Abstract
Introduction:
Human cytomegalovirus is a major cause of morbidity in kidney transplant patients.
Objectives:
We aimed to study viral replication and serological response in the first months post kidney transplant in patients undergoing universal prophylaxis or preemptive therapy and correlate the findings with the clinical course of Human cytomegalovirus infection.
Patients and methods:
Independent from the clinical strategy adopted for managing Human cytomegalovirus infection, prophylaxis versus preemptive therapy, the pp65 antigenemia assay and serological response were assessed on the day of transplantation, and then weekly during the first three months of post-transplant.
Results:
From the 32 transplant recipients, 16 were positive for pp65 antigenemia, with a similar incidence rate in each group. There were no positive results in the first three weeks of monitoring; the positivity rate peaked at week eight. There was a trend for a higher and earlier frequency of positivity in the universal prophylaxis group in which the course of the Human cytomegalovirus infection was also more severe. Despite the differences in clinical picture and in the initial immunosuppressant schedule, the serological response was similar in both groups.
Conclusion:
Routine monitoring during the first three post-transplant months has a positive impact on the early detection of Human cytomegalovirus viral replication allowing for timely treatment in order to reduce morbidity of the disease. The strategy of universal therapy employing intravenous ganciclovir was associated to a worse clinical course of the Human cytomegalovirus infection suggesting that the use of >10 cells/2 × 105 leukocytes as a cut-off in this setting may be inappropriate.
Keywords:
Cytomegalovirus; pp65 antigenemia; Kidney transplant; Universal prophylaxis; Preemptive therapy
Introduction
Human cytomegalovirus (HCMV), a DNA virus, member of herpesviridae family, is one of the major problems associated with organ transplants, directly affecting clinical outcomes and mortality.11 Varani S, Landini MP. Cytomegalovirus-induced immunopathology and its clinical consequences. Herpesviridae. 2011;2:6.
2 Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22:76-98.
3 Linares L, Sanclemente G, Cervera C, et al. Influence of cytomegalovirus disease in outcome of solid organ transplant patients. Transplant Proc. 2011;43:8.-44 Lúcia M, Crespo E, Cruzado JM, et al. Human CMV-specific T-cell responses in kidney transplantation; toward changing current risk-stratification paradigm. Transpl Int J Eur Soc Organ Transplant. 2014;27:643-56. In this context, different strategies for monitoring, prevention, and treatment have been applied.55 Fehr T, Cippà PE, Mueller NJ. Cytomegalovirus post kidney transplantation: prophylaxis versus pre-emptive therapy. Transpl Int. 2015;28:1351-6. In several centers, the HCMV screening and monitoring is generally performed using assays for viral biomarkers identification, such as phosphoprotein 65 (pp65) or viral DNA by polymerase chain reaction (PCR).66 Boaretti M, Sorrentino A, Zantedeschi C, et al. Quantification of cytomegalovirus DNA by a fully automated real-time PCR for early diagnosis and monitoring of active viral infection in solid organ transplant recipients. J Clin Virol Publ Pan Am Soc Clin Virol. 2013;56:124-8.
7 Breda G, Almeida B, Carstensen S, et al. Human cytomegalovirus detection by real-time PCR and pp65-antigen test in hematopoietic stem cell transplant recipients: a challenge in low and middle-income countries. Pathog Glob Health. 2013;107:312-9.-88 David-Neto E, Triboni AHK, Paula FJ, et al. A double-blinded, prospective study to define antigenemia and quantitative real-time polymerase chain reaction cutoffs to start preemptive therapy in low-risk, seropositive, renal transplanted recipients. Transplantation. 2014;98:1077-81.
In preemptive therapy, diagnostic tests are usually performed weekly to guide therapeutic decisions, based on previously defined cut-off. In high risk patients, however, universal prophylaxis has been used, with antiviral drugs administered immediately after transplantation, usually for at least three months.99 Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333-60.,1010 Zhang L-F, Wang Y-T, Tian J-H, et al. Preemptive versus prophylactic protocol to prevent cytomegalovirus infection after renal transplantation: a meta-analysis and systematic review of randomized controlled trials. Transpl Infect Dis Off J Transplant Soc. 2011;13:622-32.
