Abstract
The recognition of DF (DHF Dengue Hemorrhagic Fever) is very complicated due to occurrence of a wide spectrum of clinical signs and symptoms during acute phase of illness. Moreover, presence of four serotypes further complicates the prognosis. To investigate the predictors of disease severity and elucidate the prognostic markers among four dengue serotypes, this study was conducted on 320 inpatients having acute febrile illness clinically suspected as DI, over a period of five years. Dengue serotypes were confirmed by multiplex reverse transcriptase (RT)-PCR. Eighty patients were positive for DI with presence of Den-1, Den-2, Den-3, and Den-4 in 8, 35, 27 and 10 patients, respectively. The severe clinical manifestations, abdominal pain and hepatomegaly, were comparatively higher in Den-2 patients. Liver aminotransferases levels were also higher in Den-2 patients (app. 5 fold). This study clearly indicates the hyperendemicity of all dengue serotypes. Nucleotide sequencing of Envelope region revealed that the presently emerged Den-3 belongs to type III, having high homology with genotype responsible for number of outbreaks in 1980s. The re-emergence of this deadly type can be suspected to cause more outbreaks in future and is a matter of great concern.
Dengue fever; dengue infection; serotype; clinical spectra; liver transaminase
ORIGINAL ARTICLE
Correlation of disease spectrum among four Dengue serotypes: a five years hospital based study from India
Rajni Kumaria
Postdoctoral Associate - Singapore-MIT Alliance for Research and Technology, National University of Singapore, Singapore
Correspondence Correspondence to: Dr. Rajni Kumaria Singapore-MIT Alliance for Research and Technology, National University of Singapore, Singapore Phone: 65 82282240 E-mail: rankumaria@gmail.com
ABSTRACT
The recognition of DF (DHF Dengue Hemorrhagic Fever) is very complicated due to occurrence of a wide spectrum of clinical signs and symptoms during acute phase of illness. Moreover, presence of four serotypes further complicates the prognosis. To investigate the predictors of disease severity and elucidate the prognostic markers among four dengue serotypes, this study was conducted on 320 inpatients having acute febrile illness clinically suspected as DI, over a period of five years. Dengue serotypes were confirmed by multiplex reverse transcriptase (RT)-PCR. Eighty patients were positive for DI with presence of Den-1, Den-2, Den-3, and Den-4 in 8, 35, 27 and 10 patients, respectively. The severe clinical manifestations, abdominal pain and hepatomegaly, were comparatively higher in Den-2 patients. Liver aminotransferases levels were also higher in Den-2 patients (app. 5 fold). This study clearly indicates the hyperendemicity of all dengue serotypes. Nucleotide sequencing of Envelope region revealed that the presently emerged Den-3 belongs to type III, having high homology with genotype responsible for number of outbreaks in 1980s. The re-emergence of this deadly type can be suspected to cause more outbreaks in future and is a matter of great concern.
Keywords: Dengue fever, dengue infection, serotype, clinical spectra, liver transaminase.
INTRODUCTION
Dengue virus, a member of the family flaviviridae,has four serologically related but genetically distinct virus serotypes, namely, Den-1, Den-2, Den-3 and Den-4. Dengue infection (DI) is presently the most important arboviral disease of mankind.1 DI may either remain asymptomatic, or rise as mild infection called dengue fever (DF). In some instances, it results into dengue hemorrhagic fever (DHF).2,3 At present, DI is endemic in over 100 countries worldwide and causes nearly 100 million cases of DF, 500,000 cases of DHF and 24,000 deaths each year.4-7 Pathophysiological basis of hemorrhage in dengue patients still remains poorly understood. 6 A patient having DI can rapidly develop life-threatening hemorrhagic form, as early as 2 days after the onset of illness.1,8-10 Dengue outbreaks in India are now following the pattern like other high burdened countries (e.g., Thailand and Indonesia).11 The study during the last major DHF outbreak in 1996 reported Den-2 as the predominant serotype circulating in India.12 However, the extensive research during this study, since 2002, highlights the pres presence of all four serotypes, with predominance of Den-2 and Den-3 serotypes.13
Because all four dengue virus serotypes cause a number of illness, defining precisely which clinical manifestations and laboratory abnormalities are associated with different serotypes remains disappointing.14 Conducted to evaluate disease treatment, the current study, a first of its kind from this geographical region, was planned to recognize the clinical and bio chemical manifestations occurring during acute stage of infection in patients infected with vari ous serotypes. The present work presents a bet ter understanding of correlation between sero types and disease manifestations during acute phase. Further, genotypic characterization of stood.6 A patient having DI can rapidly develop Den-3, the second most predominant serotype during this study, revealed the threat associated with this newly re-emerged serotype.
