Abstract
Yacon is mainly constituted of water and carbohydrates [single sugars and fructooligosaccharides (FOS)], thus being an excellent alternative for the growth and preservation of bacterial culture. Latilactobacillus sakei ACU-2 and Staphylococcus vitulinus ACU-10 comprised the autochthonous starter culture SAS-1 designed for the manufacture of dry sausages. This study evaluated the use of yacon juice as a potential growth medium and cryoprotectant for these bacteria. The growth medium was prepared with yacon juice supplemented with peptone and dipotassium phosphate. After growing, cells were resuspended in yacon juice (5, 10 and 25 mL/100 mL) and lyophilized. Viable cells were count before, immediately after lyophilization, and along 6 months of refrigerated storage. Both bacteria grew in every yacon concentration tested; however, juice concentration affected their growth. Latilactobacillus sakei grew at μ = 0.256 ± 0.01 giving the highest bacterial density at 10 mL/100 mL (Log DOmax 0.33 ± 0.01). While 5 mL/100 mL yacon juice provided the best conditions for S. vitulinus growth (μ = 0.215 ± 0.016; Log DOmax 0.32 ± 0.01). After lyophilization, the survival rate was 91.1% for L. sakei and 65.8% for S. vitulinus. Throughout storage, high cell counts suggested good stability of both bacteria. Results revealed that yacon juice comprises a nutritive substrate for the growth and cryopreservation of tested strains from the genus Latilactobacillus and Staphylococcus.
Keywords:
Culture media; Freeze-drying process; Starter culture; Survival rates; Nutritive substrate; Cryopreservation
Resumo
O yacon é constituído principalmente por água e carboidratos (açúcares simples e fruto-oligossacarídeos), sendo uma excelente alternativa para o crescimento e a preservação da cultura bacteriana. Latilactobacillus sakei ACU-2 e Staphylococcus vitulinus ACU-10 compuseram a cultura starter autóctone SAS-1, destinada à fabricação de embutidos secos. Este estudo avaliou o uso do suco de yacon como potencial meio de crescimento e crioprotetor para essas bactérias. O meio de crescimento foi preparado com suco de yacon suplementado com peptona e fosfato dipotássico. Após o crescimento, as células foram ressuspensas em suco de yacon (5, 10 e 25 mL/100 mL) e liofilizadas. As células viáveis foram contadas antes e imediatamente após a liofilização, e ao longo de seis meses de armazenamento refrigerado. Ambas as bactérias cresceram em cada concentração de yacon testada; no entanto, a concentração de suco afetou seu crescimento. Latilactobacillus sakei cresceu a μ = 0,256 ± 0,01, dando a maior densidade bacteriana em 10 mL/100 mL (Log DOmax 0,33 ± 0,01), enquanto a concentração 5 mL/100 mL de suco de yacon apresentou as melhores condições para o crescimento de S. vitulinus (μ = 0,215 ± 0,016; Log DOmax 0,32 ± 0,01). Após a liofilização, a taxa de sobrevivência foi de 91,1% para L. sakei e 65,8% para S. vitulinus. Ao longo do armazenamento, altas contagens de células sugeriram boa estabilidade de ambas as bactérias. Os resultados revelaram que o suco de yacon é um substrato nutritivo para o crescimento e a criopreservação das cepas testadas dos gêneros Latilactobacillus e Staphylococcus.
Palavras-chave:
Meio de cultivo; Processo de liofilização; Taxas de sobrevivência; Substrato nutritivo; Criopreservação
HIGHLIGHTS
• Bacterial density from L. sakei ACU-2 was higher in presence of 10 mL/100 mL yacon juice
• S. vitulinus ACU-10 showed a better performance in the medium prepared with 5 mL/100 mL yacon juice
• Yacon juice comprises a nutritive substrate for cryopreservation of L. sakei and S. vitulinus
1 Introduction
Artisanal dry fermented sausages manufactured in Chaco, Northern Argentina, possess a distinctive sensory profile which is highly appreciated by local customers. However, as their production relies on spontaneous fermentation from house microbiota, there are significant variations in their physicochemical and microbiological characteristics throughout the year (Palavecino Prpich et al., 2015aPalavecino Prpich, N. Z. P., Castro, M. P., Cayré, M. E., Garro, O. A., & Vignolo, G. M. (2015a). Autochthonous starter culture selection to keep traditions in the manufacture of dry sausages alive. Annals of Microbiology, 65(3), 1709-1719. http://dx.doi.org/10.1007/s13213-014-1010-0
http://dx.doi.org/10.1007/s13213-014-101...
). In order to address this issue, a starter culture was designed with the autochthonous strains Latilactobacillus sakei ACU-2 and Staphylococcus vitulinus ACU-10 (Palavecino Prpich et al., 2015bPalavecino Prpich, N. Z. P., Castro, M. P., Cayré, M. E., Garro, O. A., & Vignolo, G. M. (2015b). Indigenous starter cultures to improve quality of artisanal dry fermented sausages from Chaco (Argentina). International Journal of Food Science, 2015, 931970. PMid:26955636. http://dx.doi.org/10.1155/2015/931970.
http://dx.doi.org/10.1155/2015/931970...
). This starter culture, called SAS-1, was incorporated into the production line of a small-scale facility delivering beneficial outcomes to the final product with no impact on its typical sensory profile (Palavecino Prpich et al., 2016Palavecino Prpich, N. Z. P., Garro, O. A., Romero, M., Judis, M. A., Cayré, M. E., & Castro, M. P. (2016). Evaluation of an autochthonous starter culture on the production of a traditional dry fermented sausage from Chaco (Argentina) at a small-scale facility. Meat Science, 115, 41-44. PMid:26820805. http://dx.doi.org/10.1016/j.meatsci.2016.01.005
http://dx.doi.org/10.1016/j.meatsci.2016...
). In pursuit of a potential technological transfer, the bacterial strains from SAS-1 have to be produced on a larger scale, and in addition, the best conditions for their suitable preservation have to be established.
Laboratory growth media routinely used the whole range of nutrients necessary for optimal microbial growth. Nevertheless, these media become a high price input product when biomass production is scaled up, being strongly advised to seek for other sources of raw materials to fulfill this need (Brinques et al., 2010Brinques, G. B., Peralba, M. C., & Ayub, M. A. Z. (2010). Optimization of probiotic and lactic acid production by Lactobacillus plantarum in submerged bioreactor systems. Journal of Industrial Microbiology & Biotechnology, 37(2), 205-212. PMid:19936814. http://dx.doi.org/10.1007/s10295-009-0665-1
http://dx.doi.org/10.1007/s10295-009-066...
