Abstract
Xylanases have applications in different industries, being produced by microbial cultivations using submerged (SmF) or solid-state fermentation (SSF). Precipitation stands out as a potential method for the concentration of xylanases, especially with the use as ethanol as the precipitant due to its compatibility with the biorefinery concept. This paper presents a comparative laboratory scale study of ethanol precipitation of xylanases produced by Aspergillus niger cultivated under SSF and SmF. Precipitation conditions were selected according to a central composite design. Statistical analysis showed a significant effect of pH on the recoveries of total protein and xylanase activity. The kinetic profiles showed that a relatively short period of time (up to 15 min) was sufficient to recover most of the xylanase activity precipitated under the selected conditions. Xylanase recoveries of 65 and 79% were achieved for the SSF and SmF enzymatic complexes, respectively.
Keywords:
Aspergillus niger; downstream processing; ethanol precipitation; xylanase
INTRODUCTION
Hemicellulose is the second most abundant renewable natural polymer and, together with lignin and cellulose, forms the highly complex plant cell wall structure (Pauly et al., 2013Pauly, M., Gille, S., Liu, L.F., Mansoori, N., de Souza, A., Schultink, A. and Xiong, G.Y., Hemicellulose biosynthesis. Planta, 238(4) 627-642 (2013).; Shallom and Shoham, 2003Shallom, D. and Shoham, Y., Microbial hemicellulases. Current Opinion in Microbiology, 6(3) 219-228 (2003).; Wong et al., 1988Wong, K.K.Y., Tan, L.U.L. and Saddler, J.N., Multiplicity of beta-1,4-xylanase in microorganisms - functions and applications. Microbiological Reviews, 52(3) 305-317 (1988).). Hemicelluloses are branched heteropolysaccharide complex compounds made of D-glucose, D-galactose, D-mannose, D-xylose, L-arabinose, D-glucuronic acid and 4-O-methyl-glucuronic acid. Removal of their side chains is required to increase the rate of enzymatic degradation (Santos et al., 2012Santos, F. A.; Queiróz, J. H.; Colodette, J. L.; Fernandes, S. A.; Guimarães, V. M.; and Rezende, S. T., Potencial da palha de cana-de-açúcar para produção de etanol. Química Nova, 35(5) 1004-1010 (2012).). Xylan, the major component of hemicellulose, consists of a backbone of β-xylopyranose residues joined by β-1,4-glycosidic linkages, decorated with acetyl, arabinofuranosyl, and glucuronic or 4-O-methylglucuronic acid groups (Saha, 2003Saha, B.C., Hemicellulose bioconversion. Journal of Industrial Microbiology & Biotechnology, 30(5) 279-291 (2003).). The endo-1,4-β-xylanase (xylanase) enzyme cleaves the β-1,4-glycosidic linkage between xylose residues in the backbone of xylan and is essential for the depolymerization of hemicellulose (Dodd and Cann, 2009Dodd, D. and Cann, I., Enzymatic deconstruction of xylan for biofuel production. Global Change Biology Bioenergy, 1(1) 2-17 (2009).). Xylanase enzymes have applications in the food industry, as well as in other technological sectors such as pulp and paper production (Collins et al., 2005Collins, T., Gerday, C. and Feller, G., Xylanases, xylanase families and extremophilic xylanases. Fems Microbiology Reviews, 29(3) 3-23 (2005).). In the biofuels sector, the action of xylanases improves cellulose conversion during the hydrolysis of biomass for cellulosic ethanol production by removing hemicellulose and increasing the accessibility of the substrate to cellulase enzymes (Gao et al., 2011Gao, D.H., Uppugundla, N., Chundawat, S.P.S., Yu, X.R., Hermanson, S., Gowda, K., Brumm, P., Mead, D., Balan, V. and Dale, B.E., Hemicellulases and auxiliary enzymes for improved conversion of lignocellulosic biomass to monosaccharides. Biotechnology for Biofuels, 4(5) 1-11 (2011).; Kumar and Wyman, 2009Kumar, R. and Wyman, C.E., Effect of xylanase supplementation of cellulase on digestion of corn stover solids prepared by leading pretreatment technologies. Bioresource Technology, 100(18) 4203-4213 (2009).). On an industrial scale, xylanases are mainly produced by filamentous fungi of the genera Aspergillus and Trichoderma (Park et al., 2002Park, Y.S., Kang, S.W., Lee, J.S., Hong, S.I. and Kim, S.W., Xylanase production in solid state fermentation by Aspergillus niger mutant using statistical experimental designs. Applied Microbiology and Biotechnology, 58(6) 761-766 (2002).), using submerged fermentation (SmF) or solid-state fermentation (SSF). About 80-90% of all xylanases are produced in submerged culture (Polizeli et al., 2005Polizeli, M., Rizzatti, A.C.S., Monti, R., Terenzi, H.F., Jorge, J.A. and Amorim, D.S., Xylanases from fungi: properties and industrial applications. Applied Microbiology and Biotechnology, 67(5) 577-591 (2005).). Nevertheless, the use of SSF is particularly advantageous for enzyme production by filamentous fungi, because it simulates the natural habitat of these microorganisms and, from an environmental perspective, enables the use of agro-industrial residues as sources of carbon and energy for microorganism growth and enzyme production (Farinas, 2015Farinas, C.S., Developments in solid-state fermentation for the production of biomass-degrading enzymes for the bioenergy sector. in: Renewable and Sustainable Energy Reviews, 52 179-188 (2015).; Holker and Lenz, 2005Holker, U. and Lenz, J., Solid-state fermentation - are there any biotechnological advantages? Current Opinion in Microbiology, 8(3) 301-305 (2005).).