METHODOLOGY
Patient selection and sample processing
Blood samples were collected within 5 days of fever onset (acute phase), from 320 patients clinically suspected of DI, attending the outpatient and medical wards of Lok Nayak Hospital, a tertiary care hospital at Delhi, India, from January 2002 to November 2006. On the basis of WHO inclusion criteria,1 a patient having an acute febrile illness of 2-7 days duration with two or more of the following manifestations: severe head ache, retro-orbital pain, myalgia, arthralgia, rash, hemorrhagic manifestations, and/or leucopenia was categorized as having DF. Patient with DHF is a probable case of DF, having hemorrhagic tendency along with thrombocytopenia (platelets 100,000/cu. mm or less) and evidence of plasma leakage. Fifty normal healthy age and sex matched individuals, having no history of febrile or other illness in the last three months were included as negative controls.
The prior approval has been granted by the Ethical Committee of the Institute to conduct this study. Informed consent was obtained from all patients. A case was excluded if routine laboratory testing suggested bacterial infection or any viral infection other than DI or any other disease.
Detection of dengue serotypes by multiplex RT-PCR
Reference strains specific for serotype Den-1: Hawaii; Den-2: P-23085; Den-3: 633798; and Den-4: 642069 (obtained from National Institute of Virology, Pune, India) were individually inoculated in C6/36 cells containing Leibovitz-15 media with 2% Fetal Bovine Serum.15-17 After 7 days, supernatant was separated from each culture vial at 1500 rpm for 10 minutes, 4º C and stored at -70º C, to be used as positive controls. The presence of dengue serotypes was confirmed by multiplex RT-PCR, using four serotype specific primers pairs described earlier.13,18 Serum samples from all patients were similarly processed for detection of dengue serotypes.
Amplification of E-NS1 gene region by RT-PCR
As per the method of Domingo et al.,19 5 µl of dengue viral RNA was used for RT-PCR mix to amplify E-NS1 gene region of Den-3 serotype specific strains. The reaction was carried out in two steps and the first step consisted of RT-PCR using outer sense primer S1871DEN3 and anti-sense primer AS2622DEN3 specific for Den-3. This reaction was followed by nested PCR using internal sense primer; S2176DEN3 specific for Den-3 and a common anti-sense primer AS2504DEN.
Nucleotide sequencing of E-NS1 region of Den-3 and phylogenetic analysis
The purified DNA products (25-50 ng) were sequenced by ABI 3130xl automated DNA sequencer (Applied Biosystems, USA) using dideoxy chain termination method with ABI sequencing kit following manufacturer's instructions, using primers S2176DEN3 and AS2504DEN. Sequences thus generated were submitted to Gene Bank database. Sequence identity matrices and multi-sequence alignments from DNA databases were generated using Basic Local Alignment Search Tool; available at NCBI website (http://www.ncbi.nlm.nih.gov/BLAST) and ClustalW programme (http://align.genome.jp/).
Hematological and biochemical investigations
Blood samples were collected into an EDTA-coated Vacutainer tube (Becton Dickinson Vacutainer Systems, Franklin Lakes, N.J.), for hematological investigations and plain Vacutainer tube (Becton Dickinson Vacutainer Systems, Franklin Lakes, N.J.), for biochemical investigations. All the tests were performed as per standard laboratory procedures.
Statistical analysis
Statistical analysis was performed with Epi Info version 3.3.2 (updated 2005, Center for Disease Control and Prevention, USA). Proportions of patients with abnormal clinical, hematological and biochemical findings between the two groups were compared using Chi (χ2)/Fischer exact test. Mean values between four serotype groups were compared using analysis of variance (ANOVA). Kruskal-Wallis test was applied, when variance in samples was high. Odds ratio (OR) for disease outcomes in serotypes was calculated to assess strength of association at 95% confidence intervals. The results were considered statistically significant when error was less than 5% (p < 0.05).