). Even more urgent this scenario turns out to be when biomass is intended for an industrial culture. In this sense, studies regarding the use of natural and available sources as nutrient supplements for enhanced biomass yield of bacteria are scarce.
Among microbial culture preservation methods, freeze-drying meets the most preferable features since it helps to maintain a high percentage of viable cells while preserving the morphological and biochemical characteristics of the bacterial culture (Morgan et al., 2006Morgan, C. A., Herman, N., White, P. A., & Vesey, G. (2006). Preservation of microorganisms by drying; a review. Journal of Microbiological Methods, 66(2), 183-193. PMid:16632005. http://dx.doi.org/10.1016/j.mimet.2006.02.017
http://dx.doi.org/10.1016/j.mimet.2006.0...
). However, cells are exposed to stress conditions along the process which could be able to damage their integrity (Basholli-Salihu et al., 2014Basholli-Salihu, M., Mueller, M., Salar-Behzadi, S., Unger, F. M., & Viernstein, H. (2014). Effect of lyoprotectants on b-glucosidase activity and viability of Bifidobacterium infantis after freeze-drying and storage in milk and low pH juices. Lebensmittel-Wissenschaft + Technologie, 57(1), 276-282. http://dx.doi.org/10.1016/j.lwt.2014.01.011
http://dx.doi.org/10.1016/j.lwt.2014.01....
). One of the crucial factors in cell survival throughout the freeze-drying process is related to the composition of the medium. Cell damage can be prevented with the incorporation of cryoprotective substances such as carbohydrates, proteins and polymers into the menstruum for lyophilization (Morgan et al., 2006Morgan, C. A., Herman, N., White, P. A., & Vesey, G. (2006). Preservation of microorganisms by drying; a review. Journal of Microbiological Methods, 66(2), 183-193. PMid:16632005. http://dx.doi.org/10.1016/j.mimet.2006.02.017
http://dx.doi.org/10.1016/j.mimet.2006.0...
). Many prebiotic oligosaccharides, such as galactooligosaccharides (GOS) and fructooligosaccharides (FOS), had been successfully used as cryoprotectans for the preservation of microbial cultures (Santos et al., 2014Santos, M., Gerbino, E., Araujo-Andrade, C., Tymczyszyn, E., & Gómez-Zavaglia, A. (2014). Stability of freeze-dried Lactobacillus delbrueckii subsp. Bulgaricus in the presence of galacto-oligosaccharides and lactulose as determined by near infrared spectroscopy. Food Research International, 59, 53-60. http://dx.doi.org/10.1016/j.foodres.2014.01.054
http://dx.doi.org/10.1016/j.foodres.2014...
; Romano et al., 2016Romano, N., Schebor, C., Mobili, P., & Gómez-Zavaglia, A. (2016). Role of mono- and oligosaccharides from FOS as stabilizing agents during freeze-drying and storage of Lactobacillus delbrueckii subsp. bulgaricus. Food Research International, 90, 251-258. PMid:29195879. http://dx.doi.org/10.1016/j.foodres.2016.11.003
http://dx.doi.org/10.1016/j.foodres.2016...
).
Yacon (Smallanthus sonchifolius (Poepp.) H. Rob.) is a perennial Andean plant that develops tuberous roots of sweet flavor. Mainly comprised of water and carbohydrates, these roots present, as a dry matter, 40 to 70 mL/100 mL of FOS and 15-40 mL/100 mL of single sugars, i.e., glucose, fructose and sucrose (Ojansivu et al., 2011Ojansivu, I., Ferreira, C. L., & Salminen, S. (2011). Yacon, a new source of prebiotic oligosaccharides with a history of safe use. Trends in Food Science & Technology, 22(1), 40-46. http://dx.doi.org/10.1016/j.tifs.2010.11.005
http://dx.doi.org/10.1016/j.tifs.2010.11...
). Besides this rich composition, yacon fruit had been investigated for its antidiabetic and antioxidant activities highlighting the role of yacon supplementation in promoting health and reducing the risk of chronic diseases (Caetano et al., 2016Caetano, B. F. R., Moura, N. A., Almeida, A. P. S., Dias, M. C., Sivieri, K., & Barbisan, L. F. (2016). Yacon (Smallanthus sonchifolius) as a food supplement: Health-promoting benefits of fructooligosaccharides. Nutrients, 8(7), 436. PMid:27455312. http://dx.doi.org/10.3390/nu8070436
http://dx.doi.org/10.3390/nu8070436...
; Cao et al., 2018Cao, Y., Ma, Z. F., Zhang, H., Jin, Y., Zhang, Y., & Hayford, F. (2018). Phytochemical properties and nutrigenomic implications of yacon as a potential source of prebiotic: Current evidence and future directions. Foods, 7(4), 59. PMid:29649123. http://dx.doi.org/10.3390/foods7040059
http://dx.doi.org/10.3390/foods7040059...
). Sousa et al. (2015)Sousa, S., Pinto, J., Pereira, C. F., Malcata, F. X., Pacheco, M. T. B., Gomes, A. M., & Pintado, M. (2015). In vitro evaluation of yacon (Smallanthus sonchifolius) tuber flour prebiotic potential. Food and Bioproducts Processing, 95, 96-105. http://dx.doi.org/10.1016/j.fbp.2015.04.003
http://dx.doi.org/10.1016/j.fbp.2015.04....
revealed a potential prebiotic activity of yacon tuber flour upon the growth of four probiotic strains, attributing it to its FOS content. Yacon is usually processed by drying and blanching, being hot air drying, vacuum drying, heat pump drying, osmotic dehydration, foam-mat drying, spray drying, and freeze-drying the most common methods of drying used to process its juice (Reis et al., 2021Reis, F. R., Marques, C., Moraes, A. C. S., & Masson, M. L. (2021). Effect of processing methods on yacon roots health-promoting compounds and related properties. Trends in Food Science & Technology, 113, 346-354. http://dx.doi.org/10.1016/j.tifs.2021.05.010
http://dx.doi.org/10.1016/j.tifs.2021.05...
).
The introduction of yacon into a cryopreservation process could be an excellent application for this Andean root. Thus, this study aimed to evaluate the effects of yacon juice as growth medium and cryoprotectant on the viability of L. sakei ACU-2 and S. vitulinus ACU-10 during growth, freeze-drying and subsequent storage.
2 Material and methods
2.1 Microorganisms and culture conditions
Latilactobacillus sakei ACU-2 and S. vitulinus ACU-10 composed the designed starter culture SAS-1 (Palavecino Prpich et al., 2015aPalavecino Prpich, N. Z. P., Castro, M. P., Cayré, M. E., Garro, O. A., & Vignolo, G. M. (2015a). Autochthonous starter culture selection to keep traditions in the manufacture of dry sausages alive. Annals of Microbiology, 65(3), 1709-1719. http://dx.doi.org/10.1007/s13213-014-1010-0
http://dx.doi.org/10.1007/s13213-014-101...