In transplant recipients undergoing universal prophylaxis screening is not usually performed, and data on potential early replication are unknown. However, it has been established that late disease, following completion of treatment with antiviral drugs, is more likely to occur in this group.1111 San-Juan R, Navarro D, García-Reyne A, et al. Effect of delaying prophylaxis against CMV in D+/R- solid organ transplant recipients in the development of CMV-specific cellular immunity and occurrence of late CMV disease. J Infect. 2015;71:561-70.,1212 Jamal AJ, Husain S, Li Y, et al. Factors for late-onset cytomegalovirus infection or disease in kidney transplant recipients. Transplantation. 2014;97:569-75. In view of that, some centers use monitoring strategy similar to preemptive protocol after ending treatment.1313 Monforte V, Román A, Gavaldà J, et al. Preemptive therapy with intravenous ganciclovir for the prevention of cytomegalovirus disease in lung transplant recipients. Transplant Proc. 2005;37:4039-42.,1414 Harvala H, Stewart C, Muller K, et al. High risk of cytomegalovirus infection following solid organ transplantation despite prophylactic therapy. J Med Virol. 2013;85:893-8. The objective of the present study was to study viral replication and serological response in the first three months after kidney transplantation in patients undergoing universal prophylaxis or preemptive therapy and correlate the findings with the clinical course of HCMV infection.
Patients and methods
Blood samples of patients undergoing kidney transplantation at the Nephrology Division of a University Hospital were analyzed, from April 2014 (a time when pp65 assay was implemented in our center) to September 2015. This was an observational, prospective, longitudinal study, which analyzed the HCMV replication profile by pp65 antigenemia assay, with internal validation by qPCR assay. In addition, serum levels of anti-HCMV immunoglobulins were quantified. Clinical findings and other laboratory test results were obtained from patients' charts. The research protocol was approved by the Research Ethics Committee of the Medical School (CAAE: 18768213.1.0000.5243).
Irrespective of the clinical strategy adopted, the monitoring consisted in detection of viral replication in neutrophils infected by HCMV on the day of transplantation and weekly during the first three months. An additional evaluation was conducted at the end of the sixth month post-transplantation. Patients were grouped according to the type of the strategy adopted (preemptive therapy vs. prophylactic therapy).
The pp65 assay was performed by indirect immunofluorescence using Brite™ Turbo kit (IQ Products, Groningen, Netherlands) following the manufacturer's instructions. Results were expressed as the number of positive cells per 2 × 105 leukocytes. Sample processing and analysis were performed in a blinded fashion by the laboratory staff and the reports were made available on the electronic chart system of the hospital.
Quantification of anti-CMV IgM and IgG was performed using the commercial kits Architect CMV IgG Reagent and Architect CMV IgM Reagent, respectively (Abbott, Ireland), using a HCMV lysate (strain AD169) in a chemiluminescent microparticles immunoassay. The serum levels of anti-HCMV immunoglobulins (IgM and IgG) was measured on the day of transplantation and weekly during the first three months. An additional evaluation was conducted at the end of the sixth month post-transplantation.
The type of strategy (preemptive or universal) was chosen at the discretion of the medical assistants. In case of preemptive therapy, intravenous ganciclovir was initiated when at least 10 positive cells by 2 × 105 leukocytes (cut-off) were identified in the pp65 assay. In prophylactic therapy, antiviral treatment with ganciclovir was administered soon after transplantation, and maintained for three consecutive months, according to the following criteria: donor CMV IgG positive with CMV IgG negative receptor; donor > 60 years; serum creatinine of deceased donor >1.5 mg/dL; or whenever the induction therapy was based on thymoglobulin.
Universal prophylaxis with intravenous ganciclovir (5 mg/kg) was implemented according to previous institutional protocol: P.O. 1 to P.O. 14, twice a day; P.O. 15 to P.O. 30, three times a week; P.O. 31 to P.O. 60, twice a week, and P.O. 61 to P.O. 90, once a week. The dose of ganciclovir was adjusted to the patient's renal function.
The results were expressed as mean ± SD. Differences between groups were analyzed by unpaired Student's t test or Mann–Whitney, according to the distribution pattern. Frequencies were compared using Fisher's exact test. p values <0.05 were considered statistically significant. Statistical analysis was performed using 5.0 GraphPad Prism program.
Results
Thirty-two successful kidney transplants were performed during the study period. Baseline characteristics of donors are shown in Table 1. Their mean age was 44 ± 14 years. Intracranial bleeding (41%) was the main cause of death, and all were seropositive for HCMV. The mean cold ischemia time was 18.5 ± 8.5 hours and there were no significant differences when comparing the characteristics of donors whose organs were allocated to recipients undergoing universal prophylaxis or preemptive therapy.
Out of 32 recipients (44% male, 50% white), 18 received universal prophylaxis and 14 preemptive therapy. Baseline characteristics of recipients are shown in Table 2. Again, no statistically significant differences were found when comparing baseline parameters between groups.
Baseline characteristics of recipients, according to the strategy adopted for managing HCMV post kidney transplantation.