RESULTS
Distribution pattern of dengue serotypes among dengue patients
On the basis of multiplex RT-PCR, DI was confirmed in eighty patients. Serotypic characterization identified 35 patients being positive for Den-2, 27 for Den-3, 10 for Den-4, and 8 patients positive for Den-1. Thus, Den-2 was detected in most patients, followed by Den-3.
Correlation of dengue serotypes with clinical and hemorrhagic manifestations
Among the wide spectrum of mild and severe clinical and hemorrhagic manifestations analyzed, the incidence of only two severe manifestations, namely, abdominal pain (p < 0.05) and hepatomegaly (p < 0.05), was significantly different between four serotype groups. While frequency of clinical manifestations was most predominant in Den-2 infected cases, severe hemorrhagic manifestations were predominant in Den-4 infected group. Individual analysis of each serotype group demonstrated hepatomegaly to be significantly higher in Den-2 as well as Den-3 infected patients. Abdominal pain was significantly higher in Den-2 group and anorexia was significantly higher in Den-3 group only. The overall bleeding frequency was also significantly different between the four serotype groups (p < 0.01). However, no significant difference was observed on analysis of each bleeding manifestation individually (Table 1).
Correlation of serotypes with hematological parameters
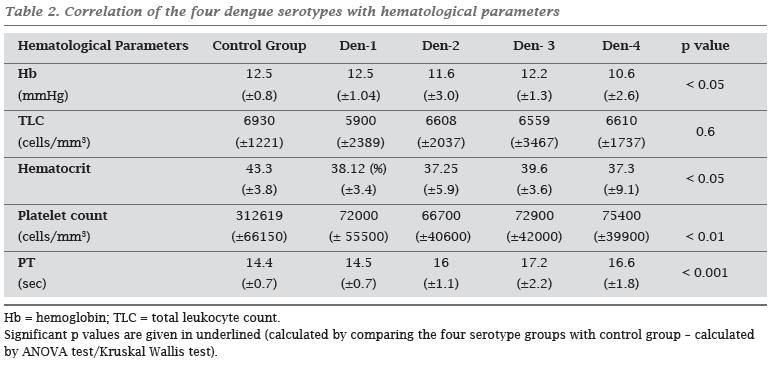
Thrombocytopenia was the common hematological manifestation during acute phase. The lowest mean platelet counts were observed in the Den-2 infected patient group. Hematological parameters (except TLC) showed significant difference in disease group when compared with control group (Table 2). However, none of the parameters showed significant difference within the disease groups. In Den-3 infected patients, a strong correlation was found between hematocrit and hemoglobin levels (p value < 0.05, r = 0.411) and also platelet counts and tlc levels (p value < 0.05, r = 0.484). In patients infected with Den-2, correlation was observed between platelet counts and hemoglobin levels (p value < 0.05, r = 0.25). However, there was no correlation between any of the hematological parameters in Den-1 and Den-4 infected patients in the present study.
Correlation of serotypes with biochemical profile
The elevation of liver aminotransferase levels was maximum in Den-2 patients. Increase of about five-fold in levels of both the aminotransferase was noticed as compared to healthy controls. The mean level of serum albumin in Den-2 infected patients was slightly reduced, as compared to the normal reference limits. However, when compared with normal healthy controls, the mean levels of all biochemical parameters were significantly altered (Table 3). Like hematological parameters, there was no significant different between biochemical parameters within serotype groups. The frequency of patients with elevated levels of SGOT was significantly higher in Den-2 group (p < 0.05) showing a strong association (O.R. 2.42; CI = 0.89-6.64).
Genotypic characterization of Den-3
There was 99-100% nucleotide homology between the sequences of five Den-3 strains isolated from dengue patients (Accession numbers: EF094024, EF119833, EF119834, EF119835, EF119836). AT composition of the sequences from the present study was between 51-58.3%. Comparison of E-NS1 region of these strains with published sequences of other strains of Den-3 categorized then into subtype III (Table 4).