, 2015bPalavecino Prpich, N. Z. P., Castro, M. P., Cayré, M. E., Garro, O. A., & Vignolo, G. M. (2015b). Indigenous starter cultures to improve quality of artisanal dry fermented sausages from Chaco (Argentina). International Journal of Food Science, 2015, 931970. PMid:26955636. http://dx.doi.org/10.1155/2015/931970.
http://dx.doi.org/10.1155/2015/931970...
). These strains are deposited in the strain repository of Laboratorio de Microbiología de Alimentos (Universidad Nacional del Chaco Austral, Argentina). Lactobacilli are kept in MRS broth (de Man, Rogosa, Sharpe) supplemented with 20 mL/100 mL of glycerol, while staphylococci are kept in Trypticase Soy Agar (TSA) + 0.6 mL/100 mL w/v yeast extract (TSBYE) added with 20 mL/100 mL of glycerol; both bacterial species are maintained frozen at −80 °C.
2.2 Culture media
Culture media were prepared with fresh yacon juice. The fruits were washed, peeled and cut into cubes. The juice was extracted with a domestic juicer (Atma, Argentina), then clarified by vacuum filtration through a Whatman N°10 filter, and subsequently, it was centrifuged at 4000 rpm in a refrigerated device (Rolco, Argentina). The supernatant was diluted with distilled water at 5 mL/100 mL, 10 mL/100 mL and 25 mL/100 mL and it was supplemented with peptone (20 g/L) and dipotassium phosphate (2 g/L). Before autoclaving, pH values of the medium were adjusted to 6.5 with sodium hydroxide (1N).
2.3 Medium inoculation and microbial growth monitoring
Medium were inoculated at 1 mL/100 mL with each of the tested bacteria. Systems inoculated with L. sakei were incubated at 30 °C while those inoculated with S. vitulinus were incubated at 37 °C. Systems prepared with de Man Rogosa and Sharpe (MRS) or Brain Heart Infusion (BHI) broths were used as growth control for lactobacilli and staphylococci, respectively. Cultures Optical Density (OD) was spectrophotometrically monitored at 600 nm (Cary UV-Visible 60, Agilent Technologies, USA) for 12 h. Viable cell counts were determined by plate counting: L. sakei ACU-2 was performed in MRS agar incubated at 30 °C for 48 h, while S. vitulinus ACU-10 was cultured in BHI agar at 37 °C for 48 h. Bacterial counts were expressed as Log (CFU/mL).
2.4 Kinetic parameters estimation
Data obtained, expressed as Log (DO600), were used to fit the modified Gompertz equation (Zwietering et al., 1990Zwietering, M. H., Jongenburger, I., Rombouts, F. M., & van 't Riet, K. (1990). Modeling of the bacterial growth curve. Applied and Environmental Microbiology, 56(6), 1875-1881. PMid:16348228. http://dx.doi.org/10.1128/aem.56.6.1875-1881.1990
http://dx.doi.org/10.1128/aem.56.6.1875-...
) (Equation 1) and to estimate the growth kinetic parameters: specific growth rate (μ, expressed at h-1) and maximum density (Log DOmax).
Where: y(t) = Log (DO600) = culture optical density at time t; yo = initial Log (DO600); ymax = maximum Log (DO600); μmax = maximum specific growth rate [time-1]; λ = lag phase length [time].
2.5 Freeze-drying process
The yacon juice concentration with the highest viable cell counts (section 2.3) was chosen to perform this process. Cells were removed by centrifugation at 3000 g at 4 °C for 10 min (Dragon Lab, China), and were washed twice with sterile saline solution. Each biomass (lactobacilli and staphylococci) was then resuspended in the same volume of sterile yacon juice 10 mL/100 mL in order to test its attributes as cryoprotectant; distilled water was used in the same fashion to constitute the control system. These systems, containing 1 mL of each of the starter strains, were frozen at -80 °C in an ultrafreezer (Presvac, Argentina) during 24 h and then were transferred to a freeze dryer (Scientz-10N, China). The freeze-drying process was performed at 10 Pa for 48 h at a condenser temperature of -50 °C. Before the freeze-drying, viable cell counts were determined as already described (section 2.3).
2.6 Bacterial cell viability
Cell viability was also determined immediately after the drying process. The freeze-dried samples were rehydrated in sterile deionized water at room temperature by adding the same volume that had been removed during freeze-drying (1 mL). Survival Rate (SR) was calculated as the ratio between CFU/mL before and after drying, expressed as a percentage (Equation 2).
2.7 Stability of microbial cells during storage
Cell viability of both freeze-dried bacteria stored at refrigerated temperature (4 °C) was investigated after storage for up to 180 days. A new vial was opened at every time interval. Viable cells were determined as before mentioned (section 2.3). The specific rate of degradation (k) of the lyophilized samples was calculated according to Equation 3 (Korakoch et al., 2005Korakoch, H., Sukyai, P., Loiseau, G., Nitisinprasert, S., Montet, D., & Wanchaitanawong, P. (2005). Prediction on the stability of spray-dried Lactobacillus reuteri KUB-AC5 by Arrhenius equation for long-term storage. Journal of Microbiology and Biotechnology, 15(6), 1178-1182. Retrieved in 2022, December 15, from https://koreascience.kr/article/JAKO200511722550148.pdf
https://koreascience.kr/article/JAKO2005...
):
Where: N0 is the number of viable cells (CFU/mL of yacon juice) at initial storage time (t = to); N is the number of viable cells (CFU/mL of yacon juice) at time t (days); k is the specific rate of degradation (days-1) and t is the storage time.
2.8 Water activity determination
After drying, the water activity of the samples was measured at 25 °C using a calibrated device (Testo 635, Testo, Germany).
2.9 Scanning Electron Microscopy (SEM)
External appearance of freeze-dried cultures was examined by SEM. The samples were mounted on aluminum SEM stubs and coated with a thin layer of gold in a Denton Vacuum Desk II sputter coater. The morphology of the samples was observed using a scanning electron microscope (JEOL 5800LV, Japan) at a 10kV acceleration voltage.
