutrition in children. International Journal of Medical Science and Public Health, vol. 6, no. 2, pp. 409-412. http://dx.doi.org/10.5455/ijmsph.2017.15082016633.
http://dx.doi.org/10.5455/ijmsph.2017.15...
, Sandeep and Krishnamurthy (2016)SANDEEP, M. and KRISHNAMURTHY, B., 2016. Thyroid hormone status in children with protein energy malnutrition. Int J Contemp Pediatr., vol. 3, no. 1, pp. 193-199.. They reported that the albumin represented one of the major proteins in rats and humans in transferring thyroid hormones to the body organs.
On the other hand, Miranda et al. (2018)MIRANDA G.H.N., GOMES B.A.Q., BITTENCOURT L.O., ARAGAO W.A.B., NOGUEIRA L.S., DIONIZIO A.S., BUZALAF M.A.R., MONTEIRO M.C. and LIMA R.R., 2018. Chronic exposure to sodium fluoride triggers oxidative biochemistry misbalance in mice: effects on peripheral blood circulation. Oxidative Medicine and Cellular Longevity, vol. 2018, pp. 1-8. http://dx.doi.org/10.1155/2018/8379123.
http://dx.doi.org/10.1155/2018/8379123...
attributed the toxic effect of fluoride due to its ability to interact with antioxidant enzymes and inhibition of protein synthesis. This lead to increasing of reactive oxygen species (ROS) that reached the mitochondria which then cause cell apoptosis.
Our study attributed the fluctuations in serum levels of TSH, (T3) and (T4) to the thyroidal reaction against an acute critical illness or an acute stress as reported by Mebis and Van den Berghe (2009)MEBIS, L. and VAN DEN BERGHE, G., 2009. The hypothalamus-pituitary-thyroid axis in critical illness. The Netherlands Journal of Medicine, vol. 67, no. 10, pp. 332-340. PMid:19915227.. They discussed the increase in serum TSH and (T4) besides the decrease in (T3) as thyroidal action against acute critical illness cases. They reported this case as a different case other than the known thyroid diseases (hyper - or hypo-thyrodism).
We can say that fluoride ions in our study exerted its harmful effect through two main routes; the first one was acting as an acute stress on the body cell organs leading to a critical illness, while the second route which came after was attacking the thyroid cells and replacing iodine ions.
Arca noae extract has not been tested before in an in vivo study as a treatment for thyroid dysfunctions. Accordingly, Arca noae is recommended for the first time in our study as a co –treatment for the Na F induced thyroid dysfunction. Generally, the group injected the extract only, i.e. the extract normal group, exhibited normal detected parameters and quite normal thyroid features as confirmed by the histological findings in Figure 3. This was confirmed also by evaluating the concentrations of some essential trace elements in the prepared extract that are needed for normal thyroid status such as copper (Cu), zinc (Zn), (Se) and magnesium (Mg) as shown in Table 2. Dahiya et al. (2016)DAHIYA, K., VERMA, M., DHANKHAR, R., GHALAUT, V.S., GHALAUT, P.S., SACHDEVA, A., MALIK, I. and KUMAR, R., 2016. Thyroid profile and iron metabolism: mutual relationship in hypothyroidism. Biomedical Research, vol. 27, no. 4, pp. 1212-1215. reported the importance of trace elements in elaborating normal thyroid functions. The extract contains also suitable amounts of proteins and carbohydrates which may compensate those were lost as result of Na F supplementation.
In the treated group, the extract succeeded in restoring the body weight, improving serum TC, TG besides reaching normal serum protein levels. The high content of proteins in the extract helped in restoring the serum level of the proteins including the albumin in rats as reported by (Thalacker-Mercer and Campbell (2008)THALACKER-MERCER, A.E. and CAMPBELL, W.W., 2008. Dietary protein intake affects albumin fractional synthesis rate in younger and older adults equally. Nutrition Reviews, vol. 66, no. 2, pp. 91-95. http://dx.doi.org/10.1111/j.1753-4887.2007.00012.x. PMid:18254875.
http://dx.doi.org/10.1111/j.1753-4887.20...
.
Surprisingly, the extract failed to restore TSH, (T4) and (T3) serum levels. A superficial and not a real improvement was detected in serum (T4) and (T3) with a continuous increase in TSH level were observed in the treated group. This pseudo-improvement was confirmed by the deteriorated case of the thyroid detected in the histological findings as shown in Figure 44b. The Pseudo- improvement in serum (T4) & (T3) was attributed to the deteriorated case of inactive thyroid gland that prevented the secretion of the hormones into the bloodstream which appeared to us as an improvement of the case.