Production of xylanases using strains of Aspergillus under both SmF and SSF have been widely reported (Betini et al., 2009Betini, J.H.A., Michelin, M., Peixoto-Nogueira, S.C., Jorge, J.A., Terenzi, H.F. and Polizeli, M., Xylanases from Aspergillus niger, Aspergillus niveus and Aspergillus ochraceus produced under solid-state fermentation and their application in cellulose pulp bleaching. Bioprocess and Biosystems Engineering, 32(6) 819-824 (2009).; Chapla et al., 2010Chapla, D., Divecha, J., Madamwar, D. and Shah, A., Utilization of agro-industrial waste for xylanase production by Aspergillus foetidus MTCC 4898 under solid state fermentation and its application in saccharification. Biochemical Engineering Journal, 49(3) 361-369 (2010).; Chipeta et al., 2008Chipeta, Z.A., du Preez, J.C. and Christopher, L., Effect of cultivation pH and agitation rate on growth and xylanase production by Aspergillus oryzae in spent sulphite liquor. Journal of Industrial Microbiology & Biotechnology, 35(6) 587-594 (2008).; Fang et al., 2010Fang, T.J., Liao, B.C. and Lee, S.C., Enhanced production of xylanase by Aspergillus carneus M34 in solid-state fermentation with agricultural waste using statistical approach. New Biotechnology, 27(1), 25-32 (2010).; Ghanem et al., 2000Ghanem, N.B., Yusef, H.H. and Mahrouse, H.K., Production of Aspergillus terreus xylanase in solid-state cultures: application of the Plackett-Burman experimental design to evaluate nutritional requirements. Bioresource Technology, 73(2) 113-121 (2000).; Pirota et al., 2013Pirota, R., Tonelotto, M., Delabona, P.D., Fonseca, R.F., Paixao, D.A.A., Baleeiro, F.C.F., Neto, V.B. and Farinas, C.S., Enhancing xylanases production by a new Amazon Forest strain of Aspergillus oryzae using solid-state fermentation under controlled operation conditions. Industrial Crops and Products, 45 465-471 (2013).; Venegas et al., 2013Venegas, I.M., Fuentes-Hernandez, J., Garcia-Rivero, M. and Martinez-Trujillo, A., Characteristics of Aspergillus niger xylanases produced on rice husk and wheat bran in submerged culture and solid-state fermentation for an applicability proposal. International Journal of Food Science and Technology, 48(9) 1798-1807 (2013).), as these fungi exhibit favorable fermentation characteristics, including high protein secretion rates and the ability to produce a wide range of extracellular enzymes (de Vries, 2003de Vries, R.P., Regulation of Aspergillus genes encoding plant cell wall polysaccharide-degrading enzymes; relevance for industrial production. Applied Microbiology and Biotechnology, 61(1) 10-20 (2003).). However, most of the aforementioned studies have mainly focused on the enzyme production step, with evaluation of different fungal strains and the effects of the operational conditions used in the cultivation process. Given that both SSF and SmF cultivations usually result in enzymatic complexes at concentration levels too low for immediate application in industrial processes, there is an urgent need for studies focusing on downstream processing (DSP) unit operations. The concentration step is especially necessary in the development of processes for on-site production of enzymes, in order to improve the catalytic efficiency of the enzymatic cocktails.
The choice of DSP unit operations is a major challenge in defining a biotechnological process, because loss of the bioproduct in the multiple operations of separation, concentration, and purification can compromise the efficiency of the entire process. Furthermore, cost reduction is a priority in the case of industrial enzymes. Given these considerations, the precipitation technique can be very effective in concentrating xylanase preparations, especially since high purity (which is typically achieved using chromatographic processes) may not be required for applications in areas such as the biofuels sector (Farinas et al., 2011Farinas, C., Scarpelini, L., Miranda, E. and Neto, V., Evaluation of operational parameters on the precipitation of endoglucanase and xylanase produced by solid state fermentation of Aspergillus niger. Brazilian Journal of Chemical Engineering, 28(1) 17-26 (2011).). Although there are numerous reports concerning the isolation and purification of xylanases, most only describe the precipitation step for a single specific condition, usually for the purposes of biochemical characterization (Ahmad et al., 2013Ahmad, Z., Butt, M.S., and Riaz M., Partial purification and characterization of xylanase produced from Aspergillus niger using wheat bran. Pakistan Journal of Agricultural Sciences, Faisalabad, 50(3) 433-437 (2013).; Bokhari et al., 2009Bokhari, S.A.I., Latif, F. and Rajoka, M.I., Purification and characterization of xylanases from Thermomyces lanuginosus and its mutant derivative possessing novel kinetic and thermodynamic properties. World Journal of Microbiology & Biotechnology, 25(3) 493-502 (2009).; Chanwicha et al., 2015Chanwicha, N., Katekaew, S., Aimi, T. and Boonlue, S., Purification and characterization of alkaline xylanase from Thermoascus aurantiacus var. levisporus KKU-PN-I2-1 cultivated by solid-state fermentation. Mycoscience, 56(3) 309-318 (2015).; Lu et al., 2008Lu, F.X., Lu, M., Lu, Z.X., Bie, X.M., Zhao, H.Z. and Wang, Y., Purification and characterization of xylanase from Aspergillus ficuum AF-98. Bioresource Technology, 99(13) 5938-5941 (2008).; Mander et al., 2014Mander, P., Choi, Y.H., Pradeep, G.C., Choi, Y.S., Hong, J.H., Cho, S.S. and Yoo, J.C., Biochemical characterization of xylanase produced from Streptomyces sp CS624 using an agro residue substrate. Process Biochemistry, 49(3) 451-456 (2014).; Ninawe et al., 2008Ninawe, S., Kapoor, M. and Kuhad, R.C., Purification and characterization of extracellular xylanase from Streptomyces cyaneus SN32. Bioresource Technology, 99(5) 1252-1258 (2008).).
From a bioprocess engineering standpoint, identification of the optimal operational conditions for the precipitation of proteins involves the study of different process variables, including the type and concentration of the precipitant, temperature, aging time, agitation, pH, and so forth. In terms of the precipitant, the use of a renewable material such as ethanol is especially attractive, because it complies with the biorefinery concept (Kamm and Kamm, 2004Kamm, B. and Kamm, M., Principles of biorefineries. Applied Microbiology and Biotechnology, 64(2) 137-145 (2004).), since it is a bioproduct of the biorefinery itself, available in site at low cost. Ethanol is one of the most important industrial precipitants, and its use can lead to a favorable balance between the solubility effect and an appropriate hydrophilicity, minimizing protein denaturation (Golunski et al., 2011Golunski, S., Astolfi, V., Carniel, N., de Oliveira, D., Di Luccio, M., Mazutti, M.A. and Treichel, H., Ethanol precipitation and ultrafiltration of inulinases from Kluyveromyces marxianus. Separation and Purification Technology, 78(3) 261-265 (2011).). However, in order to achieve high precipitation recovery of xylanase activity, a relatively high ethanol concentration (≥80%, v/v) is usually required (Fadel, 2001Fadel, M., High-level xylanase production from sorghum flour by a newly isolate of Trichoderma harzianum cultivated under solid state fermentation. Annals of Microbiology, 51(1) 61-78 (2001).; Marino et al., 2015Marino, M.A., Freitas, S. and Miranda, E.A., Ethanol precipitation of glycosyl hydrolases produced by Trichoderma harzianum P49P11. Brazilian Journal of Chemical Engineering, 32(2) 325-333 (2015).; Varma et al., 1999Varma, R.J., Nene, S., Baliga, B.A. and Elias, C., Studies on commercial aspects of xylanase from Chainia species. Journal of Scientific & Industrial Research, 58(11) 878-882 (1999).). This increases cost as well as the risk of protein denaturation, hence compromising the feasibility of the DSP. Therefore, a systematic investigation of the precipitation operational parameters has to be undertaken to enable this process to be effectively applied for each specific system.
