Abstract
This study aimed to investigate the extraction of total phenolic compounds of sugarcane bagasse using various solvents. In addition, the Sugarcane Bagasse Water Extract (SBWE) was used in refrigerated fresh meat as natural preservative. The fresh meat was dipped into water solutions containing various phenolic compounds concentrations (T1:125, T2:250 and T3:500 ppm). During 10 days of storage at 4 °C for all the treated samples were compared with untreated one. The results revealed that SBWE showed relevant values of total phenolic compounds (17.90 mg/g) and total flavonoids content (4.50 mg/g), as well as 45.90% of antioxidant content. On the other hand, microbiological examination and sensory evaluation have turned out to be the best treatment for T3:500 ppm. The SBWE showed an antibacterial impact on Staphylococcus sp. and a reduction in the Total Plate Count and in the group of Psychrotrophs. The shelf-life of refrigerated fresh meat treated with SBWE by dipping it into water solutions was also extended to more than 10 days.
Keywords:
Sugarcane bagasse; Phenolic compounds; Fresh meat; Dipping; Extraction; Pathogenic bacteria
Resumo
O objetivo deste estudo foi investigar a extração de compostos fenólicos totais do bagaço de cana usando vários solventes. Além disso, o extrato aquoso de bagaço de cana (EABC) foi utilizado como conservante natural na carne fresca refrigerada. A carne fresca foi mergulhada em soluções aquosas contendo várias concentrações de compostos fenólicos (T1: 125, T2: 250 e T3: 500 ppm). Durante 10 dias de armazenamento a 4 °C, todas as amostras tratadas foram comparadas com a carne sem tratamento. Os resultados revelaram que o EABC apresentou valores relevantes de compostos fenólicos totais (17,90 mg/g), flavonoides totais (4,50 mg/g) e 45,90% de conteúdo antioxidante. Avaliações microbiológica e sensorial indicaram que os melhores resultados foram obtidos com o tratamento T3: 500 ppm. O EABC apresentou efeito antibacteriano em Staphylococcus sp. e redução na contagem total e no grupo de psicrotróficos. A vida útil da carne fresca refrigerada tratada com EABC por imersão em solução aquosa foi estendida para mais de 10 dias.
Palavras-chave:
Bagaço de cana; Compostos fenólicos; Carne fresca; Mergulho; Extração; Bactéria patogênica
1 Introduction
Sugarcane is one of the most important crops, and the production of sugarcane generates a huge quantity of by-products and waste that may constitute a substantial extraction of useful compounds. Several products can be produced from it, as following: (1) sucrose, syrups and jaggery (as a source of food); (2) cellulose (as a source of fiber); (3) bagasse, alcohol and molasses (as a source of fuel and chemicals) (4) green leaves and sugarcane tops (as a source of fodder); (5) pressing mud and washing (as a source of fertilizer); (6) bagasse extract was utilized as natural source of phenolic compounds, antioxidants and antimicrobial agents (Solomon, 2011Solomon, S. (2011). Sugarcane by-products based industries in India. Sugar Tech, 13(4), 408-416. http://dx.doi.org/10.1007/s12355-011-0114-0
http://dx.doi.org/10.1007/s12355-011-011...
).Sugarcane bagasse is known to be one of the by-products from the sugar industry and commercial products and also being the most abundant agro-industrial by-products, with a global output of over 540 million tons per year (Bezerra & Ragauskas, 2016Bezerra, T. L., & Ragauskas, A. J. (2016). A review of sugarcane bagasse for second‐generation bioethanol and biopower production. Biofuels, Bioproducts & Biorefining, 10(5), 634-647. http://dx.doi.org/10.1002/bbb.1662
http://dx.doi.org/10.1002/bbb.1662...
). Sugarcane bagasse is the fibrous debris and also the product of grinding sugarcane. Due to the high moisture content (50%), it is named 'mill wet bagasse' after the sugarcane milling stage. Bagasse accounts for around one-third of cane land. The dry bagasse is made of cellulose (45%), hemicellulose (28%), lignin (20%), sugar (5%), minerals (1%), and ash (2%). Cellulose (45%) and pentosanes (28%) are the principal components of dry bagasse (Nikodinovic-Runic et al., 2013Nikodinovic-Runic, J., Guzik, M., Kenny, S. T., Babu, R., Werker, A., & O’Connor, K. E. (2013). Carbon-rich wastes as feedstocks for biodegradable polymer (polyhydroxyalkanoate) production using bacteria. Advances in Applied Microbiology, 84, 139-200. PMid:23763760. http://dx.doi.org/10.1016/B978-0-12-407673-0.00004-7
http://dx.doi.org/10.1016/B978-0-12-4076...
); while the composition of the aqueous extract of sugarcane bagasse contains an average 38.83% of cellulose, 27.32% of hemicellulose, 25.92% of lignin, 0.43% of ash and 10.24% of extractives (Philippini et al., 2019Philippini, R. R., Martiniano, S. E., Chandel, A. K., Carvalho, W., & Silva, S. S. (2019). Pretreatment of sugarcane bagasse from cane hybrids: Effects on chemical composition and 2G sugars recovery. Waste and Biomass Valorization, 10(6), 1561-1570. http://dx.doi.org/10.1007/s12649-017-0162-0
http://dx.doi.org/10.1007/s12649-017-016...
). Carvalho et al. (2009)Carvalho, W., Canilha, L., Castro, P., & Barbosa, L. (2009). Composition of the sugarcane bagasse. In 31st Symposium on Biotechnology for Fuels and Chemicals. San Francisco. reported that sugarcane bagasse aqueous extract contains about 42.50% of cellulose, 20.90% of lignin, 24.88% of hemicelluloses, 1.64% of ash and 5.83% of extractives.