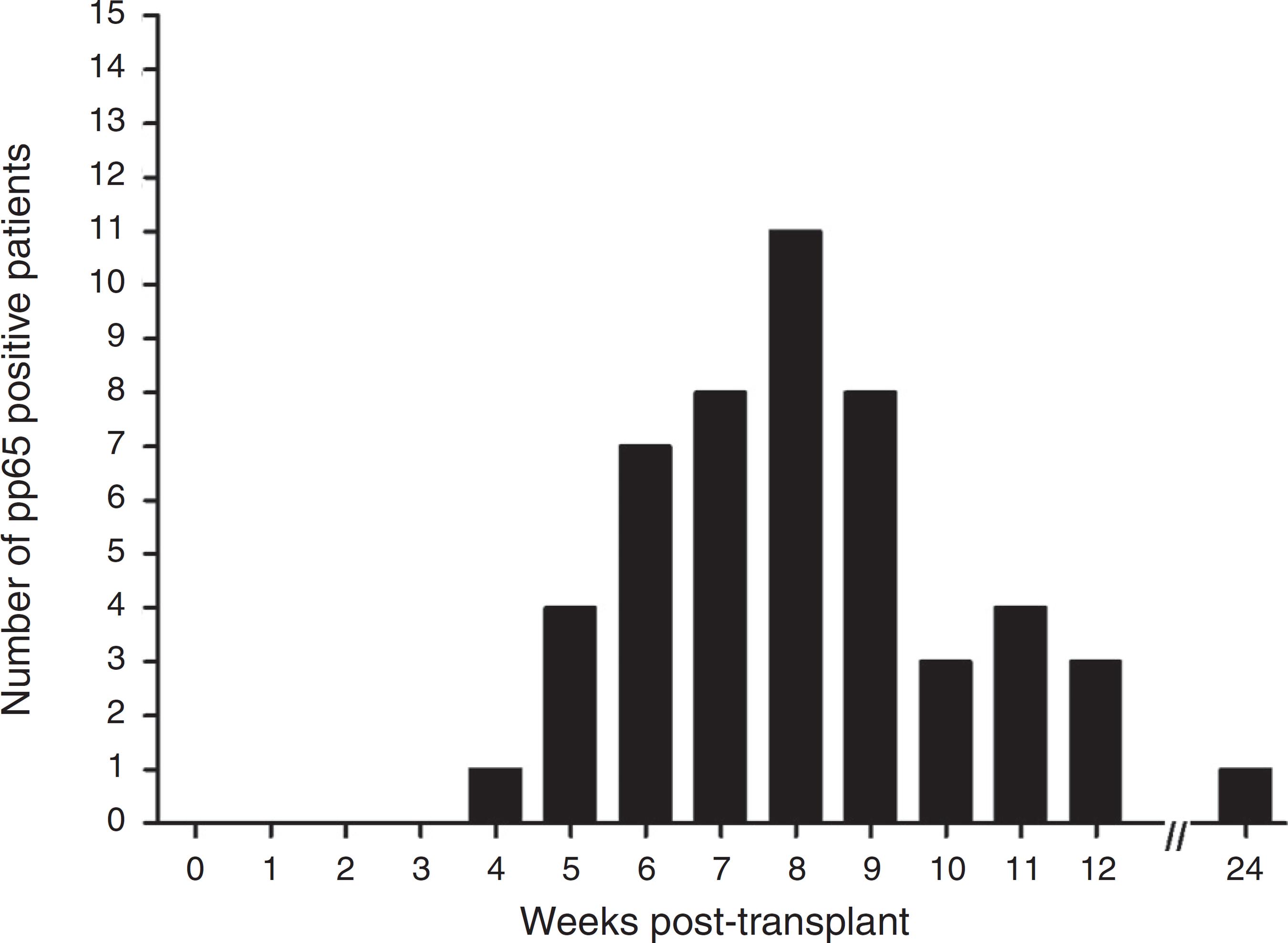
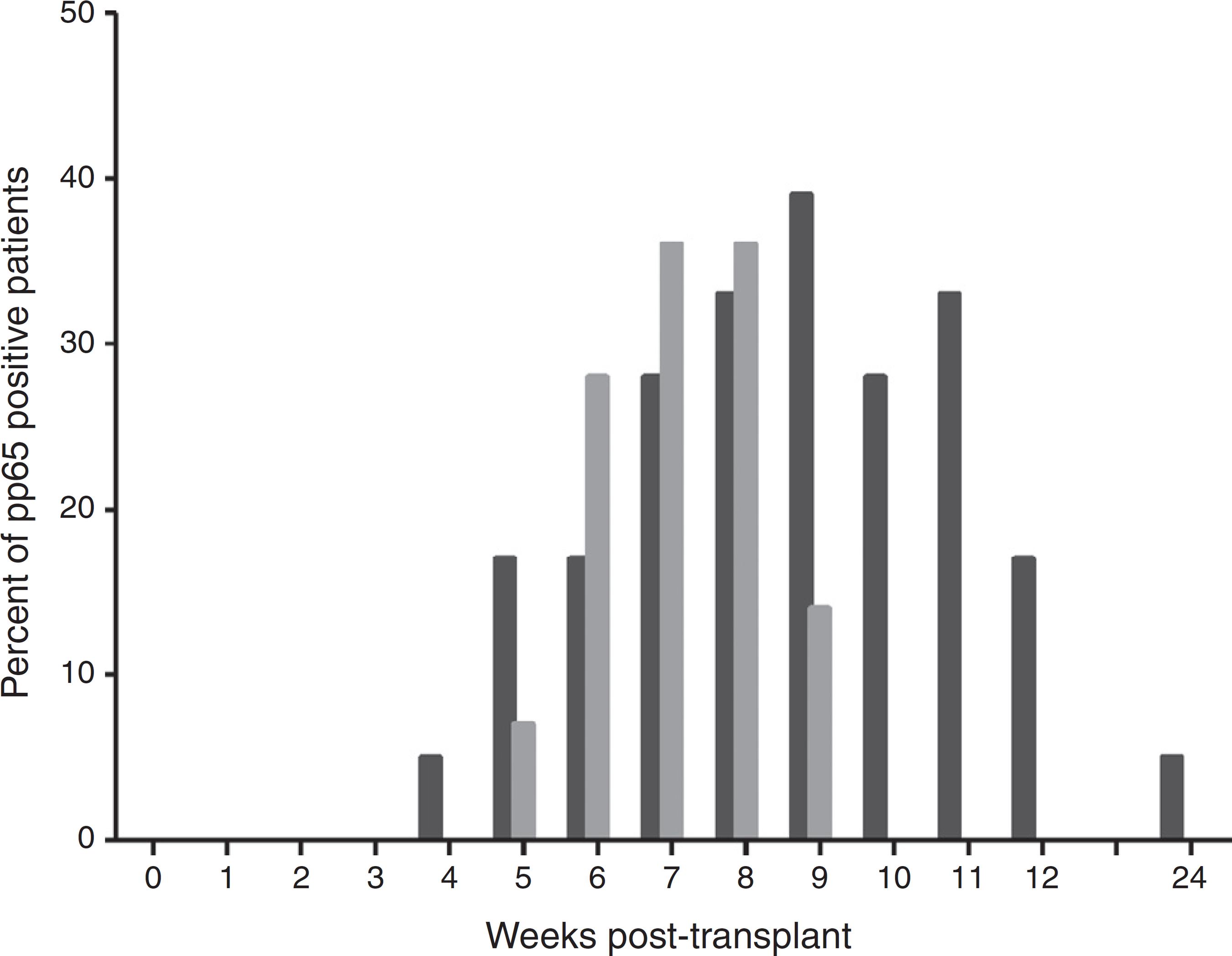
The pp65 antigenemia assay was performed weekly, for six months post-transplant. Sixteen patients were found to be positive for pp65 antigenemia, with a similar incidence between groups (Table 3). The time-line occurrence in weeks of positive cases is depicted in Fig. 1. There were no positive results in the first three weeks of monitoring and the frequency of positivity peaked on week eight. Six month post-transplant only one patient relapsed after three negative evaluations. When comparing the positivity rate along the weeks between the study groups (Fig. 2), there was a trend for a higher and earlier frequency of positivity among those on universal prophylaxis than in those on preemptive therapy. In addition, the positivity tended to last longer in the universal prophylaxis group (4.11 ± 1.83 weeks vs. 2.71 ± 0.95 weeks, p = 0.090).
Frequency of positive and negative cases for pp65 antigenemia in preemptive therapy and universal prophylaxis after kidney transplantation.
Positivity rate for antigenemia pp65 assay (cut-off > 10 positive cells/2 × 105 leukocytes) in kidney transplant patients along the study period.
Positivity rate for pp65 antigenemia assay in kidney transplant patients followed for six months, stratified by the type of strategy adopted (black columns represent the universal prophylaxis group and gray columns the preemptive therapy group).
From the nine positive patients on universal prophylaxis (mean age of 46.2 ± 9.2 years), three were male. All of them had gastrointestinal manifestations, and in three the virus was also identified by immunohistochemistry in kidney biopsies. One patient was considered clinically resistant to ganciclovir by clinicians and managed with immunoglobulin. Hemophagocytic lymphohistiocytosis was diagnosed in one case. Two of positive cases had a severe form of the disease with a fatal course. From the seven positive patients on preemptive therapy (mean age 51.4 ± 10.5 years), five were male. Here, every positive patient remained asymptomatic.