DISCUSSION
DI has now become a regular post-monsoon phenomenon in most parts of India.13,20 Most of Indian studies on clinical and laboratory profile of dengue patients have involved serologically positive patients. Because serological diagnosis demands a collection of samples five days post disease onset, it thus depicts the pattern of manifestations occurring in the convalescent phase of disease. As severe complications related to DHF may occur very early in disease, it is important to explore prognostic markers of severe disease during acute phase. The hyperendemicity with co-circulation of two or more serotypes during the same time period have been widely suspected as one of the major cause of disease severity in South East Asia.8,21 Various studies are trying to establish connection between causative serotype and disease outcome. Few of which propose that Den-2 and Den-3 may cause more severe disease,8,22-24 however, a definite link between distinct serotypes and severe manifestations has not been established yet.
This study provided direct evidence for the presence of all four serotypes in this geographical region, with Den-2 and Den-3 as two predominant serotypes. The clinical manifestations, such as abdominal pain and hepatomegaly, were higher in Den-2 patients, indicating a role in causing liver function abnormalities. Incidences of severe hemorrhagic manifestations, such as epistaxis, malena, and hemetemesis, were higher in patients having Den-4. As Den-4 positivity remained very low, there is a need of more studies to validate this finding. In a retrospective study from Thailand, Den-2 has been documented as the most frequent serotype among DHF cases (35%), followed by Den-3 (31%).24 In a study in Thailand,23 Den-2 showed association with a greater pleural effusion index and with a higher percentage of DHF cases. In congruence with the present study, Corwin et al.25 also found that Den-1 is associated with less severe disease. Several reports from other countries articulating differences in disease severity associated with distinct dengue serotypes support our results. It is suggested that screening for abdominal pain, and hepatomegaly along with severe hemorrhagic manifestations in patients tested positive by multiplex RT-PCR during acute phase of illness would be an asset in better prognosis. Hepatic dysfunction is common in patients with severe dengue manifestations.26 Liver dysfunction might be responsible for the decreased synthesis of specific factors of the intrinsic pathway, an unbalance of which may consequently cause hemorrhage.26 In our finding, mean values of both the transaminases were significantly raised in all four serotype groups, when compared with control groups. Elevation of liver aminotransferase levels was maximum in Den-2 patients (app. five-fold increase). Moreover, reduction in serum albumin levels points towards risk of pleural effusion. Higher levels of SGOT and SGPT have been earlier reported from a limited sample of Den-2 infected patients.27 However, more investigations are still required before a definite association of any serotype with hematological or biochemical profiles can be ascertained. As mortality is usually linked to delayed provision of supportive treatment, a better understanding of clinical and biochemical profile during the acute phase of illness is extremely important.
Changes in the envelope protein in particular are suggested to alter host cell susceptibility, thereby affecting virus replication and disease severity.28 The nucleotide sequence comparison of E-NS1 region of five Den-3 strains from present study with published sequences using BLAST revealed that irrespective of the year in which samples were collected, all of them belonged to genotype III. The strains isolated in our study showed ~98% homology with subtype-III of Den-3 strains isolated during epidemics in 1984 from India, in 1985 from Mozambique, in 1989, 1991 and 2000 from Sri Lanka, and in 2000 from Martinique.28,29 The identification of Den-3 during the outbreak in India during 2003 and its continued presence in later years indicates its resurgence in a dominant form and, thus, a matter of great concern. Constant and thorough epidemiological surveillance is mandatory to control the invasion and spread of dengue viruses. Studies of these types would definitely be an asset in the establishment of in time effective control strategies.
Submitted on: 06/11/2009
Approved on: 08/06/2009
We declare no conflict of interest.
- 1. World Health Organization. Prevention and control of dengue and dengue hemorrhagic fever: comprehensive guidelines. WHO Regional publication, SEARO 1999; 29.
- 2. Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 1998; 11:480-96.
- 3. Malavige GN, Fernando S, Fernando DJ, Seneviratne SL. Dengue viral infections. Post Grad Med J 2004; 80:588-01.
- 4. CDC; Centers for Disease Control and Prevention. National Center for Infectious Diseases, Division of Vector-Borne Infectious Diseases. Dengue fever home page year. www.cdc.gov/ncidod/dvbid/dengue.htm
- 5. Dengue Net-WHOs Internet-based System for Global Surveillance of Dengue Fever and Dengue Haemorrhagic Fever (Dengue / DHF).Weekly Epidemiological Record 2002; 77(36):301-304. http://www.who.int/denguenet
- 6. Gibbons RV, Vaughn DW. Dengue: an escalating problem. B M J 2002; 324:1563-66.