The phenolic quality of bagasse is already being utilized by both researchers as a natural preservative source that has greatly prevented the production of foodborne pathogens. According to Zhao et al. (2015)Zhao, Y., Chen, M., Zhao, Z., & Yu, S. (2015). The antibiotic activity and mechanisms of sugarcane (Saccharum officinarum L.) bagasse extract against food-borne pathogens. Food Chemistry, 185, 112-118. PMid:25952848. http://dx.doi.org/10.1016/j.foodchem.2015.03.120
http://dx.doi.org/10.1016/j.foodchem.201...
, phenolic content of sugarcane bagasse produced more than 4 mg/g of dry bagasse. It may also be used against other pathogenic bacteria organisms such as Staphylococcus aureus, Listeria monocytogenes, Escherichia coli and Salmonella typhimurium as a natural product with bacteriostatic operation. In fact, sugarcane bagasse extract improved the electrical conductivity of suspensions in bacterial cells, inducing Electrolyte Leakage (EL) in the cell (Zhao et al., 2015Zhao, Y., Chen, M., Zhao, Z., & Yu, S. (2015). The antibiotic activity and mechanisms of sugarcane (Saccharum officinarum L.) bagasse extract against food-borne pathogens. Food Chemistry, 185, 112-118. PMid:25952848. http://dx.doi.org/10.1016/j.foodchem.2015.03.120
http://dx.doi.org/10.1016/j.foodchem.201...
).
Therefore, phenolic compounds can scavenge free radicals, meaning that the residue has antioxidant function on the vegetable matrix. Many research have lately been focused on the effectiveness of phenolic substances such as anti-angiogenic, anti-obesity, anticancer, anti-arthritic, antiviral and anti-inflammatory activities as well as cardiovascular, immunomodulatory neuroprotective effects, and diabetes (Munro et al., 2015Munro, B., Vuong, Q. V., Chalmers, A. C., Goldsmith, C. D., Bowyer, M. C., & Scarlett, C. J. (2015). Phytochemical, antioxidant and anti-cancer properties of Euphorbia tirucalli methanolic and aqueous extracts. Antioxidants, 4(4), 647-661. PMid:26783950. http://dx.doi.org/10.3390/antiox4040647
http://dx.doi.org/10.3390/antiox4040647...
; Tsuda, 2016Tsuda, T. (2016). Recent progress in anti-obesity and anti-diabetes effect of berries. Antioxidants, 5(2), 13. PMid:27058561. http://dx.doi.org/10.3390/antiox5020013
http://dx.doi.org/10.3390/antiox5020013...
). In addition, there is another possible use in the food industry of phenolic compounds as preservative content to improve nutritional value and increase food shelf-life (Rai et al., 2016Rai, S., Dutta, P. K., & Mehrotra, G. K. (2016). Agrowaste derived phenolic compounds as additives to chitosan film for food packaging applications: Antibacterial and antioxidant study. Journal of the Indian Chemical Society, 93, 1-8.). Different methods were used to facilitate the efficient extraction of polyphenols as valuable compounds from plant sources such as Supercritical Fluid Extraction (SFE), microwave, Pressurized Fluid Extraction (PFE), Subcritical Water Extraction (SWE), Ultrasound-Assisted Extraction (UAE) and Accelerated Solvent Extraction (ASE) (Dai & Mumper, 2010Dai, J., & Mumper, R. J. (2010). Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules (Basel, Switzerland), 15(10), 7313-7352. PMid:20966876. http://dx.doi.org/10.3390/molecules15107313
http://dx.doi.org/10.3390/molecules15107...
). Solvents have been used as one of the efficient methods to recover polyphenols from their sources, such as ethanol, methanol and/or water, depending on the polarity, cost and safety of the extracted compounds (Do et al., 2014Do, Q. D., Angkawijaya, A. E., Tran-Nguyen, P. L., Huynh, L. H., Soetaredjo, F. E., Ismadji, S., & Ju, Y. H. (2014). Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. Yao Wu Shi Pin Fen Xi, 22(3), 296-302. PMid:28911418.; Smuda et al., 2018Smuda, S. S., Mohsen, S. M., Olsen, K., & Aly, M. H. (2018). Bioactive compounds and antioxidant activities of some cereal milling by-products. Journal of Food Science and Technology, 55(3), 1134-1142. PMid:29487456. http://dx.doi.org/10.1007/s13197-017-3029-2
http://dx.doi.org/10.1007/s13197-017-302...
).
Meat and meat products are an important source of several essential nutrients especially high biological value protein, which also represents the main cause for its biological spoilage (Gill & Gill, 2015Gill, A. O., & Gill, C. O. (2015). Developments in sampling and test methods for pathogens in fresh meat. In J. Sofos (Ed.), Advances in microbial food safety (pp. 257-280). Philadelphia: Woodhead Publishing. http://dx.doi.org/10.1533/9781782421153.3.257.
http://dx.doi.org/10.1533/9781782421153....
). There are two main factors affecting the commercial life of fresh meat as following: a) the growth of bacteria, which cause changes for organoleptic properties of meat products; b) moisture loss and mechanical damage during transport, distribution and storage (Fernández‐Pan et al., 2012Fernández‐Pan, I., Royo, M., & Ignacio Mate, J. (2012). Antimicrobial activity of whey protein isolate edible films with essential oils against food spoilers and foodborne pathogens. Journal of Food Science, 77(7), M383-M390. PMid:22671770. http://dx.doi.org/10.1111/j.1750-3841.2012.02752.x
http://dx.doi.org/10.1111/j.1750-3841.20...
).