Considering the industrial importance of xylanase enzymes, together with the high availability and low cost of ethanol, the purpose of this study was to carry out a systematic comparative evaluation of the precipitation with ethanol of xylanases produced by A. niger under SSF and SmF, and to determine the feasibility of applying this process as a cost-effective DSP unit operation. Prior to the precipitation studies, preliminary evaluations were made of the thermal and pH stabilities of the xylanases from both sources, together with determination of the cloud points following ethanol addition. Experimental design methodology was then used as a tool to investigate the effects of ethanol concentration and pH on xylanase precipitation recovery.
MATERIALS AND METHODS
Microorganism
The Aspergillus niger A12 strain was obtained from Embrapa Food Technology (Rio de Janeiro, Brazil) (Couri and deFarias, 1995Couri, S. and deFarias, A.X., Genetic manipulation of Aspergillus niger for increased synthesis of pectinolytic enzymes. Revista De Microbiologia, 26(4) 314-317 (1995).). The strain was kept at -18 ºC and was activated by incubation on slants of potato dextrose agar (PDA) medium for four days at 32 ºC. Suspensions of spores were prepared by the addition of 10 mL of Tween 80 (0.3%, v/v). Spore concentrations were determined using a Neubauer chamber.
Solid-state fermentation (SSF)
The solid-state fermentation cultivations were carried out in 250 mL conical flasks containing 5 g of dry wheat bran sterilized by autoclaving at 121 ºC for 15 min. The moisture content of the medium was then adjusted by the addition of 3 mL of a nutrient medium (Mandels and Sternberg, 1976Mandels, M. and Sternberg, D., Recent advances in cellulase technology. Journal of Fermentation Technology, 54(4) 267-286 (1976).). A concentration of 107 spores/g of dry solid substrate was added, and the cultivations were conducted under static conditions at 32 ºC for 72 h. The enzymes were extracted by the addition of 1:10 (w/v, mass of dry wheat bran to volume of extraction solution) 0.05 mol/L sodium acetate buffer solution (pH 4.5) and agitating the flasks in a shaker at 200 rpm, 32 ºC, for 30 min. The final enzymatic extracts were filtered, centrifuged at 12,900 g for 10 min, and kept frozen at -18 ºC prior to the analytical assays. All the cultivation experiments were carried out in triplicate, and the data were calculated as means ± standard deviations.
Submerged fermentation (SmF)
In the submerged fermentation procedure, the preculture was initiated with a 107 spores/mL conidial suspension, in 250 mL conical flasks containing 50 mL of nutrient medium (Mandels and Sternberg, 1976Mandels, M. and Sternberg, D., Recent advances in cellulase technology. Journal of Fermentation Technology, 54(4) 267-286 (1976).) enriched with 30 g/L of glucose. The incubation was carried out for 50 h in a shaker at 32 ºC, with stirring at 200 rpm. Aliquots (10 mL) of preculture suspension were transferred to 250 mL conical flasks containing 40 mL of the culture medium supplemented with 10 g/L of glucose and 1% (w/v) of wheat bran sterilized by autoclaving at 121 ºC for 15 min. The cultivations were performed for 72 h in a shaker at 32 ºC and 200 rpm. The cultivation broth was then filtered, centrifuged at 12,900 g for 10 min, and the resulting crude enzymatic extract was stored at -18 ºC prior to further analysis. All the cultivation experiments were performed in triplicate, and the data were calculated as means ± standard deviations.
Enzyme thermal and pH stability
The thermal stability of the xylanase was evaluated by measuring the residual enzymatic activity after incubation of 1.0 mL of the crude fermentation broth (SmF) or extract (SSF) with 1.0 mL of a 0.2 mol/L sodium acetate pH 5.0 buffer solution (for pH adjustment) at 15, 30, and 45 ºC for up to 180 min. For the pH stability study, the pH of the enzyme preparations was adjusted to pH 3.0, 4.0, 5.0, and 6.0 using 0.2 mol/L sodium citrate buffer, and to pH 6.0, 7.0, and 8.0 using 0.2 mol/L sodium phosphate buffer. These pH-adjusted enzyme preparations (1.0 mL volumes) were incubated at 30 ºC for a total period of 180 min. In each of these studies, sampling was performed after time intervals of 10, 30, 60, 120, and 180 min. At the end of the incubation period, the samples were centrifuged at 12,900 g for 10 min and the supernatants were used for total protein concentration and xylanase activity analyses. All the experiments were carried out in triplicate, and the data were calculated as means ± standard deviations. The data obtained were used to do linear fits for protein concentration and xylanase activity (Prot|t and Activ|t, respectively) as a function of time.
Cloud point determination
For determination of the cloud point (the minimum concentration of ethanol added to the enzyme preparation required to cause visual clouding of the solution), the pH of the enzyme preparation (2.0 mL volume) was adjusted to 5.5 by adding 9 volumes of a 2.0 mol/L sodium citrate pH 5.5 buffer in a 15 mL Falcon tube. The tube was weighed and then placed in an ice bath, followed by dropwise addition of ethanol at -7 ºC, at a flow rate of 1 mL/min, until clouding was visually detected. The mixture was allowed to rest for 10 min, and if the turbidity was maintained, the tube was weighed again in order to determine the final mass of ethanol required to reach the cloud point. Ten replicate determinations were performed for each enzyme preparation (SSF and SmF).