2.10 Determination of yacon juice chemical composition
Association of Official Analytical Chemistry (2006)Association of Official Analytical Chemistry - AOAC. (2006). Official methods of analysis (18th ed.). Gaithersburg: AOAC. methods were used to determine ash (AOAC 923.03), lipids (925.38 AOAC), nitrogen (AOAC 984.13), moisture (AOAC 934.01), total dietary fiber (AOAC 991.43) and total carbohydrates content (Association of Official Analytical Chemistry, 2000Association of Official Analytical Chemistry - AOAC. (2000). Official methods of analysis (17th ed.). Gaithersburg: AOAC.). The conversion factor of 6.25 was used to convert nitrogen into crude protein content. For moisture content determination, the temperature and time of AOAC method were modified to 60 ºC and 20 h due to sample caramelization at 105 ºC. FOS content was determined by Zuleta & Sambucetti (2001)Zuleta, A., & Sambucetti, M. E. (2001). Inulin determination for food labeling. Journal of Agricultural and Food Chemistry, 49(10), 4570-4572. PMid:11599989. http://dx.doi.org/10.1021/jf010505o
http://dx.doi.org/10.1021/jf010505o...
method. All determinations were repeated in triplicate.
2.11 Statistical analysis
Trials were run in triplicate and the resulting data were expressed as mean value ± standard deviation. Modified Gompertz Equation 1 was adjusted with growth data by means of non-linear regression using the Marquardt algorithm. The growth parameters of each different run were compared by one-way Analysis of Variance (ANOVA). Tukey’s test was applied to separate means when p value was ˂ 0.05. These analyses were conducted with the software STATGRAPHICS Plus 4.0 (Statistical Graphics Corp., Rockville, MD, USA).
3 Results and discussion
3.1 Kinetic growth parameters
In this research work, the growth medium potential of yacon juice has been explored using the strains of the starter culture SAS-1, L. sakei ACU-2 and S. vitulinus ACU-10. Kinetic growth parameters estimated by Gompertz modified Equation 1 are summarized in Table 1. Both strains grew in all the tested yacon juice concentrations; however, juice concentration significantly affected their growth. As can be observed in Table1, bacterial density from L. sakei ACU-2 was significantly higher when 10 mL/100 mL yacon juice was used. Nevertheless, the estimated parameters for yacon juice were statistically lower than the ones from the control system (MRS broth). After 12 h of incubation, lactobacilli counts were 8.48 ± 0.16 Log (CFU/mL) for 10 mL/100 mL yacon juice and 9.18 ± 0.02 Log (CFU/mL) for the control system. On the other hand, the staphylococci showed a better performance in the medium prepared with 5 mL/100 mL of yacon juice. Kinetic growth parameters estimated under this formulation showed no significant differences with the control system (BHI broth) nor the bacterial counts (Table 1). An increase in yacon juice concentration among 5 mL/100 mL was detrimental to the bacterial maximum density as indicated by a significant reduction in these values. This drop was even more pronounced for the medium with 25 mL/100 mL yacon juice.
Growth kinetic parameters and viable cell counts of L. sakei ACU-2 and S. vitulinus ACU-10 in different concentrations of yacon juice intended to be used as growth media.
The difference between the best growth medium and its respective control system was more noticeable for L. sakei ACU-2 than for S. vitulinus ACU-10 which denoted that nutrient requirements among species are distinct and reflect their specific metabolic behavior. It is well known that lactic acid bacteria are nutritionally more demanding than other bacterial genus (Axelsson, 2004Axelsson, L. (2004). Lactic acid bacteria: Classification and physiology. In: S. Salminen V. von Wright & A. Ouwehand (Eds.), Lactic acid bacteria: Microbiological and functional aspects (pp. 1-66). New York: Marcel Dekker Inc. http://dx.doi.org/10.1201/9780824752033.ch1.). Besides carbohydrates, lactobacilli require elements such as amino acids, peptides, vitamins and Mg/Mn salts to grow (Liew et al., 2005Liew, S. L., Ariff, A. B., Raha, A. R., & Ho, Y. W. (2005). Optimization of medium composition for the production of a probiotic microorganism, Lactobacillus rhamnosus, using response surface methodology. International Journal of Food Microbiology, 102(2), 137-142. PMid:15992613. http://dx.doi.org/10.1016/j.ijfoodmicro.2004.12.009
http://dx.doi.org/10.1016/j.ijfoodmicro....
; Brinques et al., 2010Brinques, G. B., Peralba, M. C., & Ayub, M. A. Z. (2010). Optimization of probiotic and lactic acid production by Lactobacillus plantarum in submerged bioreactor systems. Journal of Industrial Microbiology & Biotechnology, 37(2), 205-212. PMid:19936814. http://dx.doi.org/10.1007/s10295-009-0665-1
http://dx.doi.org/10.1007/s10295-009-066...
), while members of the genus Staphylococcus grow commonly well in simple media. This fact could explain the observed differences between both strains.
3.2 Yacon juice as cryoprotectant
Yacon juice was also evaluated as bacterial cell protectant agent during the freeze-drying process. Figure1 shows a microbial population decrease in all the freeze-dried samples. Nonetheless, the extent of the decrease depended upon the presence of protective substances and the bacterial species. An aqueous solution of yacon (10 mL/100 mL) had high Survival Rates (SR) for L. sakei ACU-2 (91.1%) and for S. vitulinus ACU-10 (65.8%). Conversely, the SR for distilled water was less than 20% for both bacteria, evidencing the protectant effect of yacon juice. On the other hand, low values of water activity were observed in the yacon juice freeze-dried samples: 0.311 ± 0.001 for L. sakei ACU-2 and 0.289 ± 0.015 for S. vitulinus ACU-10. High viability together with low water activity (< 0.6) are usually considered good quality indicators of freeze-dried cultures (Haiping et al., 2019Haiping, L., Pei, Z., Shuhai, Z., Dengyun, Z., Herong, F., Yi, S., & Xinqian, W. (2019). Protective effect of polysacharides from Pholiata nameko on Lactobacillus casei ATCC 334 subjected to freeze-drying. Lebensmittel-Wissenschaft + Technologie, 115, 108463. http://dx.doi.org/10.1016/j.lwt.2019.108463
http://dx.doi.org/10.1016/j.lwt.2019.108...
).
The survival rate of L. sakei ACU-2 and S. vitulinus ACU-10 freeze-dried in (grey) yacon juice 10 mL/100 mL and (black) deionized water.
From a marketing perspective, the preservation of freeze-dried powder stability over the shelf-life is required. According to Broeckx et al. (2016)Broeckx, G., Vandenheuvel, D., Claes, I. J. J., Lebeer, S., & Kiekens, F. (2016). Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. International Journal of Pharmaceutics, 505(1-2), 303-318. PMid:27050865. http://dx.doi.org/10.1016/j.ijpharm.2016.04.002
http://dx.doi.org/10.1016/j.ijpharm.2016...