Meat products are one of the most food industries using chemical preservatives (sodium nitrite or sodium nitrate) to extend the shelf-life, which acts as antimicrobial agents against many pathogenic bacteria, seeing that it causes health problems. Recently, different studies have been investigated the use of natural preservatives such as phytochemicals (mainly phenolic compounds), which showed significant antibacterial, antiviral, and antiseptic effects (Li et al., 2010Li, X., Yao, S., Tu, B., Li, X., Jia, C., & Song, H. (2010). Determination and comparison of flavonoids and anthocyanins in Chinese sugarcane tips, stems, roots and leaves. Journal of Separation Science, 33(9), 1216-1223. PMid:20235128. http://dx.doi.org/10.1002/jssc.200900567
http://dx.doi.org/10.1002/jssc.200900567...
).
The agribusiness encompasses an important economic activity in the world, and sugarcane bagasse was used as a strengthening in phenolic thermoset matrices. Therefore, this study aimed (i) to convert fiber from plant waste into a usable resource (sugarcane bagasse extract) by using different solvents; and (ii) to treat the refrigerated fresh meat by aqueous sugarcane bagasse extract as a neutral preservative to extend the shelf-life.
2 Materials and methods
2.1 Chemicals
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) and all solvents were obtained from Sigma Chemical Company (St. Louis, Mo. USA). Aluminum chloride, Folin reagent, Gallic acid, potassium acetate and catechin were obtained from Fluka Chemie AG (Buchs, Switzerland). Plate Count Agar (PCA) and mannitol salt agar gar were obtained from Merck (Darmstadt, Germany).
2.2 Raw material
Sugarcane bagasse was collected from El-Hawamdeya Sugar Plant (Giza, Egypt). The sugarcane bagasse was dried at 65 °C for 24 h, milled and then screened (by a sieve with 0.50 mm). Finally, the particle size less than 0.50 mm was collected and kept dry at -20 °C in glass bottles of 250 mL with Blue Screw Cap autoclavable and sealed (Glass Schott Duran Bottle).
2.3 Preparation of sugarcane bagasse extract
The phytochemical profiles of Dried Sugarcane Bagasse (DSB) were extracted using different solvents as follows: Water Extract (WE); Methanol Extract (ME 95%); Ethanol Extract (EE 95%); Water-Ethanol Extract (WEE, 1:2); and Water-Methanol Extract (WME, 1:2) for 24 h at 4 °C. The DSB extracts were centrifuged at 1000g for 10 min and then filtrated. After that, the supernatant for each solvent was concentrated using a rotary evaporator (EYELA, SB-1000, Rikakikai Co. Ltd., Tokyo, Japan) at 65 °C for a different time to quantify the volume of sugarcane bagasse extract.
2.4 Phytochemical analysis of sugarcane bagasse extracts
2.4.1 Total Phenolic Compound (TPC)
Total Phenolic Compound (TPC) was carried out by the Folin-Ciocalteu method according to Altemimi et al. (2021)Altemimi, A. B., Al-Hilphy, A. R., Abedelmaksoud, T. G., Aboud, S. A., Badwaik, L. S., Noore, S., & Pratap-Singh, A. (2021). Infrared radiation favorably influences the quality characteristics of key lime juice. Applied Sciences, 11(6), 2842. http://dx.doi.org/10.3390/app11062842
http://dx.doi.org/10.3390/app11062842...
with some modification. In brief, 1 mL of each sample (extracts before evaporation) was added to a test tube and then 1 mL of Folin reagent was added. After 3 min, 1 mL of sodium carbonate (7.50%) was added. The mixture was stood for 1 h in the dark and the absorbance at 760 nm was measured. The TPC was calculated using a standard curve of gallic acid and expressed as mg gallic acid/g sample.
2.4.2 Total Flavonoids Content (TFC)
The Total Flavonoids Content (TFC) was carried out using the method of Abedelmaksoud et al. (2019a)Abedelmaksoud, T. G., Mohsen, S. M., Duedahl-Olesen, L., Elnikeety, M. M., & Feyissa, A. H. (2019a). Optimization of ohmicsonication for overall quality characteristics of NFC apple juice. Journal of Food Processing and Preservation, 43(9), e14087. http://dx.doi.org/10.1111/jfpp.14087
http://dx.doi.org/10.1111/jfpp.14087...
. In brief, 0.25 mL of each sample was added to 0.75 mL of methanol and then 50 μL of aluminum chloride 10% and 50 μL of potassium acetate were added. The mixture was completed to 1.40 mL with distilled water and kept for 40 min in the dark. The absorbance was measured at 430 nm. The TFC was calculated using a standard curve of catechin acid and expressed as mg catechin/g sample.
2.4.3 Antioxidant activity
The antioxidant activity of each sample was evaluated according to DPPH method according to El-Mogy et al. (2019)El-Mogy, M. M., Ali, M. R., Darwish, O. S., & Rogers, H. J. (2019). Impact of salicylic acid, abscisic acid, and methyl jasmonate on postharvest quality and bioactive compounds of cultivated strawberry fruit. Journal of Berry Research, 9(2), 333-348. http://dx.doi.org/10.3233/JBR-180349
http://dx.doi.org/10.3233/JBR-180349...
and calculated according to Equation 1:
2.5 Preparation of meat samples
Regarding the preparation of meat samples, four samples were used. Three of them were dipped into the sugarcane bagasse extract (WE) at different concentrations of TPC for 2 min, filtered and then packaged in polypropylene packages (T1: meat sample treated with sugarcane bagasse extract containing 125 ppm of TPC, T2: meat sample treated with sugarcane bagasse extract containing 250 ppm of TPC, and T3: meat sample treated with sugarcane bagasse extract containing 500 ppm of TPC and the last one is a control (fresh meat sample). All treated samples were stored at 4 °C for 10 days.