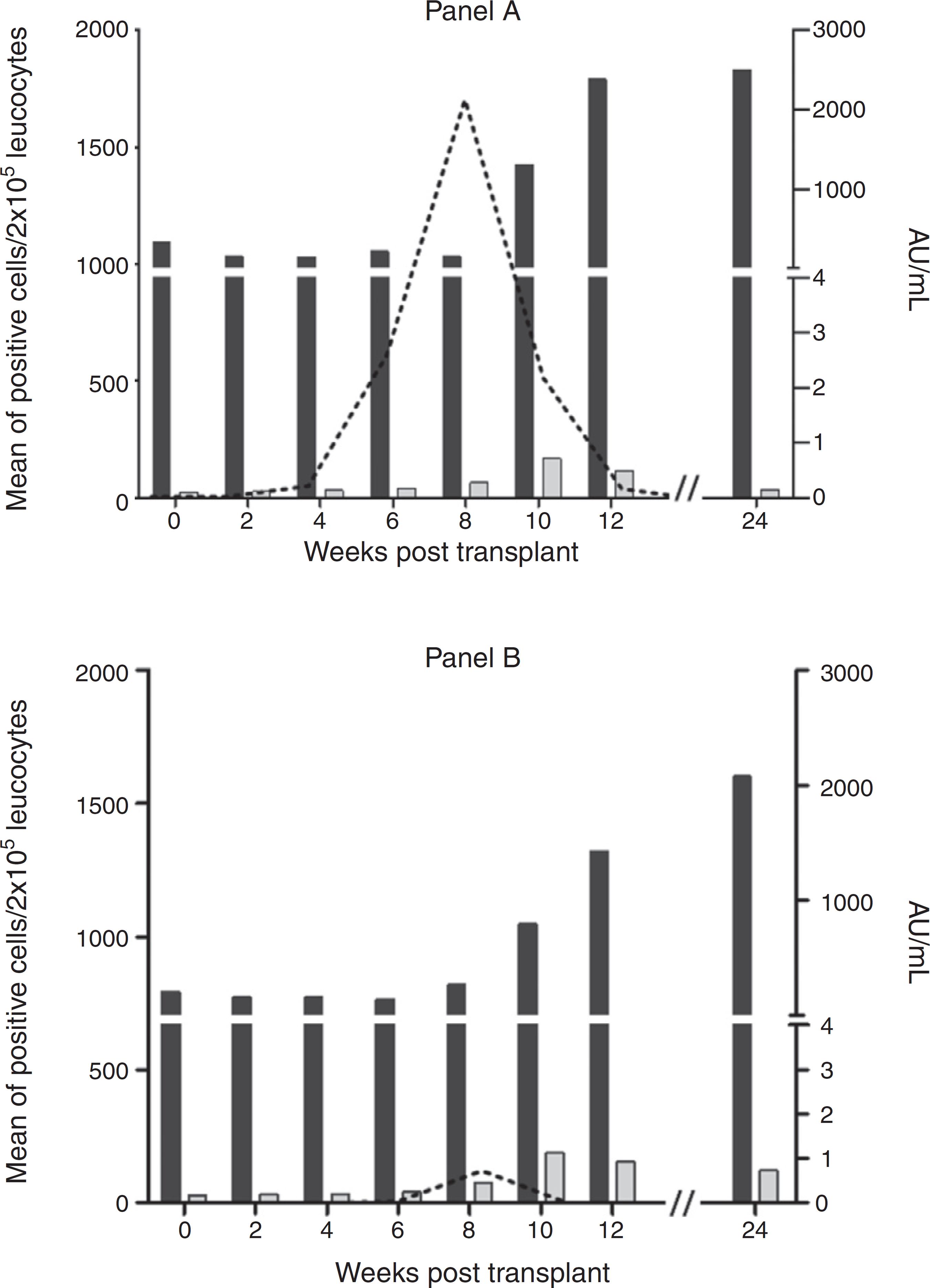
The mean number of cells of the positive patients and the serological response to HCMV infection are shown in Fig. 3. A significant difference in the number of positive cells/2 × 105 leukocytes between groups in favor of universal prophylaxis was found (123 ± 423 vs. 23 ± 87, p < 0.01). No difference was observed between groups regarding serum levels of either IgG or IgM anti-HCMV.
Comparison between viral replication profile of HCMV by pp65 antigenemia assay (dashed line) and IgM and IgG levels (gray and black columns, respectively) during the study period in kidney transplant patients on universal prophylaxis (Panel A) or preemptive therapy (Panel B).