, storage temperature, moisture content, water activity, relative humidity, oxygen presence and exposure to light, amongst others, are factors that can influence the shelf-life of the finished product. Besides, other authors highlighted that: i) temperature is one of the most critical parameter for microbial survival during storage (Carvalho et al., 2004Carvalho, A. S., Silva, J., Ho, P., Teixeira, P., Malcata, F. X., & Gibbs, P. (2004). Relevant factors for the preparation of freeze-dried lactic acid bacteria. International Dairy Journal, 14(10), 835-847. http://dx.doi.org/10.1016/j.idairyj.2004.02.001
http://dx.doi.org/10.1016/j.idairyj.2004...
) and; ii) storage at refrigerated temperatures generally provides the best stability (Meng et al., 2008Meng, X. C., Stanton, C., Fitzgerald, G. F., Daly, C., & Ross, R. P. (2008). Anhydrobiotics: The challenges of drying probiotic cultures. Food Chemistry, 106(4), 1406-1416. http://dx.doi.org/10.1016/j.foodchem.2007.04.076
http://dx.doi.org/10.1016/j.foodchem.200...
; Lapsiri et al., 2012Lapsiri, W., Bhandari, B., & Wanchaitanawong, P. (2012). Viability of Lactobacillus plantarum TISTR 2075 in different protectants during spray drying and storage. Drying Technology, 30(13), 1407-1412. http://dx.doi.org/10.1080/07373937.2012.684226
http://dx.doi.org/10.1080/07373937.2012....
; Chotiko & Sathivel, 2014Chotiko, A., & Sathivel, S. (2014). Effects of enzymatically-extracted purple rice bran fiber as aprotectant of L. plantarum NRRL B-4496 during freezing, freeze drying, and storage. Lebensmittel-Wissenschaft + Technologie, 59(1), 59-64. http://dx.doi.org/10.1016/j.lwt.2014.05.056
http://dx.doi.org/10.1016/j.lwt.2014.05....
). In this sense, the stability over 180 days of storage was performed at a refrigerated temperature of 4 °C. Figure 2 shows the viability reduction over storage for both bacteria, where 0.77 and 1.65 Log reductions can be observed for L. sakei ACU-2 and S. vitulinus ACU-2, respectively. The specific rate of degradation for the lactobacilli (0.0044 ± 0.0010 day-1) was significantly lower than the one for the staphylococci (0.0088 ± 0.0012 day-1), thus evidencing lower stability of the latter. Despite this difference, the residual viability of both bacteria was high (7.59 ± 0.07 and 7.08 ± 0.01 CFU/mL for the bacilli and the cocci, respectively). These results suggested that freeze-dried bacteria from the starter culture SAS-1 imbibed in yacon juice were stable after six months of storage time at 4 °C.
Log (N/N0) from lyophilized L. sakei ACU-2 (●) and S. vitulinus ACU-10 (Δ) in 10 mL/100 mL yacon juice as a function of storage time at 4 °C.
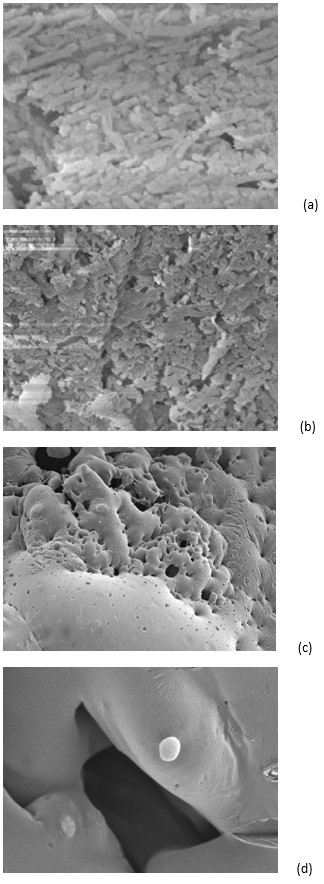
The protectant effect reckoned could be supported by microscope observation of freeze-dried samples (Figure 3). The appearance of freeze-dried cultures in water (Figure 3a-3b) appeared less dense than that the freeze-dried cultures in yacon juice (Figure 3c-3d). The freeze-dried cocci with yacon juice were visible under SEM (Figure 3d), but no lactobacilli cells were clearly seen in the SEM micrographs (Figure 3c). It could be assumed that these cells were hidden inside the vitreous matrix. The latter observation is in keeping with Etchepare et al. (2016)Etchepare, M. A., Raddatz, G. C., Cichoski, A. J., Flores, É. M. M., Barin, J. S., Zepka, L.Q., Jacob-Lopes, E., Grosso, C. R. F., & Menezes, C. R. (2016). Effect of resistant starch (Hi-maize) on the survival of Lactobacillus acidophilus microencapsulated with sodium alginate. Journal of Functional Foods, 21, 321-329. http://dx.doi.org/10.1016/j.jff.2015.12.025
http://dx.doi.org/10.1016/j.jff.2015.12....
, who stated that L. acidophillus La-14 was probably entrapped inside the capsules of Hi-maize.
Scanning electron micrographs (SEM) of freeze-dried samples. (a) L. sakei ACU-2 in water (10000 x). (b) S. vitulinus ACU-10 in water (10000 x). (c) L. sakei ACU-2 in yacon juice (500 x). (d) S. vitulinus ACU-10 in yacon juice (5000 x).
In short words, the protectant effect of yacon juice could be equally attributed to the single sugars and the FOS present in the menstruum. It is consistent with FOS and carbohydrate contents evaluated in the juice (Table 2), which are in keeping with González (2021)González, M. (2021). Optimización microbiológica y sensorial del uso de aceites esenciales en la formulación de un jugo de yacón (Doctoral dissertation). Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires.. The protectant activity of the saccharides is assigned to the ability of these substances to stabilize membranes and proteins by replacing the water surrounding the polar residues within these macromolecules’ structures (Golovina et al., 2009Golovina, E. A., Golovin, A. V., Hoekstra, F. A., & Faller, R. (2009). Water replacement hypothesis in atomic detail-factors determining the structure of dehydrated bilayer stacks. Biophysical Journal, 97(2), 490-499. PMid:19619463. http://dx.doi.org/10.1016/j.bpj.2009.05.007
http://dx.doi.org/10.1016/j.bpj.2009.05....
). Another protective mechanism would be related to the capacity of these sugars to build vitreous matrices. High viscosity and low molecular mobility -characteristics of the amorphous state- delay deteriorative reactions that take place by diffusion (Grasmeijer et al., 2013Grasmeijer, N., Stankovic, M., Waard, H., Frijlink, H. W., & Hinrichs, W. L. J. (2013). Unraveling protein stabilization mechanisms: Vitrification and water replacement in a glass transition temperature controlled system. Biochimica et Biophysica Acta, 1834(4), 763-769. PMid:23360765. http://dx.doi.org/10.1016/j.bbapap.2013.01.020
http://dx.doi.org/10.1016/j.bbapap.2013....