2.6 Microbiological analysis
Thirty grams of meat were aseptically taken from each sample and homogenized with Peptone water (0.10%) to reach a final dilution at 1/10 in a Lab-Blender for 3 min. Serial dilutions were made using the same diluents and then plated each dilution in duplicate for bacterial counts. Total plate count was determined by PCA after 48 h incubation at 30 °C (Abedelmaksoud et al., 2019bAbedelmaksoud, T. G., Mohsen, S. M., Duedahl‐Olesen, L., Elnikeety, M. M., & Feyissa, A. H. (2019b). Impact of ohmicsonication treatment on pectinmethylesterase in not-from-concentrate orange juice. Journal of Food Science and Technology, 56(8), 3951-3956. PMid:31413420. http://dx.doi.org/10.1007/s13197-019-03834-2
http://dx.doi.org/10.1007/s13197-019-038...
). Psychrotrophs were determined by PCA after 10 days of incubation at 7 °C. Staphylococcus sp. was determined by using isolates from mannitol salt agar after 48 h incubation at 37 °C (Busta et al., 2003Busta, F. F., Suslow, T. V., Parish, M. E., Beuchat, L. R., Farber, J. N., Garrett, E. H., & Harris, L. J. (2003). The use of indicators and surrogate microorganisms for the evaluation of pathogens in fresh and fresh‐cut produce. Comprehensive Reviews in Food Science and Food Safety, 2(s1), 179-185. http://dx.doi.org/10.1111/j.1541-4337.2003.tb00035.x
http://dx.doi.org/10.1111/j.1541-4337.20...
).
2.7 Sensory evaluation
Samples were assessed for several sensory characteristics by 50 members of Faculty of Agriculture, Cairo University, Egypt. They were selected regarding the basis of experience and interest in sensory evaluation. Panelists were instructed to evaluate color, smell and appearance using 9-point hedonic scale for grading the quality of samples where nine (9) points indicated the highest acceptability. On the other hand, five (5) points indicated unacceptable samples (Berry, 1992Berry, B. W. (1992). Low fat level effects on sensory, shear, cooking, and chemical properties of ground beef patties. Journal of Food Science, 57(3), 537. http://dx.doi.org/10.1111/j.1365-2621.1992.tb08037.x
http://dx.doi.org/10.1111/j.1365-2621.19...
).
2.8 Statistical analysis
The experiments were carried out in triplicate for each treatment and the statistical analysis system used in this research was SPSS v.22 (SPSS Inc., Chicago, IL, USA). Duncan’s multiple comparison tests were used to compare means (differences were considered significant at p < 0.05).
3 Results and discussion
3.1 Effect of different solvents on the extraction of phytochemical profiles from sugarcane bagasse
The presented data in Table 1 showed the effect of different solvents on TPC, TFC and antioxidant activity of dried sugarcane bagasse extracts.
The highest TPC of DSB was obtained with WME (21.20) followed by WEE (19.50) and WE (17.90). In addition, the results showed that antioxidant activity % values of DSB were 54, 51.10 and 45.90 with WME, WEE and WE, respectively. The TFC of the samples was consistent with the previous results of 6.50, 5.90 and 4.50 mg/g of DSB for WME, WEE and WE, respectively. On the other hand, ME 95% and EE 95% observed the lowest values for TPC, TFC and antioxidant activity %. From Table 1, the results showed that the polarity had a clear effect on the extraction efficiency of TPC. Indeed, it could be noted that the concentrated solvents were the least efficient in the extraction. On the other hand, mixing both ethanol and methanol with water led to an increase in the hydrogen bonds, thus increasing the extraction efficiency (Zheng et al., 2017Zheng, R., Su, S., Li, J., Zhao, Z., Wei, J., Fu, X., & Liu, R. H. (2017). Recovery of phenolics from the ethanolic extract of sugarcane (Saccharum officinarum L.) baggase and evaluation of the antioxidant and antiproliferative activities. Industrial Crops and Products, 107, 360-369. http://dx.doi.org/10.1016/j.indcrop.2017.05.050
http://dx.doi.org/10.1016/j.indcrop.2017...
). With respect to Zhao et al. (2015)Zhao, Y., Chen, M., Zhao, Z., & Yu, S. (2015). The antibiotic activity and mechanisms of sugarcane (Saccharum officinarum L.) bagasse extract against food-borne pathogens. Food Chemistry, 185, 112-118. PMid:25952848. http://dx.doi.org/10.1016/j.foodchem.2015.03.120
http://dx.doi.org/10.1016/j.foodchem.201...
, they reported that the flavonoid content was 0.47 g of quercetin/g regarding DSB.
According to the obtained result in Table 1, it could be clarified that WME (1:2) was the best solvent for phytochemicals extraction compared to other used solvents, and this result was probably due to the presence of hydrogen bonds that improves the efficiency of the extraction process of the phenolic compounds and flavonoids, thus increasing the antioxidant activity. Based on previous studies, it has been reported that the most abundant phenolic acids which could be identified using High Performance Liquid Chromatography (HPLC) in sugarcane bagasse water extract were 4.36 mg/g gallic acid, 1.87 mg/g ferulic acid, 1.66 mg/g coumaric acid and 1.63 mg/g chlorogenic acid (Zhao et al., 2015Zhao, Y., Chen, M., Zhao, Z., & Yu, S. (2015). The antibiotic activity and mechanisms of sugarcane (Saccharum officinarum L.) bagasse extract against food-borne pathogens. Food Chemistry, 185, 112-118. PMid:25952848. http://dx.doi.org/10.1016/j.foodchem.2015.03.120
http://dx.doi.org/10.1016/j.foodchem.201...