Experimental design
A full factorial design followed by response surface analysis was used to evaluate the effects of two variables (pH and ethanol concentration, individually and in interaction) on the precipitation of the xylanases. The experimental design selected was a central composite design (CCD) comprising 11 runs, corresponding to four cube points, four axial points, and three central points, with the experiments carried out in random order. The pH varied from 3.4 to 7.6 and the ethanol concentration varied from 78 to 92% (v/v) (a preliminary study showed that, below 70% ethanol, recovering of activity and protein in the precipitate were too low for precise quantification). The dependent variables (responses) were xylanase activity recovery, total protein recovery, specific activity, and purification factor. Statistica v. 8.0 software (Statsoft) was used to analyze the experimental data, perform analysis of variance (ANOVA) calculations, and plot the response surfaces.
For this entire set of precipitation experiments, the pH of the enzyme preparations was first adjusted to the values established in the experimental design, using 2.0 mol/L sodium citrate buffer at a 1:9 (v/v) sample:buffer ratio. A 1.0 mL volume of each sample was transferred to a 50 mL Falcon tube. The tubes were then continuously stirred in an ice bath at -7 ºC, while ethanol was added dropwise, at a flow rate of 1 mL/min, until the desired concentration was reached. The tubes were then incubated at 15 ºC for 3 h, followed by centrifugation at 12,900 g for 10 min. The precipitate was suspended in 5.0 mL of 0.2 mol/L sodium citrate buffer (pH 5.0) for the quantification of total protein and xylanase activity. All the experiments were carried out in triplicate, and the data were calculated as means ± standard deviations.
Kinetics of xylanase precipitation with ethanol
For the kinetic study of xylanase precipitation with ethanol under the selected process conditions, the pH values of the crude SSF and SmF enzyme preparations were adjusted to pH 5.5 (as described before) and 1.0 mL volumes were transferred to 15 mL Falcon tubes. The tubes were kept under stirring in an ice bath at -7.0 ºC and ethanol was added dropwise until reaching a concentration of 85% (v/v). The tubes were then incubated at 15 ºC, withdrawn at different times, and centrifuged at 12,900 g for 10 min. The precipitates were dissolved in 5.0 mL of 0.2 mol/L sodium citrate buffer (pH 5.0) for the quantification of total protein and xylanase activity. All the experiments were carried out in triplicate, and the data were calculated as means ± standard deviations.
Enzyme activity assay
The xylanase activity was measured according to the methodology described by Bailey and Poutanen (Bailey and Poutanen, 1989Bailey, M.J. and Poutanen, K., Production of xylanolytic enzymes by strains of Aspergillus. Applied Microbiology and Biotechnology, 30(1) 5-10 (1989).), with incubation of 1.0 mL of the suitably diluted samples at 50 ºC for 30 min with a substrate of 1% beechwood xylan (Sigma, USA) solution prepared in 0.20 mol/L sodium acetate buffer (pH 5.0). Here, one unit of xylanase activity corresponds to 1 µmol of xylose released per minute at pH 5.0 and 50 ºC. Quantification of the reducing groups was performed according to the dinitrosalicylic acid (DNS) method (Miller, 1959Miller, G., Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3) 426-428 (1959).).
Total protein
The total protein concentrations in the enzymatic samples were determined by the Bradford method (Bradford, 1976Bradford, M.M., Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248-254 (1976).), using bovine serum albumin as a standard.
RESULTS AND DISCUSSION
Thermal and pH stability of xylanase
A preliminary set of experiments was carried out to evaluate the thermal and pH stability of the xylanase present in the crude enzymatic preparations obtained by cultivation of A. niger under SSF and SmF. These analyses were required in order to establish the ranges of pH and temperature that would be appropriate in the subsequent precipitation studies. The xylanase stability results are presented in terms of the linear fitting parameters obtained by plotting the residual activity as a function of incubation time of the crude enzyme preparations at different temperatures and pH.
In the case of thermal stability (Table 1), no significant changes in xylanase activity or protein concentration were detected up to 180 min of incubation at 15, 30, or 45 ºC, for both SSF and SmF enzyme preparations, as shown by the very low angular coefficient values obtained for all the temperatures tested. These results indicated that the xylanases produced by A. niger presented good thermal stability (for the temperatures and time periods tested). In addition, it is noteworthy that no difference was observed between the thermal stabilities of the xylanases obtained from the different cultivation systems.
Thermal stability study of xylanase enzymes produced by A. niger under SSF and SmF: parameters of the linear fits for residual enzyme activity (Ativ|t) or protein concentration (Prot|t), as a function of time.
These findings are in agreement with literature reports on the thermal stability of xylanases from different Aspergillus strains. For instance, Guimarães et al. (2013)Guimaraes, N.C.D., Sorgatto, M., Peixoto-Nogueira, S.D., Betini, J.H.A., Zanoelo, F.F., Marques, M.R., Polizeli, M. and Giannesi, G.C., Bioprocess and biotechnology: effect of xylanase from Aspergillus niger and Aspergillus flavus on pulp biobleaching and enzyme production using agroindustrial residues as substract. Springerplus, 2 1-7 (2013). found that the xylanase produced by A. niger under SmF retained over 85% activity after 2 h of incubation at 50 ºC. Xylanase produced by A. terreus under SmF showed no significant decrease in activity during 3 h incubation at 30 and 45 ºC (Bakri et al., 2010Bakri, Y., Masson, M. and Thonart, P., Isolation and Identification of Two New Fungal Strains for Xylanase Production. Applied Biochemistry and Biotechnology, 162(6) 1626-1634 (2010).). Similar thermal stability has also been reported for xylanases produced under SSF by A. niger, A. ochraceus, and A. niveus, which remained stable for up to 1 h at 50 ºC (Betini et al., 2009Betini, J.H.A., Michelin, M., Peixoto-Nogueira, S.C., Jorge, J.A., Terenzi, H.F. and Polizeli, M., Xylanases from Aspergillus niger, Aspergillus niveus and Aspergillus ochraceus produced under solid-state fermentation and their application in cellulose pulp bleaching. Bioprocess and Biosystems Engineering, 32(6) 819-824 (2009).).
Table 2 presents the pH stability results obtained for incubation of the SSF and SmF enzyme preparations at different pH. Similar to the previous results for thermal stability, no significant changes were observed in xylanase activity or protein concentration after incubation of the crude enzyme preparations for 180 min at 30 ºC, using pH ranging from 3.0 to 8.0. It is interesting to note that for pH 6.0, similar stability results were obtained using two different buffers, indicating that the nature of the salts present in the buffers did not affect the analysis.