), increasing the physical and chemical stability of the imbibed cells. It has been reported that either the GOS (Tymczyszyn et al., 2012Tymczyszyn, E. E., Sosa, N., Gerbino, E., Hugo, A. A., Gómez-Zavaglia, A., & Schebor, C. (2012). Effect of physical properties on the stability of Lactobacillus bulgaricus in a freeze-dried galacto-oligosaccharides matrix. International Journal of Food Microbiology, 155(3), 217-221. PMid:22410267. http://dx.doi.org/10.1016/j.ijfoodmicro.2012.02.008
http://dx.doi.org/10.1016/j.ijfoodmicro....
; Santos et al., 2014Santos, M., Gerbino, E., Araujo-Andrade, C., Tymczyszyn, E., & Gómez-Zavaglia, A. (2014). Stability of freeze-dried Lactobacillus delbrueckii subsp. Bulgaricus in the presence of galacto-oligosaccharides and lactulose as determined by near infrared spectroscopy. Food Research International, 59, 53-60. http://dx.doi.org/10.1016/j.foodres.2014.01.054
http://dx.doi.org/10.1016/j.foodres.2014...
), as well as the FOS (Romano et al., 2015Romano, N., Tymczyszyn, E., Mobili, A., & Gómez-Zavaglia, A. (2015). Prebiotics as protectants of lactic acid bacteria. In R. R. Watson & V. R. Preedy (Eds.), Bioactive foods in promoting health: Probiotics, prebiotics, and synbiotics (2nd ed., pp. 155-164). London: Academic Press.) can be optimum protectant agents due to their ability to form vitreous matrices. Though cell inactivation is low beneath the vitreous transition temperature (Tg), it does not completely stop (Higl et al., 2007Higl, B., Kurtmann, L., Carlsen, C. U., Ratjen, J., Forst, P., Skibsted, L. H., Kulozik, U., & Risbo, J. (2007). Impact of water activity, temperature, and physical state on the storage stability of Lactobacillus paracasei ssp. paracasei freeze-dried in a lactose matrix. Biotechnology Progress, 23(4), 794-800. PMid:17636886. http://dx.doi.org/10.1002/bp070089d
http://dx.doi.org/10.1002/bp070089d...
); hence, the emergence of this matrix would not be enough to achieve an efficient protection for the cells. In this regard, Romano et al. (2016)Romano, N., Schebor, C., Mobili, P., & Gómez-Zavaglia, A. (2016). Role of mono- and oligosaccharides from FOS as stabilizing agents during freeze-drying and storage of Lactobacillus delbrueckii subsp. bulgaricus. Food Research International, 90, 251-258. PMid:29195879. http://dx.doi.org/10.1016/j.foodres.2016.11.003
http://dx.doi.org/10.1016/j.foodres.2016...
suggested the joint use of polysaccharides of high Tg and simple carbohydrates of low Tg, being the latter able to interact with biological membranes, they constitute a reasonable strategy for bacterial stabilization. These authors reported that the protectant effect of FOS towards L. delbrueckii subsp. bulgaricus CIDCA 333 was the result of the equilibrium between monosaccharides, sucrose and FOS, where the smallest sugars might be more efficient in the protection of lipid membranes while the biggest would contribute to the formation of vitreous states.
4 Conclusion
Along with the results shown herein, the use of yacon juice as an alternative source of nutrients for biomass production and cryopreservation would be promising. Culture media prepared with yacon juice (10 mL/100 mL) could be suitable for both the growth and freeze-drying process of the strains comprising the autochthonous starter culture SAS-1, L. sakei ACU-2 and S. vitulinus ACU-10. Regarding the emphasis given nowadays to functional foods, the introduction of yacon juice into the cryopreservation process would be advantageous to improve its application and future perspectives within the food industry, bearing in mind the health promoting issues of these products, i.e., the presence of FOS from yacon as prebiotic sources to improve beneficial gut microbiota (Scott et al., 2013Scott, K. P., Gratz, S. W., Sheridan, P. O., Flint, H. J., & Duncan, S. H. (2013). The influence of diet on the gut microbiota. Pharmacological Research, 69(1), 52-60. PMid:23147033. http://dx.doi.org/10.1016/j.phrs.2012.10.020
http://dx.doi.org/10.1016/j.phrs.2012.10...
; Caetano et al., 2016Caetano, B. F. R., Moura, N. A., Almeida, A. P. S., Dias, M. C., Sivieri, K., & Barbisan, L. F. (2016). Yacon (Smallanthus sonchifolius) as a food supplement: Health-promoting benefits of fructooligosaccharides. Nutrients, 8(7), 436. PMid:27455312. http://dx.doi.org/10.3390/nu8070436
http://dx.doi.org/10.3390/nu8070436...
; Pacheco et al., 2020Pacheco, T., Hernández-Hernández, O., Moreno, F. J., & Villamiel, M. (2020). Andean tubers grown in Ecuador: New sources of functional ingredients. Food Bioscience, 35, 100601. http://dx.doi.org/10.1016/j.fbio.2020.100601
http://dx.doi.org/10.1016/j.fbio.2020.10...
). Even though the information provided by these results is still scarce, the potential use of yacon juice, or its by-products, could be expanded once media formulation was optimized according to the specific needs of each microorganism.
Acknowledgements
This work was supported by Consejo Nacional de Inestigaciones Científicas y Técnicas de la República Argentina (CONICET) (PIP N°112-201301-00078CO) and Universidad Nacional del Chaco Austral (UNCAus) (PI N° 69).
-
Cite as: Palavecino Prpich, N., Sanabria, E., Gliemmo, M. F., Cayré, M. E., & Castro, M. P. (2023). Yacon juice as culture and cryoprotectant medium for Latilactobacillus sakei and Staphylococcus vitulinus autochthonous strains. Brazilian Journal of Food Technology, 26, e2022119. https://doi.org/10.1590/1981-6723.11922
-
Funding: Ministerio de Ciencia, Tecnología e Innovación Productiva/Agencia Nacional de Promoción Científica y Tecnológica/Fondo para la Investigación Científica y Tecnológica (2018-0290).
References
- Association of Official Analytical Chemistry - AOAC. (2000). Official methods of analysis (17th ed.). Gaithersburg: AOAC.
- Association of Official Analytical Chemistry - AOAC. (2006). Official methods of analysis (18th ed.). Gaithersburg: AOAC.
- Axelsson, L. (2004). Lactic acid bacteria: Classification and physiology. In: S. Salminen V. von Wright & A. Ouwehand (Eds.), Lactic acid bacteria: Microbiological and functional aspects (pp. 1-66). New York: Marcel Dekker Inc. http://dx.doi.org/10.1201/9780824752033.ch1.