This deterioration in the thyroid parameters could be attributed to the interaction between the fluoride ions that attacked the thyroid cells and some of the heavy metals found in the extract. Zhang et al. (2013)ZHANG, J., SONG, J., ZHANG, J., CHEN, X., ZHOU, M., CHENG, G. and XIE, X., 2013. Combined effects of fluoride and cadmium on liver and kidney function in male rats. Biological Trace Element Research, vol. 155, no. 3, pp. 396-402. http://dx.doi.org/10.1007/s12011-013-9807-4. PMid:24006106.
http://dx.doi.org/10.1007/s12011-013-980...
, Olszowski et al. (2016)OLSZOWSKI T., SIKORA M. and CHLUDEK D., 2016. Combined toxicity of fluoride and cadmium. Fluorid, vol. 49, no. 3, pp. 194-203. observed the toxic effect of the interactions of fluoride and heavy metals on body organs.
Our study also tried to detect the relation between fluoride induced thyroid dysfunction and the presence of serum immune parameters. Many cytokines were detected in the chronic sites of inflammations such as in case of thyroiditis. IL-6 is one of the important cytokines that are considered as a marker of inflammatory status and is a major mediator of host response to tissue injury and infection.
IL-6 was found to be overproduced in most autoimmune diseases such as in Graves’ disease, subacute thyroiditis and aminodarone induced thyrotoxicosis. IL-6 plays a great role in growth and differentiation of thyroid cells and its expression in thyrocytes is positively correlated to the lymphocyte infiltration in the site of inflammation (El-Shenawy et al., 2011EL-SHENAWY S.Z., HELMI M.H. and ATTIA H., 2011. Serum levels of proinflammatory cytokines (Interleukin 6 & Interleukin 15) and adiponectin in Hashimoto’s Thyroiditis with different thyroid function states. Journal of American Science, vol. 7, no. 6, pp. 1156-1162.; Mala et al., 2017MALA, S., KUMAR, S., PIRYANKA, K. and SHAIKH, Z.A., 2017. Serum inflammatory markers in subclinical hypothyroidism. European Journal Pharmaceutical and Medical Research, vol. 4, no. 9, pp. 855-859.).
We reported in our study that there was no relation between fluoride induced thyroid dysfunction and IL-6 secretion. Serum levels of IL-6 in the group supplemented with Na F recorded similar levels of that detected in the control group. It was also noticed that the extract had no inflammatory impact in both of the extract injected group and in the treated one. These results were confirmed by the histological findings that showed the absence of any cell infiltration in the thyroid tissues of all the tested groups.
5. Conclusion
-
Fluoride represents a real environmental stress and exhibited a dangerous effect on the HPT axis functions resulting a case of acute illness that can be changed to a chronic case if there is no real and safe treatment.
-
Applying the water extract of the marine organism; Arca noae as a co-treatment for the daily and neglected attack of fluoride supplementation corrected the protein and the lipid profile in serum.
-
The extract failed to regain the efficiency of the HPT axis. This was shown in the expressed high serum TSH level in the treated group in spite of the noticeable decreasing level of TSH in the Arca noae injected normal group.
-
Fluoride - heavy metal interactions may represent a toxic effect on thyroid gland and brain more than that existed during the supplementation of fluoride alone.
-
For thyroid gland safety, man must control the consumed quantities of marine organisms or seafood and eating them with caution and in different intervals in the presence of excess fluoride.
Acknowledgements
Financial support provided by the National Research Centre, Giza, Egypt, (Project AR110220) is gratefully acknowledged.
-
(With 4 figures)
References
- ABDUL RAHMAN, M.M. and FETOUH, F.A., 2013. Effect of sodium fluoride on the thyroid follicular cells and the amelioration by calcium supplementation in albino rats: A light and an electron microscopic study. The Journal of American Science, vol. 9, no. 10, pp. 107-114.
- BANCHROFT, J.D., STEVENS, A.A. and TURNER, D.R., 1996. Theory and practice of histological techniques 4th ed. Churchil Livingstone: Elsevier.