Stability, at different pH, of xylanase enzymes produced by A. niger under SSF and SmF: parameters of the linear fits for residual enzyme activity or protein concentration, as a function of time.
The results corroborated the findings of other studies concerning the pH stability of xylanase from Aspergillus. Guimarães et al. (2013) found that A. niger xylanase retained over 95% of the initial activity after incubation at pH in the range from 3.0 to 8.0 for up to 1 h. Xylanases from A. ochraceus and A. terricola presented stability greater than 70% when incubated in the pH range from 2.5 to 8.0 for up to 1 h (Michelin et al., 2010Michelin, M., Peixoto-Nogueira, S.C., Betini, J.H.A., da Silva, T.M., Jorge, J.A., Terenzi, H.F. and Polizeli, M., Production and properties of xylanases from Aspergillus terricola Marchal and Aspergillus ochraceus and their use in cellulose pulp bleaching. Bioprocess and Biosystems Engineering, 33(7) 813-821 (2010).). Xylanase from A. niger was stable at pH in the range from 2.0 to 7.0, retaining over 75% activity after 1 h, while xylanases of A. ochraceus and A. niveus were stable at pH from 3.0 to 7.0, with more than 70% activity retained (Betini et al., 2009Betini, J.H.A., Michelin, M., Peixoto-Nogueira, S.C., Jorge, J.A., Terenzi, H.F. and Polizeli, M., Xylanases from Aspergillus niger, Aspergillus niveus and Aspergillus ochraceus produced under solid-state fermentation and their application in cellulose pulp bleaching. Bioprocess and Biosystems Engineering, 32(6) 819-824 (2009).).
In light of all the previous results concerning the thermal and pH stability of A. niger xylanase from both SSF and SmF cultivation systems, temperatures between 15 and 45 ºC and pH values in the range from 3.0 to 8.0 were selected for evaluation of ethanol precipitation. The xylanases were quite stable over these ranges of temperature and pH, within the period studied (up to 3 h). This information is important in order to avoid any misinterpretation of the following ethanol precipitation results.
Determination of the cloud point
The proteins from SSF and SmF showed slightly different behaviors in terms of the minimum concentration of ethanol required for detection of a visible haze in the medium (the cloud point). The crude enzyme preparation from SSF required 50.8% (v/v) of ethanol to reach the cloud point, while the SmF preparation required an average of 56.2% (v/v) of ethanol. This could have been due to the higher concentration of proteins in the SSF medium (0.24 mg/mL), compared to the SmF medium (0.11 mg/mL), which enabled supersaturation to be achieved at a lower ethanol concentration. A higher initial concentration of proteins in the medium leads to higher supersaturation, at a given concentration of ethanol, which in turn leads to increased protein precipitation (Nakadai and Nasuno, 1989Nakadai, T. and Nasuno, S., Enzyme preparation from extract of wheat bran koji by alcohol precipitation. Journal of Fermentation and Bioengineering, 67(4) 253-257 (1989).). Therefore, it was important to know the cloud point in order to determine the minimum concentration of ethanol required in the subsequent precipitation studies.
Ethanol precipitation of xylanases
An overall analysis of the results of xylanase precipitation using ethanol, performed according to the 22 central composite design, showed that protein recoveries in the precipitates from SSF were mostly higher than in those from SmF (Table 3). This could have been due to the 2.4-fold higher initial concentration of proteins in the SSF medium, compared to the SmF medium. The same rationale could be extended to the xylanase activity, since the highest recoveries were obtained from the SmF medium, with initial activity values of 7.09 and 5.16 IU/mL for SmF and SSF, respectively. The crude enzyme preparations from SSF and SmF also presented different initial xylanase specific activities (17.79 IU/mg for SSF and 59.08 IU/mg for SmF), as well as different final xylanase specific activities in the precipitates, hence resulting in different purification factors.
Matrix of coded and real values of the central composite design (CCD) and responses for ethanol precipitation of the xylanase enzymes produced by A. niger under SSF and SmF.
For the precipitation with ethanol using the SSF medium, the highest recoveries of protein (between 78.9 and 86.2%) and xylanase activity (between 59.8 and 64.4%) were obtained in test runs 7-11, in which the initial pH was 5.5, irrespective of the concentration of ethanol used (Table 3). In the case of the precipitations carried out with the SmF broth, adjustment of the pH to 5.5 and increasing the ethanol concentration from 78 to 92% (v/v) resulted in 22% and 16% increases in the recoveries of protein and xylanase activity, respectively.