). Although, it could be observed that the results indicated that the water extract was not the best solvent in the extraction process. However, the water extract was preferred for meat preservation. Indeed, using water as a solvent for the extraction of phenolic compounds depends on natural material which can be nontoxic, safe and low-priced.
3.2 Microbiological assessment
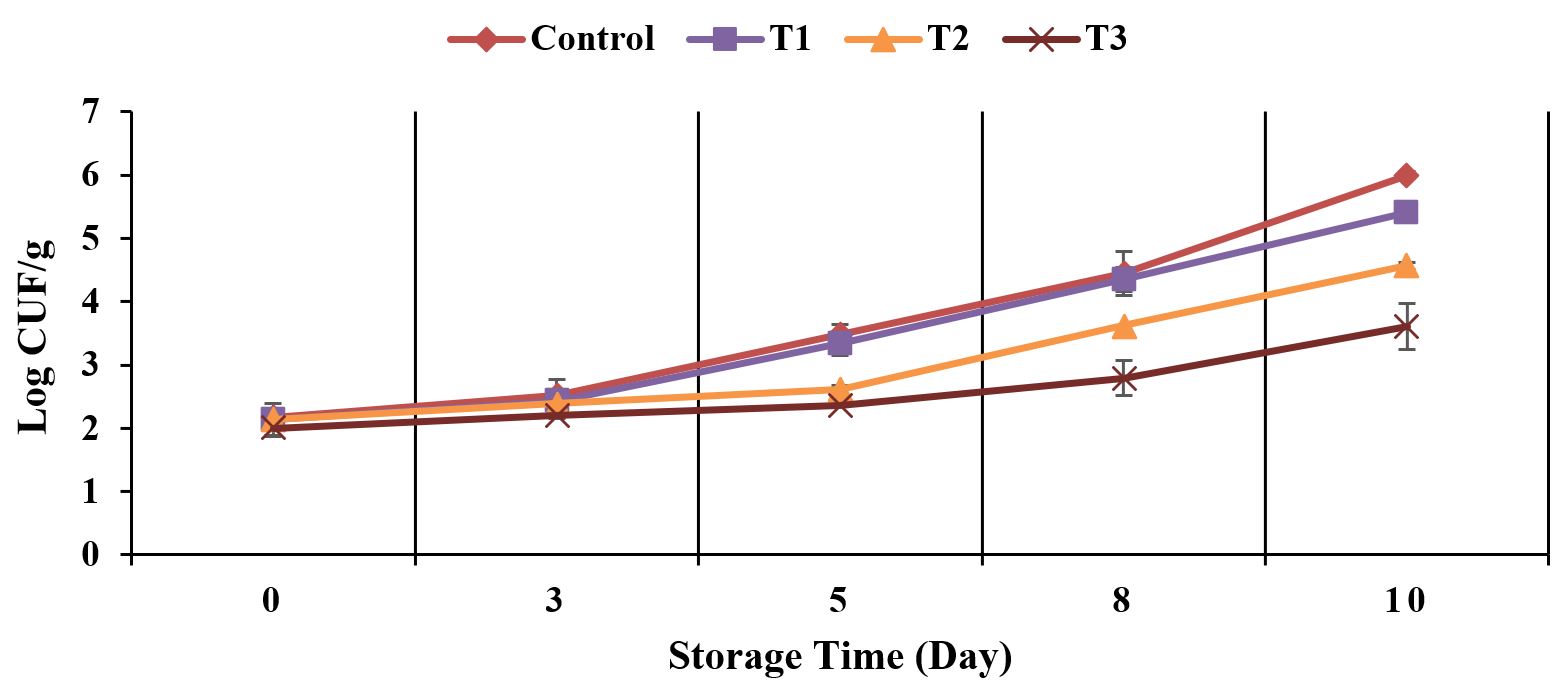
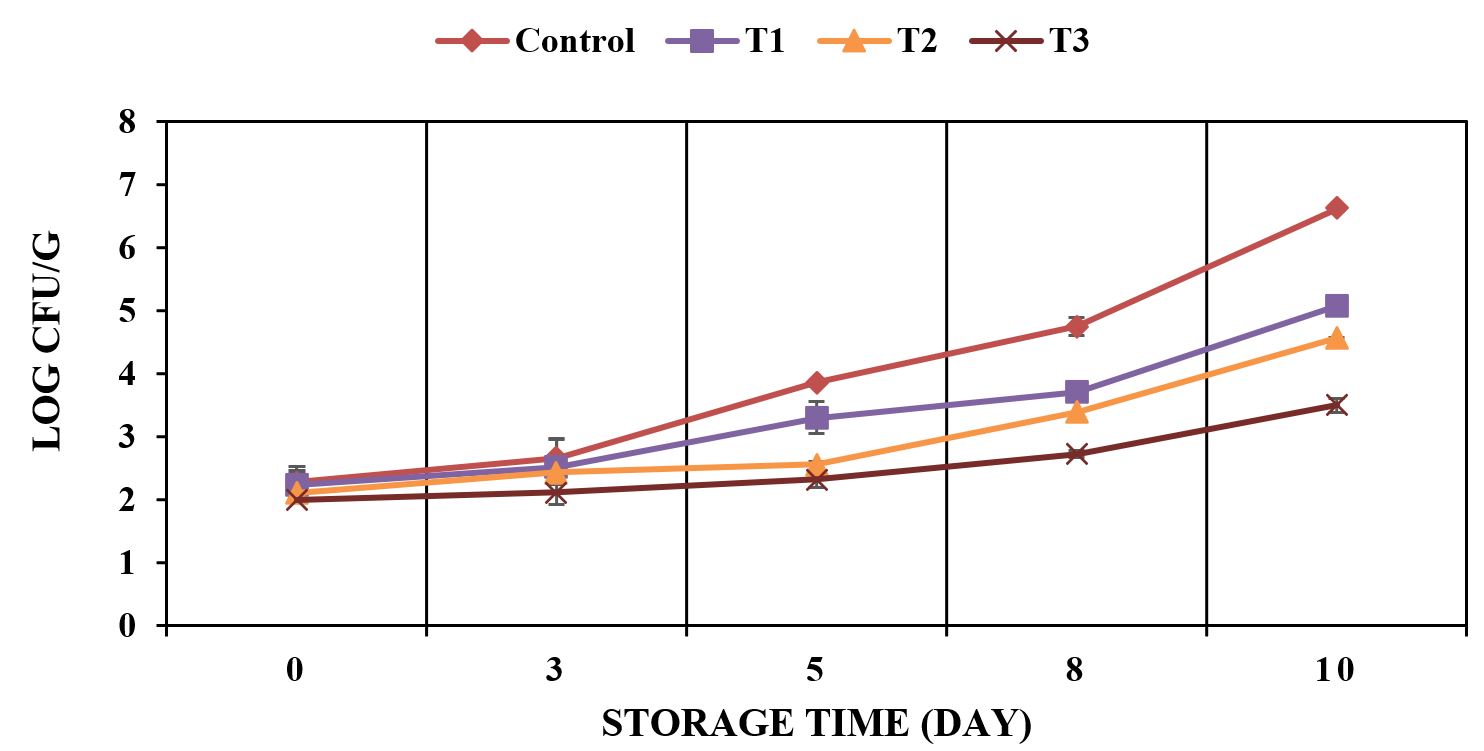
Figures 1 to 3 indicated a microbiological analysis of the treated meat samples (T1: meat sample treated with sugarcane bagasse extract containing 125 ppm of TPC, T2: meat sample treated with sugarcane bagasse extract containing 250 ppm of TPC, and T3: meat sample treated with sugarcane bagasse extract containing 500 ppm of TPC.
Total plate count (log CFU/g) of treated and untreated meat samples during storage at 4 °C.
Psychrotrophic bacterial count (log CFU/g) of treated and untreated meat samples during storage at 4 °C.
Staphylococcus sp. (log CFU/g) of treated and untreated meat samples during storage at 4 °C.
Figures 1 to 3 demonstrated the relationship between the microbial count (total plate count, Staphylococcus sp. and psychrotrophic bacteria) and the concentration of the used polyphenols during storage (4 °C, 10 days). Results indicated no significant (p < 0.05) changes in the initial number of total plate count, Staphylococcus sp. and psychrotrophic bacteria for all treated samples when compared to control at zero time. The effect of phenolic extracts at different concentrations (T1, T2 and T3) could be observed after 3 days of the storage at 4 °C. All treated meat samples were remained in accordance with the guidelines for the maximum limits of total plate count (≤ 7 Log CFU/g) (Korea, 2015Korea. Ministry of Food and Drug Safety – MFDS. (2015). Microbiological examination tip in meat (Notification 11; 2014-135). Korea.) during storage at 4 °C for 10 days. On the other hand, the control exceeded the maximum limits after 8 days of storage. At the end of storage concerning the fresh control meat