- Basholli-Salihu, M., Mueller, M., Salar-Behzadi, S., Unger, F. M., & Viernstein, H. (2014). Effect of lyoprotectants on b-glucosidase activity and viability of Bifidobacterium infantis after freeze-drying and storage in milk and low pH juices. Lebensmittel-Wissenschaft + Technologie, 57(1), 276-282. http://dx.doi.org/10.1016/j.lwt.2014.01.011
» http://dx.doi.org/10.1016/j.lwt.2014.01.011 - Brinques, G. B., Peralba, M. C., & Ayub, M. A. Z. (2010). Optimization of probiotic and lactic acid production by Lactobacillus plantarum in submerged bioreactor systems. Journal of Industrial Microbiology & Biotechnology, 37(2), 205-212. PMid:19936814. http://dx.doi.org/10.1007/s10295-009-0665-1
» http://dx.doi.org/10.1007/s10295-009-0665-1 - Broeckx, G., Vandenheuvel, D., Claes, I. J. J., Lebeer, S., & Kiekens, F. (2016). Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. International Journal of Pharmaceutics, 505(1-2), 303-318. PMid:27050865. http://dx.doi.org/10.1016/j.ijpharm.2016.04.002
» http://dx.doi.org/10.1016/j.ijpharm.2016.04.002 - Caetano, B. F. R., Moura, N. A., Almeida, A. P. S., Dias, M. C., Sivieri, K., & Barbisan, L. F. (2016). Yacon (Smallanthus sonchifolius) as a food supplement: Health-promoting benefits of fructooligosaccharides. Nutrients, 8(7), 436. PMid:27455312. http://dx.doi.org/10.3390/nu8070436
» http://dx.doi.org/10.3390/nu8070436 - Cao, Y., Ma, Z. F., Zhang, H., Jin, Y., Zhang, Y., & Hayford, F. (2018). Phytochemical properties and nutrigenomic implications of yacon as a potential source of prebiotic: Current evidence and future directions. Foods, 7(4), 59. PMid:29649123. http://dx.doi.org/10.3390/foods7040059
» http://dx.doi.org/10.3390/foods7040059 - Carvalho, A. S., Silva, J., Ho, P., Teixeira, P., Malcata, F. X., & Gibbs, P. (2004). Relevant factors for the preparation of freeze-dried lactic acid bacteria. International Dairy Journal, 14(10), 835-847. http://dx.doi.org/10.1016/j.idairyj.2004.02.001
» http://dx.doi.org/10.1016/j.idairyj.2004.02.001 - Chotiko, A., & Sathivel, S. (2014). Effects of enzymatically-extracted purple rice bran fiber as aprotectant of L. plantarum NRRL B-4496 during freezing, freeze drying, and storage. Lebensmittel-Wissenschaft + Technologie, 59(1), 59-64. http://dx.doi.org/10.1016/j.lwt.2014.05.056
» http://dx.doi.org/10.1016/j.lwt.2014.05.056 - Etchepare, M. A., Raddatz, G. C., Cichoski, A. J., Flores, É. M. M., Barin, J. S., Zepka, L.Q., Jacob-Lopes, E., Grosso, C. R. F., & Menezes, C. R. (2016). Effect of resistant starch (Hi-maize) on the survival of Lactobacillus acidophilus microencapsulated with sodium alginate. Journal of Functional Foods, 21, 321-329. http://dx.doi.org/10.1016/j.jff.2015.12.025
» http://dx.doi.org/10.1016/j.jff.2015.12.025 - Golovina, E. A., Golovin, A. V., Hoekstra, F. A., & Faller, R. (2009). Water replacement hypothesis in atomic detail-factors determining the structure of dehydrated bilayer stacks. Biophysical Journal, 97(2), 490-499. PMid:19619463. http://dx.doi.org/10.1016/j.bpj.2009.05.007
» http://dx.doi.org/10.1016/j.bpj.2009.05.007 - González, M. (2021). Optimización microbiológica y sensorial del uso de aceites esenciales en la formulación de un jugo de yacón (Doctoral dissertation). Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires.
- Grasmeijer, N., Stankovic, M., Waard, H., Frijlink, H. W., & Hinrichs, W. L. J. (2013). Unraveling protein stabilization mechanisms: Vitrification and water replacement in a glass transition temperature controlled system. Biochimica et Biophysica Acta, 1834(4), 763-769. PMid:23360765. http://dx.doi.org/10.1016/j.bbapap.2013.01.020
» http://dx.doi.org/10.1016/j.bbapap.2013.01.020 - Haiping, L., Pei, Z., Shuhai, Z., Dengyun, Z., Herong, F., Yi, S., & Xinqian, W. (2019). Protective effect of polysacharides from Pholiata nameko on Lactobacillus casei ATCC 334 subjected to freeze-drying. Lebensmittel-Wissenschaft + Technologie, 115, 108463. http://dx.doi.org/10.1016/j.lwt.2019.108463
» http://dx.doi.org/10.1016/j.lwt.2019.108463 - Higl, B., Kurtmann, L., Carlsen, C. U., Ratjen, J., Forst, P., Skibsted, L. H., Kulozik, U., & Risbo, J. (2007). Impact of water activity, temperature, and physical state on the storage stability of Lactobacillus paracasei ssp. paracasei freeze-dried in a lactose matrix. Biotechnology Progress, 23(4), 794-800. PMid:17636886. http://dx.doi.org/10.1002/bp070089d
» http://dx.doi.org/10.1002/bp070089d - Korakoch, H., Sukyai, P., Loiseau, G., Nitisinprasert, S., Montet, D., & Wanchaitanawong, P. (2005). Prediction on the stability of spray-dried Lactobacillus reuteri KUB-AC5 by Arrhenius equation for long-term storage. Journal of Microbiology and Biotechnology, 15(6), 1178-1182. Retrieved in 2022, December 15, from https://koreascience.kr/article/JAKO200511722550148.pdf
» https://koreascience.kr/article/JAKO200511722550148.pdf - Lapsiri, W., Bhandari, B., & Wanchaitanawong, P. (2012). Viability of Lactobacillus plantarum TISTR 2075 in different protectants during spray drying and storage. Drying Technology, 30(13), 1407-1412. http://dx.doi.org/10.1080/07373937.2012.684226
» http://dx.doi.org/10.1080/07373937.2012.684226 - Liew, S. L., Ariff, A. B., Raha, A. R., & Ho, Y. W. (2005). Optimization of medium composition for the production of a probiotic microorganism, Lactobacillus rhamnosus, using response surface methodology. International Journal of Food Microbiology, 102(2), 137-142. PMid:15992613. http://dx.doi.org/10.1016/j.ijfoodmicro.2004.12.009