- 7. Sapir DG, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerging Themes in Epidemiology 2005, 2:1 doi:10.1186/1742-7622-2-1 online http://www.eteonline.com/content/2/1/1
- 8. Endy TP, Nisalak A, Chunsuttiwat S et al Spatial and temporal circulation of dengue virus serotypes: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol 2002; 56:52-9.
- 9. Guzman MG, Kouri G. Dengue and dengue hemorrhagic fever in Americas: lessons and challenges. J Clin Virol 2003; 27:1-13.
- 10. World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control, 2nd ed. World Health Organization, Geneva, Switzerland. 1997.
- 11. Dengue Net in India. Weekly Epidemiological Record 2004; 79 (6):57-62.
- 12. Singh UB, Maitra A, Broor S, Rai A, Pasha ST, Seth P. Partial nucleotide sequencing and molecular evolution of epidemic causing dengue 2 strains. J Infect Dis 1999; 180:959-65.
- 13. Kumaria R, Chakravarti A. Molecular detection and serotypic characterization of dengue viruses by single tube multiplex RT-PCR. Diagn Microbiol Infect Dis 2005; 52(4):311-6.
- 14. Balmaseda A, Hammond SN, Pérez L et al Serotype-specific differences in clinical manifestations of dengue. Am J Trop Med. Hyg 2006; 74(3):449-56.
- 15. Harris E, Roberts G, Smith L et al Typing of dengue viruses in clinical specimens and mosquitoes by single-tube multiplex reverse transcriptase PCR. J Clin Microbiol 1998; 36(9):2634-9.
- 16. Igarashi A. Mosquito cell cultures and study of arthropodborne togaviruses. Adv Virus Res 1985; 30:21-39.
- 17. Tesh RB. A method for the isolation and identification of dengue viruses, using mosquito cell cultures. Am J Trop Med Hyg 1979; 28:1053-9.
- 18. Chakravarti A,Kumaria R,Kar P,Batra VV,Verma V.Improved Detection of Dengue Virus Serotypes From Serum Samples -Evaluation of Single Tube Multiplex RT-PCR with Cell Culture. WHO Dengue Bull 2006; 30:133-40.
- 19. Domingo C, Palacios G, Niedrig M et al A New Tool for the Diagnosis and Molecular Surveillance of Dengue Infections in Clinical Samples. WHO Dengue Bull 2004; 28:87-95.
- 20. Chakravarti A, Kumaria R. Eco-epidemiological analysis of dengue infection during an outbreak of dengue fever, India. Virology J 2005; 2:32; 1-7. (Online).
- 21 . Graham RR, Juffrie M, Tan R et al A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia Studies in 1995-1996. Am J Trop Med Hyg 1999; 61:412-19.
- 22. Kalayanarooj S, Nimmannitya S. Clinical and laboratory presentations of dengue patients with different serotypes. WHO Dengue Bulletin. 2000; 24:53-9.
- 23. Vaughn DW, Green S, Kalayanarooj S et al Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 2000; 181:2-9.
- 24. Nisalak A, Endy TP, Nimmanniya S et al Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973-1979. Am J Trop Med Hyg 2003; 68:191-202.
- 25. Corwin AL, Larasati RP, Bangs MJ et al Epidemic dengue transmission in southern Sumatra, Indonesia. Trans R Soc Trop Med Hyg 2001; 95:257-65.
- 26. Lei HY, Yeh TM, Liua HS, Lin YS, Chen SH, Liu CC. Immunopathogenesis of Dengue Virus Infection. J Biomed Sci 2001; 8:377-88.
- 27. Huang YH, Liu CC, Wang ST, Lei HY, Liu HL, Lin YS. Activation of coagulation and fibrinolysis during dengue virus infection. J Med Virol 2001; 63:247-51.
- 28. Lanciotti RS, Lewis JL, Gubler DJ, Trent DW. Molecular evolution and epidemiology of dengue-3 viruses. J Gen Virol 1994; 75:65-75.
- 29. Peyrefitte CN, Couissinier-Paris P, Mercier-Perennec V et al Genetic Characterization of Newly Reintroduced Dengue Virus Type 3 in Martinique (French West Indies). J Clin Microbio 2003; 41 (11):5195-8.
Publication Dates
-
Publication in this collection
11 June 2010 -
Date of issue
Apr 2010
History
-
Received
06 Nov 2009 -
Accepted
08 June 2009