samples (4 °C, 10 days), total plate count, Staphylococcus sp. and psychrotrophic bacteria were detected in 9.56, 6.62 and 6.00 Log CFU/g, respectively. While a significant reduction in the count of all stated microbiological analysis was observed in T3 (5.33, 3.50 and 3.61 Log CFU/g) followed by T2 (5.94, 4.57 and 4.57 Log CFU/g) and T1 (6.93, 5.08 and 5.41 Log CFU/g), respectively, when compared to control. It was noted significant differences (p < 0.05) between all tested concentrations (125 ppm, 250 ppm, 500 ppm). Bacteriostatic mechanism of the sugarcane bagasse extract may have been attributed to the toxicity of polyphenolic compounds on these microorganisms (Borges et al., 2013Borges, A., Ferreira, C., Saavedra, M. J., & Simoes, M. (2013). Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microbial Drug Resistance, 19(4), 256-265. http://dx.doi.org/10.1089/mdr.2012.0244
http://dx.doi.org/10.1089/mdr.2012.0244...
; Abbas et al., 2014Abbas, S. R., Sabir, S. M., Ahmad, S. D., Boligon, A. A., & Athayde, M. L. (2014). Phenolic profile, antioxidant potential and DNA damage protecting activity of sugarcane (Saccharum officinarum). Food Chemistry, 147, 10-16. PMid:24206679. http://dx.doi.org/10.1016/j.foodchem.2013.09.113
http://dx.doi.org/10.1016/j.foodchem.201...
).
The sugarcane bagasse extract (SCBE) has also shown bacteriostatic activity against the growth of variable strains such as S. aureus, L. monocytogenes, E. coli and S. typhimurium. Additionally, the antibacterial effect was possibly underestimated insofar as the SCBE may inactivate cellular proteins. The SCBE can increase the electrical conductivity of bacterial cell suspensions and induce EL in the cell (Zheng et al., 2017Zheng, R., Su, S., Li, J., Zhao, Z., Wei, J., Fu, X., & Liu, R. H. (2017). Recovery of phenolics from the ethanolic extract of sugarcane (Saccharum officinarum L.) baggase and evaluation of the antioxidant and antiproliferative activities. Industrial Crops and Products, 107, 360-369. http://dx.doi.org/10.1016/j.indcrop.2017.05.050
http://dx.doi.org/10.1016/j.indcrop.2017...
).