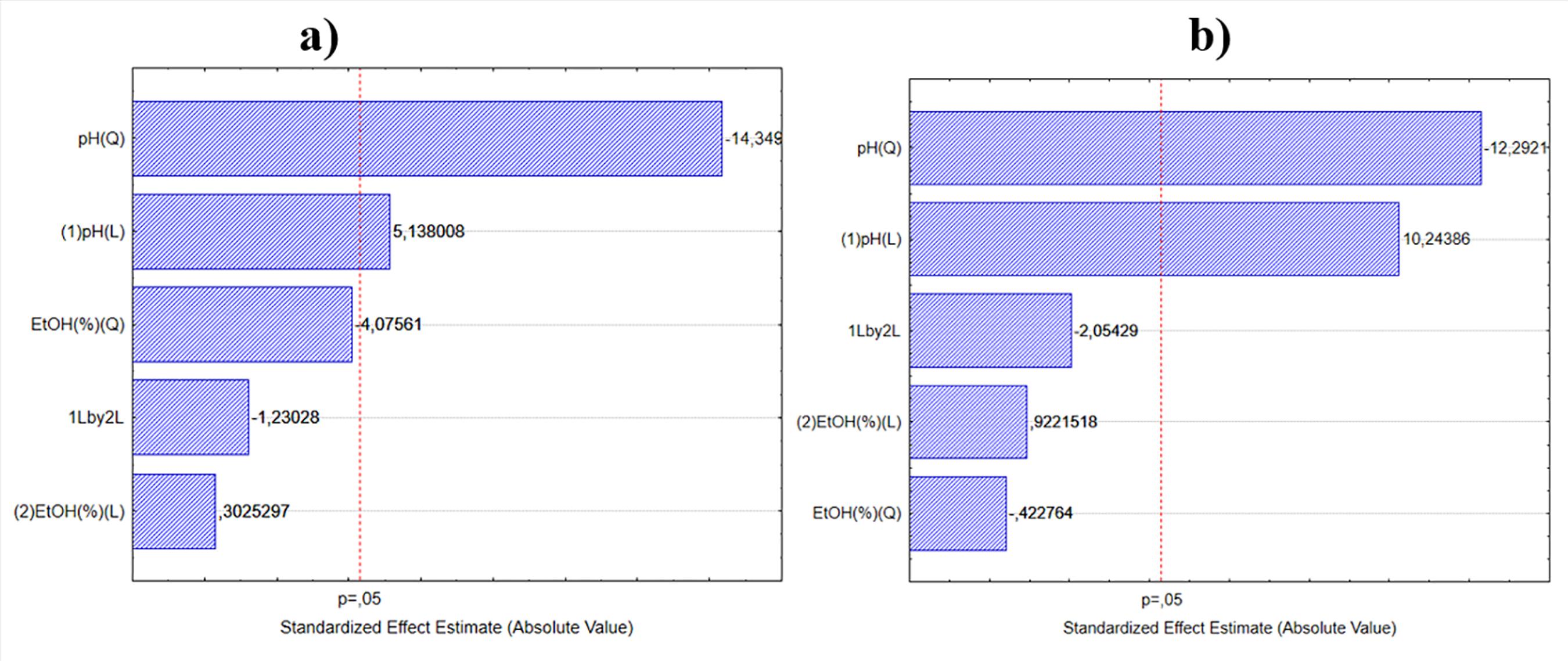
Pareto chart analysis was used to determine the degree of significance of the effect of each variable on the recoveries of xylanase activity and protein, for SSF (Figure 1) and SmF (Figure 2). For SSF, only the effect of pH was statistically significant (p<0.05) for the recoveries of both xylanase activity (Figure 1A) and protein (Figure 1B). For xylanase recovery from precipitation using the SmF broth, only the quadratic pH effect was significant, within the range evaluated (Figure 2A). For protein recovery from SmF (Figure 2B), the quadratic pH effect and the linear ethanol concentration effect were both significant at the 95% confidence level (p<0.05). This quadratic effect - that leads to a pH of maximun recovery for a fixed ethanol concentration - is due to the charge of the molecules. Most of proteins have their pI in the range of neutrality to slightly acidic pH. Values of pH distant from this range lead to charged molecules that tend to repel each other.
Pareto chart for the effects of the variables ethanol concentration and pH on the recovery of xylanase activity (A) and total protein (B) for the enzymatic complex from A. niger cultivated under SSF.
Pareto chart for the effects of the variables ethanol concentration and pH on the recovery of xylanase activity (A) and total protein (B) for the enzymatic complex from A. niger cultivated under SmF.
Table 4 provides the coefficients of the mathematical model and the statistical parameters obtained by analysis of the CCD responses for the xylanase activity and protein recoveries after ethanol precipitation of the SSF and SmF crude preparations. The low value for the coefficient of determination (R) for acitivity recovery may be explained by the fact that A. niger xylanases precipitated are not a single type of molecule, but xylanases with different molecular masses and pI values.
Coefficient values and statistical analysis for total protein concentration and xylanase activity.
In general, the pH effect was greater than the ethanol concentration effect, as can be seen from the coefficient values listed in Table 4. The ANOVA correlation coefficient and F-test values indicated satisfactory prediction by the models used to describe the response surface plots of the xylanase activity and protein recoveries for SSF (Figure 3) and SmF (Figure 4). The response surface plots showed that, in the case of ethanol precipitation of the enzyme complex produced by SSF, higher xylanase activity and total protein recoveries were obtained for an initial extract pH in the range from 5.5 to 7.0. Under these conditions, there was no significant influence of the ethanol concentration (Figure 3). For the ethanol precipitation using the SmF broth extract, the results showed that, for both xylanase activity and total protein, the best recovery was obtained when the pH was adjusted to the center point value, 5.5 (Figure 4). However, unlike the precipitation using the SSF extract, the concentration of the precipitant affected the precipitation process of the SmF broth extract, at any pH, with the effect being greater at lower pH.
Response surface plots for ethanol precipitation of the enzymatic complex from A. niger cultivated under SSF, varying the ethanol concentration and pH values. Recovery of xylanase activity (A) and total protein (B).
Response surface plots for ethanol precipitation of the enzymatic complex from A. niger cultivated under SmF, varying the ethanol concentration and pH values. Recovery of xylanase activity (A) and total protein (B).
Overall, the values for the conditions at the central point (85% (v/v) ethanol; pH 5.5) resulted in the best recoveries of xylanase from both the SSF and the SmF preparations, with average values of 63% and 67%, respectively. These precipitation conditions were therefore selected in the subsequent kinetic study. The recoveries were superior to those previously reported in studies of xylanase precipitation. For instance, Farinas et al. (2011)Farinas, C., Scarpelini, L., Miranda, E. and Neto, V., Evaluation of operational parameters on the precipitation of endoglucanase and xylanase produced by solid state fermentation of Aspergillus niger. Brazilian Journal of Chemical Engineering, 28(1) 17-26 (2011). reported that the use of 80% (v/v) ethanol to precipitate xylanases from A. niger cultivated under SSF resulted in up to 23% recovery. This difference could have been due to the different precipitation operational conditions employed, especially the temperature, which must be controlled in order to avoid protein refolding and denaturation.
Kinetic study of xylanase precipitation with ethanol
The kinetic curves for xylanase precipitation with ethanol using the SSF and SmF crude enzyme preparations (pH 5.5) are shown in Figures 5 A and B, respectively. These correspond to the temporal profiles for the xylanase activity and protein concentration recoveries obtained using precipitation at 15 ºC with 85% (v/v) of ethanol. The recovery of total protein from the SSF medium showed a rapid initial increase, reaching 90% after 75 min, and then remained stable. The recovery of xylanase activity from the SSF medium also remained stable (at around 60%) from the start of the period up to 360 min, after which there was a slight decrease (Figure 5A). The decrease could have been due to enzyme denaturation following exposure to a high concentration of ethanol for such a long period. For the precipitation of proteins from the SmF broth (Figure 5B), the recovery of total protein increased in the first 45 min and then remained quite stable. For the SmF broth, the xylanase activity recovery was stable (at around 80%) from the beginning of the period up to 360 min.