- CHENG, S.Y., LEONARD, J.L. and DAVIS, P.J., 2010. Molecular aspects of thyroid hormone actions. Endocrine Reviews, vol. 31, no. 2, pp. 139-170. http://dx.doi.org/10.1210/er.2009-0007 PMid:20051527.
» http://dx.doi.org/10.1210/er.2009-0007 - CHOI, W.J. and KIM, J., 2014. Dietary factors and the risk of thyroid cancer: a review. Clinical Nutrition Research, vol. 3, no. 2, pp. 75-88. http://dx.doi.org/10.7762/cnr.2014.3.2.75 PMid:25136535.
» http://dx.doi.org/10.7762/cnr.2014.3.2.75 - CHOKSI N.Y., JAHNKE G.D., HILAIRE C. ST. and SHELBY M., 2003. Role of thyroid hormones in human and laboratory animal reproductive health. Birth Defects Research, vol. 68(Pt B), pp. 479-491.
- CINAR, A. and SELCUK, M., 2005. Effects of chronic fluorosis on thyroxin, tri-iodothyronine, and protein-bound iodine in cows. Fluoride, vol. 38, pp. 65-68.
- COLE, T.G., KLOTZSCH, S.G. and MCNAMARA, J., 1997. Measurment of triglyceride concentration. In: N. RIFAI, G.R. WARNICK and M.H. DOMINICZAK, eds. Handbook of lipoprotein testing Washington: AACC Press, pp. 155.
- DAHIYA, K., VERMA, M., DHANKHAR, R., GHALAUT, V.S., GHALAUT, P.S., SACHDEVA, A., MALIK, I. and KUMAR, R., 2016. Thyroid profile and iron metabolism: mutual relationship in hypothyroidism. Biomedical Research, vol. 27, no. 4, pp. 1212-1215.
- DUPCIC RADIC, I., CARIĆ, M., NAJDEK, M., JASPRICA, N., BOLOTIN, J., PEHARDA, M. and BRATOŠ CETINIĆ, A., 2014. Biochemical and fatty acid composition of Arca noae (Bivalvia: Arcidae) from the Mali Ston Bay, Adriatic Sea. Mediterranean Marine Science, vol. 15, no. 3, pp. 520-531. http://dx.doi.org/10.12681/mms.436
» http://dx.doi.org/10.12681/mms.436 - ECONOMIDOU, F., DOUKA, E., TZANELA, M., NANAS, S. and KOTANIDOU, A., 2011. Thyroid function during critical illness. Hormones (Athens, Greece), vol. 10, no. 2, pp. 117-124. http://dx.doi.org/10.14310/horm.2002.1301 PMid:21724536.
» http://dx.doi.org/10.14310/horm.2002.1301 - EGAN, H., KIRK, R.S. and SAWYER, R., 1981. Pearson’s chemical analysis of food Edinburgh: Churchill Livingstone.
- EKINS, R., 1993. Free hormone assays. Nuclear Medicine Communications, vol. 14, no. 8, pp. 676-688. http://dx.doi.org/10.1097/00006231-199308000-00008 PMid:8371893.
» http://dx.doi.org/10.1097/00006231-199308000-00008 - EL-SHENAWY S.Z., HELMI M.H. and ATTIA H., 2011. Serum levels of proinflammatory cytokines (Interleukin 6 & Interleukin 15) and adiponectin in Hashimoto’s Thyroiditis with different thyroid function states. Journal of American Science, vol. 7, no. 6, pp. 1156-1162.
- FISHER, D.A., 1996. Physiological variations in thyroid hormones: physiological and pathophysiological considerations. Clinical Chemistry, vol. 42, no. 1, pp. 135-139. http://dx.doi.org/10.1093/clinchem/42.1.135 PMid:8565215.
» http://dx.doi.org/10.1093/clinchem/42.1.135 - GAMIT, A.M., KHUBCHANDANI, A.S., GAMIT, M.R., PARMAR, U., ADARSH, A. and GAADHE, P., 2017. A study of serum total protein, serum albumin and thyroid hormones in protein energy malnutrition in children. International Journal of Medical Science and Public Health, vol. 6, no. 2, pp. 409-412. http://dx.doi.org/10.5455/ijmsph.2017.15082016633
» http://dx.doi.org/10.5455/ijmsph.2017.15082016633 - GHRIBI, F., BOUSSOUFA, D., AOUINI, F., BEJAOUI, S., CHETOUI, I., RABEH, I. and EL CAFSI, M., 2018. Seasonal variation of biochemical composition of Noah’s ark shells (Arca noae L. 1758) in a Tunisian coastal lagoon in relation to its reproductive cycle and environmental conditions. Aquatic Living Resources, vol. 31, pp. 14-15. http://dx.doi.org/10.1051/alr/2018002
» http://dx.doi.org/10.1051/alr/2018002 - GORNALL, A.G., BARDAWILL, C.J. and DAVID, M.M., 1949. Determination of serum proteins by means of the biuret reaction. The Journal of Biological Chemistry, vol. 177, no. 2, pp. 751-766. PMid:18110453.