3.3 Sensory evaluation
The data presented in Table 2 indicated that a sensory analysis of both samples concerning treated and untreated meat was compatible with the microbiological assessment findings. Sensory evaluation of all samples (treated and untreated meat) during storage (4 °C, 10 days) was analyzed in terms of color and odor, and appearance. No substantial differences were observed between all samples except from the control and T1 after 3 days of storage, with respect to odor. This fact may be associated to all treated samples that were below the maximum limits of acceptable level of microorganisms present in meat (below 7 Log CFU/g), meanwhile the control sample surpassed that limit and reached 8 Log CFU/g after 8 days of storage, and at that limit the odor begins to develop 9 (Barnes, 1957Barnes, E. M. (1957). New methods in food preservation. (a) Antibiotics. Royal Society of Health Journal, 77(8), 446-457. PMid:13454642.). On the other hand, there were significant differences between all the samples after storage for 5, 8 and 10 days, except from T3, which was the best sample regarding all sensory evaluation terms. Increasing the concentration of phenolic compounds resulted in inhibiting microbial growth, which kept the sensorial properties at acceptable levels to consumers and led to extend shelf-life.
4 Conclusion
The obtained results provided that water was the appropriate solvent to extract phenolic compounds from dried sugarcane bagasse. Meat sample treated with sugarcane bagasse extract by water containing 500 ppm of TPC was the best treatment, which resulted in relevant preservation effect (low microbial count) and enhancement of sensorial properties. Therefore, the shelf-life of fresh meat was extended to more than 10 days at 4 °C. Finally, sugarcane bagasse water extract (with high content of phenolic compounds) could be considered as one of the new technologies used for preserving fresh meat. Moreover, sugarcane bagasse extracts required further experiments for being tested in other food products.
Acknowledgements
All authors would like to thank Food Science Department and Food Processing Technology Program (in English) at Faculty of Agriculture, Cairo University, in Egypt for conducting experiments in the laboratories.
-
Cite as: Mohamed, R. M., Ali, M. R., Smuda, S. S., & Abedelmaksoud, T. G. (2021). Utilization of sugarcane bagasse aqueous extract as a natural preservative to extend the shelf life of refrigerated fresh meat. Brazilian Journal of Food Technology, 24, e2020167. https://doi.org/10.1590/1981-6723.16720
-
Funding: None.
References
- Abbas, S. R., Sabir, S. M., Ahmad, S. D., Boligon, A. A., & Athayde, M. L. (2014). Phenolic profile, antioxidant potential and DNA damage protecting activity of sugarcane (Saccharum officinarum). Food Chemistry, 147, 10-16. PMid:24206679. http://dx.doi.org/10.1016/j.foodchem.2013.09.113
» http://dx.doi.org/10.1016/j.foodchem.2013.09.113 - Abedelmaksoud, T. G., Mohsen, S. M., Duedahl-Olesen, L., Elnikeety, M. M., & Feyissa, A. H. (2019a). Optimization of ohmicsonication for overall quality characteristics of NFC apple juice. Journal of Food Processing and Preservation, 43(9), e14087. http://dx.doi.org/10.1111/jfpp.14087
» http://dx.doi.org/10.1111/jfpp.14087 - Abedelmaksoud, T. G., Mohsen, S. M., Duedahl‐Olesen, L., Elnikeety, M. M., & Feyissa, A. H. (2019b). Impact of ohmicsonication treatment on pectinmethylesterase in not-from-concentrate orange juice. Journal of Food Science and Technology, 56(8), 3951-3956. PMid:31413420. http://dx.doi.org/10.1007/s13197-019-03834-2
» http://dx.doi.org/10.1007/s13197-019-03834-2 - Altemimi, A. B., Al-Hilphy, A. R., Abedelmaksoud, T. G., Aboud, S. A., Badwaik, L. S., Noore, S., & Pratap-Singh, A. (2021). Infrared radiation favorably influences the quality characteristics of key lime juice. Applied Sciences, 11(6), 2842. http://dx.doi.org/10.3390/app11062842
» http://dx.doi.org/10.3390/app11062842 - Barnes, E. M. (1957). New methods in food preservation. (a) Antibiotics. Royal Society of Health Journal, 77(8), 446-457. PMid:13454642.
- Berry, B. W. (1992). Low fat level effects on sensory, shear, cooking, and chemical properties of ground beef patties. Journal of Food Science, 57(3), 537. http://dx.doi.org/10.1111/j.1365-2621.1992.tb08037.x
» http://dx.doi.org/10.1111/j.1365-2621.1992.tb08037.x - Bezerra, T. L., & Ragauskas, A. J. (2016). A review of sugarcane bagasse for second‐generation bioethanol and biopower production. Biofuels, Bioproducts & Biorefining, 10(5), 634-647. http://dx.doi.org/10.1002/bbb.1662
» http://dx.doi.org/10.1002/bbb.1662 - Borges, A., Ferreira, C., Saavedra, M. J., & Simoes, M. (2013). Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microbial Drug Resistance, 19(4), 256-265. http://dx.doi.org/10.1089/mdr.2012.0244
» http://dx.doi.org/10.1089/mdr.2012.0244 - Busta, F. F., Suslow, T. V., Parish, M. E., Beuchat, L. R., Farber, J. N., Garrett, E. H., & Harris, L. J. (2003). The use of indicators and surrogate microorganisms for the evaluation of pathogens in fresh and fresh‐cut produce. Comprehensive Reviews in Food Science and Food Safety, 2(s1), 179-185. http://dx.doi.org/10.1111/j.1541-4337.2003.tb00035.x
» http://dx.doi.org/10.1111/j.1541-4337.2003.tb00035.x - Carvalho, W., Canilha, L., Castro, P., & Barbosa, L. (2009). Composition of the sugarcane bagasse. In 31st Symposium on Biotechnology for Fuels and Chemicals San Francisco.