» http://dx.doi.org/10.1016/j.ijfoodmicro.2004.12.009 - Meng, X. C., Stanton, C., Fitzgerald, G. F., Daly, C., & Ross, R. P. (2008). Anhydrobiotics: The challenges of drying probiotic cultures. Food Chemistry, 106(4), 1406-1416. http://dx.doi.org/10.1016/j.foodchem.2007.04.076
» http://dx.doi.org/10.1016/j.foodchem.2007.04.076 - Morgan, C. A., Herman, N., White, P. A., & Vesey, G. (2006). Preservation of microorganisms by drying; a review. Journal of Microbiological Methods, 66(2), 183-193. PMid:16632005. http://dx.doi.org/10.1016/j.mimet.2006.02.017
» http://dx.doi.org/10.1016/j.mimet.2006.02.017 - Ojansivu, I., Ferreira, C. L., & Salminen, S. (2011). Yacon, a new source of prebiotic oligosaccharides with a history of safe use. Trends in Food Science & Technology, 22(1), 40-46. http://dx.doi.org/10.1016/j.tifs.2010.11.005
» http://dx.doi.org/10.1016/j.tifs.2010.11.005 - Pacheco, T., Hernández-Hernández, O., Moreno, F. J., & Villamiel, M. (2020). Andean tubers grown in Ecuador: New sources of functional ingredients. Food Bioscience, 35, 100601. http://dx.doi.org/10.1016/j.fbio.2020.100601
» http://dx.doi.org/10.1016/j.fbio.2020.100601 - Palavecino Prpich, N. Z. P., Castro, M. P., Cayré, M. E., Garro, O. A., & Vignolo, G. M. (2015a). Autochthonous starter culture selection to keep traditions in the manufacture of dry sausages alive. Annals of Microbiology, 65(3), 1709-1719. http://dx.doi.org/10.1007/s13213-014-1010-0
» http://dx.doi.org/10.1007/s13213-014-1010-0 - Palavecino Prpich, N. Z. P., Castro, M. P., Cayré, M. E., Garro, O. A., & Vignolo, G. M. (2015b). Indigenous starter cultures to improve quality of artisanal dry fermented sausages from Chaco (Argentina). International Journal of Food Science, 2015, 931970. PMid:26955636. http://dx.doi.org/10.1155/2015/931970
» http://dx.doi.org/10.1155/2015/931970 - Palavecino Prpich, N. Z. P., Garro, O. A., Romero, M., Judis, M. A., Cayré, M. E., & Castro, M. P. (2016). Evaluation of an autochthonous starter culture on the production of a traditional dry fermented sausage from Chaco (Argentina) at a small-scale facility. Meat Science, 115, 41-44. PMid:26820805. http://dx.doi.org/10.1016/j.meatsci.2016.01.005
» http://dx.doi.org/10.1016/j.meatsci.2016.01.005 - Reis, F. R., Marques, C., Moraes, A. C. S., & Masson, M. L. (2021). Effect of processing methods on yacon roots health-promoting compounds and related properties. Trends in Food Science & Technology, 113, 346-354. http://dx.doi.org/10.1016/j.tifs.2021.05.010
» http://dx.doi.org/10.1016/j.tifs.2021.05.010 - Romano, N., Schebor, C., Mobili, P., & Gómez-Zavaglia, A. (2016). Role of mono- and oligosaccharides from FOS as stabilizing agents during freeze-drying and storage of Lactobacillus delbrueckii subsp. bulgaricus. Food Research International, 90, 251-258. PMid:29195879. http://dx.doi.org/10.1016/j.foodres.2016.11.003
» http://dx.doi.org/10.1016/j.foodres.2016.11.003 - Romano, N., Tymczyszyn, E., Mobili, A., & Gómez-Zavaglia, A. (2015). Prebiotics as protectants of lactic acid bacteria. In R. R. Watson & V. R. Preedy (Eds.), Bioactive foods in promoting health: Probiotics, prebiotics, and synbiotics (2nd ed., pp. 155-164). London: Academic Press.
- Santos, M., Gerbino, E., Araujo-Andrade, C., Tymczyszyn, E., & Gómez-Zavaglia, A. (2014). Stability of freeze-dried Lactobacillus delbrueckii subsp. Bulgaricus in the presence of galacto-oligosaccharides and lactulose as determined by near infrared spectroscopy. Food Research International, 59, 53-60. http://dx.doi.org/10.1016/j.foodres.2014.01.054
» http://dx.doi.org/10.1016/j.foodres.2014.01.054 - Scott, K. P., Gratz, S. W., Sheridan, P. O., Flint, H. J., & Duncan, S. H. (2013). The influence of diet on the gut microbiota. Pharmacological Research, 69(1), 52-60. PMid:23147033. http://dx.doi.org/10.1016/j.phrs.2012.10.020
» http://dx.doi.org/10.1016/j.phrs.2012.10.020 - Sousa, S., Pinto, J., Pereira, C. F., Malcata, F. X., Pacheco, M. T. B., Gomes, A. M., & Pintado, M. (2015). In vitro evaluation of yacon (Smallanthus sonchifolius) tuber flour prebiotic potential. Food and Bioproducts Processing, 95, 96-105. http://dx.doi.org/10.1016/j.fbp.2015.04.003
» http://dx.doi.org/10.1016/j.fbp.2015.04.003 - Tymczyszyn, E. E., Sosa, N., Gerbino, E., Hugo, A. A., Gómez-Zavaglia, A., & Schebor, C. (2012). Effect of physical properties on the stability of Lactobacillus bulgaricus in a freeze-dried galacto-oligosaccharides matrix. International Journal of Food Microbiology, 155(3), 217-221. PMid:22410267. http://dx.doi.org/10.1016/j.ijfoodmicro.2012.02.008
» http://dx.doi.org/10.1016/j.ijfoodmicro.2012.02.008 - Zuleta, A., & Sambucetti, M. E. (2001). Inulin determination for food labeling. Journal of Agricultural and Food Chemistry, 49(10), 4570-4572. PMid:11599989. http://dx.doi.org/10.1021/jf010505o
» http://dx.doi.org/10.1021/jf010505o - Zwietering, M. H., Jongenburger, I., Rombouts, F. M., & van 't Riet, K. (1990). Modeling of the bacterial growth curve. Applied and Environmental Microbiology, 56(6), 1875-1881. PMid:16348228. http://dx.doi.org/10.1128/aem.56.6.1875-1881.1990
» http://dx.doi.org/10.1128/aem.56.6.1875-1881.1990
Edited by
Publication Dates
-
Publication in this collection
06 Feb 2023 -
Date of issue
2023
History
-
Received
02 Oct 2022 -
Accepted
15 Dec 2022