Discussion
HCMV infection is a major concern in kidney transplantation especially during the first three months of post-transplant when higher doses of immunosuppressant are required.1515 Puttini C, Carmellini M, Garosi G, et al. HCMV infection in renal transplant recipients: a retrospective cohort study. New Microbiol. 2013;36:363-71. The profile of both pp65 antigenemia and serological studies were evaluated under universal prophylaxis or preemptive therapy, and the clinical courses of the two groups were compared. The positivity of pp65 antigenemia test can precede clinical symptoms; therefore, it is a useful tool to promptly support clinical decisions and has been considered the gold standard for monitoring HCMV viral replication in solid organ transplant recipients.99 Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333-60.,1616 Schroeder R, Michelon T, Fagundes I, et al. Cytomegalovirus disease latent and active infection rates during the first trimester after kidney transplantation. Transplant Proc. 2004;36:896-8.,1717 Kamei H, Ito Y, Onishi Y, et al. Cytomegalovirus (CMV) Monitoring After Liver Transplantation: Comparison of CMV Pp65 Antigenemia Assay with Real-Time PCR Calibrated to WHO International Standard. Ann Transplant. 2016;21:131-6.
The mean age of the donors was similar to other studies,1818 Couzi L, Helou S, Bachelet T, et al. Preemptive therapy versus valgancyclovir prophylaxis in cytomegalovirus-positive kidney transplant recipients receiving antithymocyte globulin induction. Transplant Proc. 2012;44:2809-13.,1919 Jung GO, Kim S-J, Choi G-S, et al. The effect of cytomegalovirus antigenemia titer on the efficacy of preemptive therapy for the prevention of cytomegalovirus disease after kidney transplantation. Transplant Proc. 2010;42:804-10. and the positivity rate of IgG anti-HCMV was 100%. More importantly, the analyzed parameters did not differ between groups. The mean age of recipients was also in the range reported by most of the studies.2020 Ozaki KS, Pestana JOM, Granato CFH, et al. Sequential cytomegalovirus antigenemia monitoring in kidney transplant patients treated with antilymphocyte antibodies. Transpl Infect Dis. 2004;6:63-8.
21 Kliem V, Fricke L, Wollbrink T, et al. Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: results of a randomized clinical trial. Am J Transplant. 2008;8:975-83.-2222 Kesiraju S, Paritala P, Rao Ch, et al. Anti-thymocyte globulin versus basiliximab induction in renal transplant recipients: long-term outcome. Saudi J Kidney Dis Transplant. 2014;25:9-15. The profile of the underlying disease was comparable to that described by the Brazilian census of dialysis but with a high percent of ADPKD patients.2323 Sesso RC, Lopes AA, Thomé FS, et al. Brazilian Chronic Dialysis Census 2014. J Bras Nefrol Orgao. 2016;38:54-61. As expected, the proportion of sensitized patients tended to be higher in the universal prophylaxis group.
Consistent with the reported frequency of HCMV infection in the renal transplant population (30–70%),2424 Caring for Australians with Renal Impairment (CARI). The CARI guidelines. CMV disease and kidney transplant: prophylaxis for cytomegalovirus infection in patients following renal transplantation. Nephrol Carlton Vic. 2004;9 Suppl. 3:S27-31.,2525 Nishida H, Ishida H, Tanaka T, et al. Cytomegalovirus infection following renal transplantation in patients administered low-dose rituximab induction therapy. Transpl Int. 2009;22:961-9. the overall frequency of HCMV infection in our sample was 50%. Unexpectedly, the rate of HCMV infection in universal prophylaxis and preemptive therapy was the same.
The first positive patient for pp65 antigenemia in our study was detected at week 4, in line with previous reports.1616 Schroeder R, Michelon T, Fagundes I, et al. Cytomegalovirus disease latent and active infection rates during the first trimester after kidney transplantation. Transplant Proc. 2004;36:896-8.,2626 Wirgart BZ, Claesson K, Eriksson B-M, et al. Cytomegalovirus (CMV) DNA amplification from plasma compared with CMV pp65 antigen (ppUL83) detection in leukocytes for early diagnosis of symptomatic CMV infection in kidney transplant patients. Clin Diagn Virol. 1996;7:99-110. The peak of affected patients occurred at week 8. In some way surprising, the first positive patient was on universal prophylaxis. Indeed, the positivity rate was higher in the first weeks as well as in the last weeks of the study in the group on universal prophylaxis compared to those on preemptive therapy. It should be pointed out that HCMV infection was more severe in those on universal prophylaxis resulting in two fatalities. In the preemptive therapy group, none of the patient had symptoms. Accordingly, the number of infected cells and the duration of positivity were both higher in the universal prophylaxis group. Interestingly, despite the differences in clinical picture and in the initial immunosuppressant schedule, the serological response, as evaluated by the serum levels of specific IgG and IgM, was similar in both groups.