- HU, X., SONG, L., HUANG, L., ZHENG, Q. and YU, R., 2012. Antitumor Effect of a polypeptide fraction from Arca subcrenata in vitro and in vivo. Marine Drugs, vol. 10, no. 12, pp. 2782-2794. http://dx.doi.org/10.3390/md10122782 PMid:23342393.
» http://dx.doi.org/10.3390/md10122782 - IWEN, K.A., SCHRODER, E. and BRABANT, G., 2013. Thyroid hormone and the metabolic syndrome. European Thyroid Journal, vol. 2, no. 2, pp. 83-92. http://dx.doi.org/10.1159/000351249 PMid:24783045.
» http://dx.doi.org/10.1159/000351249 - LEONARD, P.J., PERSAUD, J. and MOTWANI, R., 1971. The estimation of plasma albumin by BCG dye binding on the Technicon SMA 12/60 analyzer and a comparison with the HABA dye binding technique. Clinica Chimica Acta; International Journal of Clinical Chemistry, vol. 35, no. 2, pp. 409-412. http://dx.doi.org/10.1016/0009-8981(71)90214-2 PMid:5125326.
» http://dx.doi.org/10.1016/0009-8981(71)90214-2 - LUO, Q., CUI, H., DENG, H., KUANG, P., LIU, H., LU, Y., FANG, J., ZUO, Z., DENG, J., LI, Y., WANG, X. and ZHAO, L., 2017. Sodium fluoride induces renal inflammatory responses by activating NF-κB signaling pathway and reducing anti-inflammatory cytokine expression in mice. Oncotarget, vol. 8, no. 46, pp. 80192-80207. http://dx.doi.org/10.18632/oncotarget.19006 PMid:29113295.
» http://dx.doi.org/10.18632/oncotarget.19006 - MAIA, A.M.L.R., MEDEIROS, E. and HENRY-SILVA, G.G., 2018. Distribution and density of the bivalve Anomalocardia brasiliana in the estuarine region of Northeastern Brazil. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 78, no. 1, pp. 32-40. http://dx.doi.org/10.1590/1519-6984.02316 PMid:28678977.
» http://dx.doi.org/10.1590/1519-6984.02316 - MALA, S., KUMAR, S., PIRYANKA, K. and SHAIKH, Z.A., 2017. Serum inflammatory markers in subclinical hypothyroidism. European Journal Pharmaceutical and Medical Research, vol. 4, no. 9, pp. 855-859.
- MEBIS, L. and VAN DEN BERGHE, G., 2009. The hypothalamus-pituitary-thyroid axis in critical illness. The Netherlands Journal of Medicine, vol. 67, no. 10, pp. 332-340. PMid:19915227.
- MIRANDA G.H.N., GOMES B.A.Q., BITTENCOURT L.O., ARAGAO W.A.B., NOGUEIRA L.S., DIONIZIO A.S., BUZALAF M.A.R., MONTEIRO M.C. and LIMA R.R., 2018. Chronic exposure to sodium fluoride triggers oxidative biochemistry misbalance in mice: effects on peripheral blood circulation. Oxidative Medicine and Cellular Longevity, vol. 2018, pp. 1-8. http://dx.doi.org/10.1155/2018/8379123
» http://dx.doi.org/10.1155/2018/8379123 - NELSON, J.C. and WILCOX, R.B., 1996. Analytical performance of free and total thyroxine assays. Clinical Chemistry, vol. 42, no. 1, pp. 146-154. http://dx.doi.org/10.1093/clinchem/42.1.146 PMid:8565218.
» http://dx.doi.org/10.1093/clinchem/42.1.146 - OLSZOWSKI T., SIKORA M. and CHLUDEK D., 2016. Combined toxicity of fluoride and cadmium. Fluorid, vol. 49, no. 3, pp. 194-203.