- Dai, J., & Mumper, R. J. (2010). Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules (Basel, Switzerland), 15(10), 7313-7352. PMid:20966876. http://dx.doi.org/10.3390/molecules15107313
» http://dx.doi.org/10.3390/molecules15107313 - Do, Q. D., Angkawijaya, A. E., Tran-Nguyen, P. L., Huynh, L. H., Soetaredjo, F. E., Ismadji, S., & Ju, Y. H. (2014). Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. Yao Wu Shi Pin Fen Xi, 22(3), 296-302. PMid:28911418.
- El-Mogy, M. M., Ali, M. R., Darwish, O. S., & Rogers, H. J. (2019). Impact of salicylic acid, abscisic acid, and methyl jasmonate on postharvest quality and bioactive compounds of cultivated strawberry fruit. Journal of Berry Research, 9(2), 333-348. http://dx.doi.org/10.3233/JBR-180349
» http://dx.doi.org/10.3233/JBR-180349 - Fernández‐Pan, I., Royo, M., & Ignacio Mate, J. (2012). Antimicrobial activity of whey protein isolate edible films with essential oils against food spoilers and foodborne pathogens. Journal of Food Science, 77(7), M383-M390. PMid:22671770. http://dx.doi.org/10.1111/j.1750-3841.2012.02752.x
» http://dx.doi.org/10.1111/j.1750-3841.2012.02752.x - Gill, A. O., & Gill, C. O. (2015). Developments in sampling and test methods for pathogens in fresh meat. In J. Sofos (Ed.), Advances in microbial food safety (pp. 257-280). Philadelphia: Woodhead Publishing. http://dx.doi.org/10.1533/9781782421153.3.257
» http://dx.doi.org/10.1533/9781782421153.3.257 - Korea. Ministry of Food and Drug Safety – MFDS. (2015). Microbiological examination tip in meat (Notification 11; 2014-135). Korea.
- Li, X., Yao, S., Tu, B., Li, X., Jia, C., & Song, H. (2010). Determination and comparison of flavonoids and anthocyanins in Chinese sugarcane tips, stems, roots and leaves. Journal of Separation Science, 33(9), 1216-1223. PMid:20235128. http://dx.doi.org/10.1002/jssc.200900567
» http://dx.doi.org/10.1002/jssc.200900567 - Munro, B., Vuong, Q. V., Chalmers, A. C., Goldsmith, C. D., Bowyer, M. C., & Scarlett, C. J. (2015). Phytochemical, antioxidant and anti-cancer properties of Euphorbia tirucalli methanolic and aqueous extracts. Antioxidants, 4(4), 647-661. PMid:26783950. http://dx.doi.org/10.3390/antiox4040647
» http://dx.doi.org/10.3390/antiox4040647 - Nikodinovic-Runic, J., Guzik, M., Kenny, S. T., Babu, R., Werker, A., & O’Connor, K. E. (2013). Carbon-rich wastes as feedstocks for biodegradable polymer (polyhydroxyalkanoate) production using bacteria. Advances in Applied Microbiology, 84, 139-200. PMid:23763760. http://dx.doi.org/10.1016/B978-0-12-407673-0.00004-7
» http://dx.doi.org/10.1016/B978-0-12-407673-0.00004-7 - Philippini, R. R., Martiniano, S. E., Chandel, A. K., Carvalho, W., & Silva, S. S. (2019). Pretreatment of sugarcane bagasse from cane hybrids: Effects on chemical composition and 2G sugars recovery. Waste and Biomass Valorization, 10(6), 1561-1570. http://dx.doi.org/10.1007/s12649-017-0162-0
» http://dx.doi.org/10.1007/s12649-017-0162-0 - Rai, S., Dutta, P. K., & Mehrotra, G. K. (2016). Agrowaste derived phenolic compounds as additives to chitosan film for food packaging applications: Antibacterial and antioxidant study. Journal of the Indian Chemical Society, 93, 1-8.
- Smuda, S. S., Mohsen, S. M., Olsen, K., & Aly, M. H. (2018). Bioactive compounds and antioxidant activities of some cereal milling by-products. Journal of Food Science and Technology, 55(3), 1134-1142. PMid:29487456. http://dx.doi.org/10.1007/s13197-017-3029-2
» http://dx.doi.org/10.1007/s13197-017-3029-2 - Solomon, S. (2011). Sugarcane by-products based industries in India. Sugar Tech, 13(4), 408-416. http://dx.doi.org/10.1007/s12355-011-0114-0
» http://dx.doi.org/10.1007/s12355-011-0114-0 - Tsuda, T. (2016). Recent progress in anti-obesity and anti-diabetes effect of berries. Antioxidants, 5(2), 13. PMid:27058561. http://dx.doi.org/10.3390/antiox5020013
» http://dx.doi.org/10.3390/antiox5020013 - Zhao, Y., Chen, M., Zhao, Z., & Yu, S. (2015). The antibiotic activity and mechanisms of sugarcane (Saccharum officinarum L.) bagasse extract against food-borne pathogens. Food Chemistry, 185, 112-118. PMid:25952848. http://dx.doi.org/10.1016/j.foodchem.2015.03.120
» http://dx.doi.org/10.1016/j.foodchem.2015.03.120 - Zheng, R., Su, S., Li, J., Zhao, Z., Wei, J., Fu, X., & Liu, R. H. (2017). Recovery of phenolics from the ethanolic extract of sugarcane (Saccharum officinarum L.) baggase and evaluation of the antioxidant and antiproliferative activities. Industrial Crops and Products, 107, 360-369. http://dx.doi.org/10.1016/j.indcrop.2017.05.050
» http://dx.doi.org/10.1016/j.indcrop.2017.05.050
Publication Dates
-
Publication in this collection
06 Aug 2021 -
Date of issue
2021
History
-
Received
04 July 2020 -
Accepted
19 Mar 2021