Kinetics of ethanol precipitation of the crude enzymatic SSF extract (a) and SmF broth (b) from A. niger. Initial xylanase activities: 4.15 IU/mL (a) and 7.68 UI/mL (b); initial protein concentrations: 0.23 mg/mL (a) and 0.12 mg/mL (b). Precipitation conditions: 15 ºC, pH 5.5, 85% (v/v) ethanol.
The differences in the initial total protein concentrations and xylanase activities of the crude enzyme preparations obtained from SSF and SmF were reflected in the specific activity values and, consequently, in the purification factors achieved after precipitation. For example, the maximum purification factors obtained for SSF and SmF were 0.88 and 1.12, respectively. For both SSF and SmF, the highest purification factor values were found using short precipitation periods, when much of the total protein was still in the soluble phase. These results showed that relatively short periods of time (up to 15 min) were sufficient to recover most of the xylanase activity precipitated under the operational conditions employed (15 ºC; pH 5.5; 85% (v/v) ethanol). Overall, the results achieved here for xylanase precipitation with ethanol were very satisfactory, when compared to the literature (Table 5), indicating that the procedure can be considered as a potential DSP unit operation for the concentration of A. niger xylanases from different cultivation systems.
Comparative data from ethanol precipitation studies of xylanase enzymes produced by different fungal strains cultivated under SSF and SmF.
CONCLUSIONS
A systematic comparative study was undertaken of the ethanol precipitation of xylanases produced by A. niger cultivated under SSF and SmF. The process was evaluated using a central composite design, considering the effects of pH and ethanol concentration on recovery yields. There was a significant effect of pH on the total protein and xylanase activity recoveries, with the best results for an initial pH of 5.5. The ethanol concentration had no significant effect on the precipitation of xylanase from the SSF extract, within the range tested, but had a more pronounced effect in the case of the SmF extract, especially at acidic pH. The kinetic profiles showed that relatively short periods of time (15 min) were sufficient to recover most of the xylanase activity precipitated under the conditions employed, reaching xylanase recoveries of 65% and 79% for the SSF and SmF enzymatic complexes, respectively. In summary, our findings demonstrate that ethanol precipitation is a potential DSP unit operation for the concentration of xylanases from different cultivation systems.
ACKNOWLEDGMENTS
The authors thank the Brazilian institutions CAPES, FAPESP, CNPq, and EMBRAPA for financial support.
REFERENCES
- Ahmad, Z., Butt, M.S., and Riaz M., Partial purification and characterization of xylanase produced from Aspergillus niger using wheat bran. Pakistan Journal of Agricultural Sciences, Faisalabad, 50(3) 433-437 (2013).
- Bailey, M.J. and Poutanen, K., Production of xylanolytic enzymes by strains of Aspergillus Applied Microbiology and Biotechnology, 30(1) 5-10 (1989).
- Bakri, Y., Masson, M. and Thonart, P., Isolation and Identification of Two New Fungal Strains for Xylanase Production. Applied Biochemistry and Biotechnology, 162(6) 1626-1634 (2010).
- Betini, J.H.A., Michelin, M., Peixoto-Nogueira, S.C., Jorge, J.A., Terenzi, H.F. and Polizeli, M., Xylanases from Aspergillus niger, Aspergillus niveus and Aspergillus ochraceus produced under solid-state fermentation and their application in cellulose pulp bleaching. Bioprocess and Biosystems Engineering, 32(6) 819-824 (2009).
- Bokhari, S.A.I., Latif, F. and Rajoka, M.I., Purification and characterization of xylanases from Thermomyces lanuginosus and its mutant derivative possessing novel kinetic and thermodynamic properties. World Journal of Microbiology & Biotechnology, 25(3) 493-502 (2009).
- Bradford, M.M., Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248-254 (1976).
- Chanwicha, N., Katekaew, S., Aimi, T. and Boonlue, S., Purification and characterization of alkaline xylanase from Thermoascus aurantiacus var. levisporus KKU-PN-I2-1 cultivated by solid-state fermentation. Mycoscience, 56(3) 309-318 (2015).
- Chapla, D., Divecha, J., Madamwar, D. and Shah, A., Utilization of agro-industrial waste for xylanase production by Aspergillus foetidus MTCC 4898 under solid state fermentation and its application in saccharification. Biochemical Engineering Journal, 49(3) 361-369 (2010).
- Chipeta, Z.A., du Preez, J.C. and Christopher, L., Effect of cultivation pH and agitation rate on growth and xylanase production by Aspergillus oryzae in spent sulphite liquor. Journal of Industrial Microbiology & Biotechnology, 35(6) 587-594 (2008).
- Collins, T., Gerday, C. and Feller, G., Xylanases, xylanase families and extremophilic xylanases. Fems Microbiology Reviews, 29(3) 3-23 (2005).
- Couri, S. and deFarias, A.X., Genetic manipulation of Aspergillus niger for increased synthesis of pectinolytic enzymes. Revista De Microbiologia, 26(4) 314-317 (1995).
- de Vries, R.P., Regulation of Aspergillus genes encoding plant cell wall polysaccharide-degrading enzymes; relevance for industrial production. Applied Microbiology and Biotechnology, 61(1) 10-20 (2003).
- Dodd, D. and Cann, I., Enzymatic deconstruction of xylan for biofuel production. Global Change Biology Bioenergy, 1(1) 2-17 (2009).
- Fadel, M., High-level xylanase production from sorghum flour by a newly isolate of Trichoderma harzianum cultivated under solid state fermentation. Annals of Microbiology, 51(1) 61-78 (2001).
- Fang, T.J., Liao, B.C. and Lee, S.C., Enhanced production of xylanase by Aspergillus carneus M34 in solid-state fermentation with agricultural waste using statistical approach. New Biotechnology, 27(1), 25-32 (2010).
- Farinas, C., Scarpelini, L., Miranda, E. and Neto, V., Evaluation of operational parameters on the precipitation of endoglucanase and xylanase produced by solid state fermentation of Aspergillus niger Brazilian Journal of Chemical Engineering, 28(1) 17-26 (2011).
- Farinas, C.S., Developments in solid-state fermentation for the production of biomass-degrading enzymes for the bioenergy sector. in: Renewable and Sustainable Energy Reviews, 52 179-188 (2015).