There is almost a consensus in the literature regarding a higher incidence of HCMV following the end of the universal prophylaxis period.2727 Kelly J, Hurley D, Raghu G. Comparison of the efficacy and cost effectiveness of pre-emptive therapy as directed by CMV antigenemia and prophylaxis with ganciclovir in lung transplant recipients. J Heart Lung Transplant. 2000;19:355-9.,2828 Florescu DF, Qiu F, Schmidt CM, et al. A direct and indirect comparison meta-analysis on the efficacy of cytomegalovirus preventive strategies in solid organ transplant. Clin Infect Dis. 2014;58:785-803. In view of that the period of prophylaxis initially recommended as three months was extended to six months.99 Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333-60. However, if universal prophylaxis is more effective than preemptive therapy in terms of the prevention of the infection and its morbidity is a matter of controversy. The use of different drugs, protocols and routes of administration for universal prophylaxis with potential different results may add some fuel to the debate. In a recent meta-analysis, ganciclovir was found to be comparable to either valganciclovir or valacyclovir regarding the prevention of CMV disease.2828 Florescu DF, Qiu F, Schmidt CM, et al. A direct and indirect comparison meta-analysis on the efficacy of cytomegalovirus preventive strategies in solid organ transplant. Clin Infect Dis. 2014;58:785-803. In the present study, universal prophylaxis, when implemented with intravenous ganciclovir, was found to be inferior to preemptive therapy: the rate of HCMV infection was not reduced and the morbidity of the infection was substantially higher with universal prophylaxis. The explanation for the particularities of the clinical course of HCMV infection under the two management strategies is uncertain but two possibilities could be proposed. Patients under universal prophylaxis underwent more aggressive immunosuppression, which may have impaired their immune response against HCMV. In addition, the use of suboptimal doses of intravenous ganciclovir may have perturbed the process of leukocyte invasion by the virus. As a result, an underlying HCMV infection could remain unrevealed and the diagnosis would only be made in a relatively advanced stage of the disease. In this circumstance, the use of more than 10 cells as a cutoff could be inappropriate. We wonder if adopting 1 cell/2 × 105 leukocytes as a cutoff for a positive test, as already recommended in other solid organ transplantation,77 Breda G, Almeida B, Carstensen S, et al. Human cytomegalovirus detection by real-time PCR and pp65-antigen test in hematopoietic stem cell transplant recipients: a challenge in low and middle-income countries. Pathog Glob Health. 2013;107:312-9.,2929 Greanya ED, Partovi N, Yoshida EM, et al. The role of the cytomegalovirus antigenemia assay in the detection and prevention of cytomegalovirus syndrome and disease in solid organ transplant recipients: a review of the British Columbia experience. Can J Infect Dis Med Microbiol. 2005;16:335-41. would have changed the course of disease of those on universal prophylaxis.
Reports using intravenous ganciclovir as universal prophylaxis are scarce in the literature since oral administration of drugs is the preferred route in this setting. However, consistent with our findings, a study with intravenous ganciclovir as universal therapy in lung transplantation reported a rate of 68% of HCMV infection in the first year post-transplant.3030 Schröeder R, Michelon T, Wurdig J, et al. The incidence of cytomegalovirus infection in lung transplant recipients under universal prophylaxis with intravenous ganciclovir. Braz J Infect Dis. 2007;11:212-4. Perhaps not accidently, the study was also carried out in our country, in which oral drugs for HCMV prophylaxis are not made available by the public health system. Considering the mentioned specificity of the present study, our findings cannot be used to argue the concept that universal prophylaxis with oral drugs reduces and delays HCMV infection.1818 Couzi L, Helou S, Bachelet T, et al. Preemptive therapy versus valgancyclovir prophylaxis in cytomegalovirus-positive kidney transplant recipients receiving antithymocyte globulin induction. Transplant Proc. 2012;44:2809-13.,2121 Kliem V, Fricke L, Wollbrink T, et al. Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: results of a randomized clinical trial. Am J Transplant. 2008;8:975-83.,3131 Limaye AP, Bakthavatsalam R, Kim HW, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006;81:1645-52. It does suggest though that when implemented using intravenous ganciclovir, universal prophylaxis does not seem to be warranted in light of our findings.