- PAŁKOWSKA-GOŹDZIK, E., LACHOWICZ, K. and ROSOŁOWSKA-HUSZCZ, D., 2017. Effects of dietary protein on thyroid axis activity. Nutrients, vol. 10, no. 5, pp. 1-15. PMid:29271877.
- PATIL V.V. and DHURVEY V.T., 2015. Exposure to sodium fluoride affects thyroid follicular cells in albino rats. International Journal of Plant, Animal and Environmental Sciences, vol. 5, no. 1, pp. 56-61.
- SAAD EL-DIN, M.I. and GAD EL-HAK, H.N., 2017. Impact of heavy metals contamination on spring abundance of aquatic macro-invertebrates inhabiting lake Timsah, Egypt. Journal of Water Security, vol. 3, no. 1, pp. 1-6. http://dx.doi.org/10.15544/jws.2017.003
» http://dx.doi.org/10.15544/jws.2017.003 - SANDEEP, M. and KRISHNAMURTHY, B., 2016. Thyroid hormone status in children with protein energy malnutrition. Int J Contemp Pediatr., vol. 3, no. 1, pp. 193-199.
- SARKAR, C. and PAL, S., 2014. Ameliorative effect of Resveratrol against fluoride-induced alteration of thyroid function in male wistar rats. Biological Trace Element Research, vol. 162, no. 1-3, pp. 278-287. http://dx.doi.org/10.1007/s12011-014-0108-3 PMid:25164033.
» http://dx.doi.org/10.1007/s12011-014-0108-3 - SEARCY, R.L., 1969. Diagnostic biochemistry New York: McGraw-Hill.
- SHARMA, R., BHARTI, S. and KUMAR, K.V.S.H., 2014. Diet and thyroid – myths and facts. J Med Nutr Nutraceut., vol. 3, no. 2, pp. 60-65.
- SONG, L., LI, T., YU, R., YAN, C., REN, S. and ZHAO, Y., 2008. Antioxidant activities of hydrolysates of Arca subcrenata prepared with three proteases. Marine Drugs, vol. 6, no. 4, pp. 607-619. http://dx.doi.org/10.3390/md6040607 PMid:19172198.
» http://dx.doi.org/10.3390/md6040607 - THALACKER-MERCER, A.E. and CAMPBELL, W.W., 2008. Dietary protein intake affects albumin fractional synthesis rate in younger and older adults equally. Nutrition Reviews, vol. 66, no. 2, pp. 91-95. http://dx.doi.org/10.1111/j.1753-4887.2007.00012.x PMid:18254875.
» http://dx.doi.org/10.1111/j.1753-4887.2007.00012.x - WANG, H., YANG, Z., ZHOU, B., GAO, H., YAN, X. and WANG, J., 2009. Fluoride-induced thyroid dysfunction in rats: roles of dietary protein and calcium level. Toxicology and Industrial Health, vol. 25, no. 1, pp. 49-57. http://dx.doi.org/10.1177/0748233709102720 PMid:19318504.
» http://dx.doi.org/10.1177/0748233709102720 - WAUGH, D.T., GODFREY, M., LIMEBACK, H. and POTTER, W., 2017. Black tea source, production, and consumption: assessment of health risks of fluoride intake in New Zealand. Journal of Environmental and Public Health, vol. 2017, Article ID 5120504. http://dx.doi.org/10.1155/2017/5120504 PMid:28713433.
» http://dx.doi.org/10.1155/2017/5120504 - ZHANG, J., SONG, J., ZHANG, J., CHEN, X., ZHOU, M., CHENG, G. and XIE, X., 2013. Combined effects of fluoride and cadmium on liver and kidney function in male rats. Biological Trace Element Research, vol. 155, no. 3, pp. 396-402. http://dx.doi.org/10.1007/s12011-013-9807-4 PMid:24006106.
» http://dx.doi.org/10.1007/s12011-013-9807-4 - ZOELLER, R.T., 2007. Environmental Chemicals Impacting the Thyroid: targets and Consequences. Thyroid, vol. 17, no. 9, pp. 811-817. http://dx.doi.org/10.1089/thy.2007.0107 PMid:17956155.
» http://dx.doi.org/10.1089/thy.2007.0107
Publication Dates
-
Publication in this collection
11 Sept 2020 -
Date of issue
Jul-Sep 2021
History
-
Received
31 July 2019 -
Accepted
17 Jan 2020 -
Published
31 Aug 2021