- Gao, D.H., Uppugundla, N., Chundawat, S.P.S., Yu, X.R., Hermanson, S., Gowda, K., Brumm, P., Mead, D., Balan, V. and Dale, B.E., Hemicellulases and auxiliary enzymes for improved conversion of lignocellulosic biomass to monosaccharides. Biotechnology for Biofuels, 4(5) 1-11 (2011).
- Ghanem, N.B., Yusef, H.H. and Mahrouse, H.K., Production of Aspergillus terreus xylanase in solid-state cultures: application of the Plackett-Burman experimental design to evaluate nutritional requirements. Bioresource Technology, 73(2) 113-121 (2000).
- Golunski, S., Astolfi, V., Carniel, N., de Oliveira, D., Di Luccio, M., Mazutti, M.A. and Treichel, H., Ethanol precipitation and ultrafiltration of inulinases from Kluyveromyces marxianus Separation and Purification Technology, 78(3) 261-265 (2011).
- Guimaraes, N.C.D., Sorgatto, M., Peixoto-Nogueira, S.D., Betini, J.H.A., Zanoelo, F.F., Marques, M.R., Polizeli, M. and Giannesi, G.C., Bioprocess and biotechnology: effect of xylanase from Aspergillus niger and Aspergillus flavus on pulp biobleaching and enzyme production using agroindustrial residues as substract. Springerplus, 2 1-7 (2013).
- Holker, U. and Lenz, J., Solid-state fermentation - are there any biotechnological advantages? Current Opinion in Microbiology, 8(3) 301-305 (2005).
- Kamm, B. and Kamm, M., Principles of biorefineries. Applied Microbiology and Biotechnology, 64(2) 137-145 (2004).
- Kumar, R. and Wyman, C.E., Effect of xylanase supplementation of cellulase on digestion of corn stover solids prepared by leading pretreatment technologies. Bioresource Technology, 100(18) 4203-4213 (2009).
- Lu, F.X., Lu, M., Lu, Z.X., Bie, X.M., Zhao, H.Z. and Wang, Y., Purification and characterization of xylanase from Aspergillus ficuum AF-98. Bioresource Technology, 99(13) 5938-5941 (2008).
- Mandels, M. and Sternberg, D., Recent advances in cellulase technology. Journal of Fermentation Technology, 54(4) 267-286 (1976).
- Mander, P., Choi, Y.H., Pradeep, G.C., Choi, Y.S., Hong, J.H., Cho, S.S. and Yoo, J.C., Biochemical characterization of xylanase produced from Streptomyces sp CS624 using an agro residue substrate. Process Biochemistry, 49(3) 451-456 (2014).
- Marino, M.A., Freitas, S. and Miranda, E.A., Ethanol precipitation of glycosyl hydrolases produced by Trichoderma harzianum P49P11. Brazilian Journal of Chemical Engineering, 32(2) 325-333 (2015).
- Michelin, M., Peixoto-Nogueira, S.C., Betini, J.H.A., da Silva, T.M., Jorge, J.A., Terenzi, H.F. and Polizeli, M., Production and properties of xylanases from Aspergillus terricola Marchal and Aspergillus ochraceus and their use in cellulose pulp bleaching. Bioprocess and Biosystems Engineering, 33(7) 813-821 (2010).
- Miller, G., Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3) 426-428 (1959).
- Nakadai, T. and Nasuno, S., Enzyme preparation from extract of wheat bran koji by alcohol precipitation. Journal of Fermentation and Bioengineering, 67(4) 253-257 (1989).
- Ninawe, S., Kapoor, M. and Kuhad, R.C., Purification and characterization of extracellular xylanase from Streptomyces cyaneus SN32. Bioresource Technology, 99(5) 1252-1258 (2008).
- Park, Y.S., Kang, S.W., Lee, J.S., Hong, S.I. and Kim, S.W., Xylanase production in solid state fermentation by Aspergillus niger mutant using statistical experimental designs. Applied Microbiology and Biotechnology, 58(6) 761-766 (2002).
- Pauly, M., Gille, S., Liu, L.F., Mansoori, N., de Souza, A., Schultink, A. and Xiong, G.Y., Hemicellulose biosynthesis. Planta, 238(4) 627-642 (2013).
- Pirota, R., Tonelotto, M., Delabona, P.D., Fonseca, R.F., Paixao, D.A.A., Baleeiro, F.C.F., Neto, V.B. and Farinas, C.S., Enhancing xylanases production by a new Amazon Forest strain of Aspergillus oryzae using solid-state fermentation under controlled operation conditions. Industrial Crops and Products, 45 465-471 (2013).
- Polizeli, M., Rizzatti, A.C.S., Monti, R., Terenzi, H.F., Jorge, J.A. and Amorim, D.S., Xylanases from fungi: properties and industrial applications. Applied Microbiology and Biotechnology, 67(5) 577-591 (2005).
- Saha, B.C., Hemicellulose bioconversion. Journal of Industrial Microbiology & Biotechnology, 30(5) 279-291 (2003).
- Santos, F. A.; Queiróz, J. H.; Colodette, J. L.; Fernandes, S. A.; Guimarães, V. M.; and Rezende, S. T., Potencial da palha de cana-de-açúcar para produção de etanol. Química Nova, 35(5) 1004-1010 (2012).
- Shallom, D. and Shoham, Y., Microbial hemicellulases. Current Opinion in Microbiology, 6(3) 219-228 (2003).
- Varma, R.J., Nene, S., Baliga, B.A. and Elias, C., Studies on commercial aspects of xylanase from Chainia species. Journal of Scientific & Industrial Research, 58(11) 878-882 (1999).
- Venegas, I.M., Fuentes-Hernandez, J., Garcia-Rivero, M. and Martinez-Trujillo, A., Characteristics of Aspergillus niger xylanases produced on rice husk and wheat bran in submerged culture and solid-state fermentation for an applicability proposal. International Journal of Food Science and Technology, 48(9) 1798-1807 (2013).
- Wong, K.K.Y., Tan, L.U.L. and Saddler, J.N., Multiplicity of beta-1,4-xylanase in microorganisms - functions and applications. Microbiological Reviews, 52(3) 305-317 (1988).
Publication Dates
-
Publication in this collection
Apr-Jun 2018
History
-
Received
21 Aug 2016 -
Reviewed
12 Feb 2017 -
Accepted
02 Mar 2017