In conclusion, the present study reiterate that routine monitoring during the first three post-transplant months has a positive impact on early detection of HCMV replication allowing timely treatment to reduce the morbidity of the disease. The strategy of universal therapy employing intravenous ganciclovir was associated to a more severe clinical course of HCMV infection allowing us to think that the use of >10 cells/2 × 105 leukocytes as a cutoff in this setting may be inappropriate. Further studies, with larger sample sizes, may be needed to confirm our results.
-
FundingSupport from CAPES and FAPERJ, E-26/111.225/2013.
Acknowledgements
The authors thanks of Andrezza Cucinelli and Fabiana de Araujo Pinto, for the laboratorial support.
References
-
1Varani S, Landini MP. Cytomegalovirus-induced immunopathology and its clinical consequences. Herpesviridae. 2011;2:6.
-
2Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22:76-98.
-
3Linares L, Sanclemente G, Cervera C, et al. Influence of cytomegalovirus disease in outcome of solid organ transplant patients. Transplant Proc. 2011;43:8.
-
4Lúcia M, Crespo E, Cruzado JM, et al. Human CMV-specific T-cell responses in kidney transplantation; toward changing current risk-stratification paradigm. Transpl Int J Eur Soc Organ Transplant. 2014;27:643-56.
-
5Fehr T, Cippà PE, Mueller NJ. Cytomegalovirus post kidney transplantation: prophylaxis versus pre-emptive therapy. Transpl Int. 2015;28:1351-6.
-
6Boaretti M, Sorrentino A, Zantedeschi C, et al. Quantification of cytomegalovirus DNA by a fully automated real-time PCR for early diagnosis and monitoring of active viral infection in solid organ transplant recipients. J Clin Virol Publ Pan Am Soc Clin Virol. 2013;56:124-8.
-
7Breda G, Almeida B, Carstensen S, et al. Human cytomegalovirus detection by real-time PCR and pp65-antigen test in hematopoietic stem cell transplant recipients: a challenge in low and middle-income countries. Pathog Glob Health. 2013;107:312-9.
-
8David-Neto E, Triboni AHK, Paula FJ, et al. A double-blinded, prospective study to define antigenemia and quantitative real-time polymerase chain reaction cutoffs to start preemptive therapy in low-risk, seropositive, renal transplanted recipients. Transplantation. 2014;98:1077-81.
-
9Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333-60.
-
10Zhang L-F, Wang Y-T, Tian J-H, et al. Preemptive versus prophylactic protocol to prevent cytomegalovirus infection after renal transplantation: a meta-analysis and systematic review of randomized controlled trials. Transpl Infect Dis Off J Transplant Soc. 2011;13:622-32.
-
11San-Juan R, Navarro D, García-Reyne A, et al. Effect of delaying prophylaxis against CMV in D+/R- solid organ transplant recipients in the development of CMV-specific cellular immunity and occurrence of late CMV disease. J Infect. 2015;71:561-70.
-
12Jamal AJ, Husain S, Li Y, et al. Factors for late-onset cytomegalovirus infection or disease in kidney transplant recipients. Transplantation. 2014;97:569-75.
-
13Monforte V, Román A, Gavaldà J, et al. Preemptive therapy with intravenous ganciclovir for the prevention of cytomegalovirus disease in lung transplant recipients. Transplant Proc. 2005;37:4039-42.
-
14Harvala H, Stewart C, Muller K, et al. High risk of cytomegalovirus infection following solid organ transplantation despite prophylactic therapy. J Med Virol. 2013;85:893-8.
-
15Puttini C, Carmellini M, Garosi G, et al. HCMV infection in renal transplant recipients: a retrospective cohort study. New Microbiol. 2013;36:363-71.
-
16Schroeder R, Michelon T, Fagundes I, et al. Cytomegalovirus disease latent and active infection rates during the first trimester after kidney transplantation. Transplant Proc. 2004;36:896-8.
-
17Kamei H, Ito Y, Onishi Y, et al. Cytomegalovirus (CMV) Monitoring After Liver Transplantation: Comparison of CMV Pp65 Antigenemia Assay with Real-Time PCR Calibrated to WHO International Standard. Ann Transplant. 2016;21:131-6.
-
18Couzi L, Helou S, Bachelet T, et al. Preemptive therapy versus valgancyclovir prophylaxis in cytomegalovirus-positive kidney transplant recipients receiving antithymocyte globulin induction. Transplant Proc. 2012;44:2809-13.
-
19Jung GO, Kim S-J, Choi G-S, et al. The effect of cytomegalovirus antigenemia titer on the efficacy of preemptive therapy for the prevention of cytomegalovirus disease after kidney transplantation. Transplant Proc. 2010;42:804-10.
-
20Ozaki KS, Pestana JOM, Granato CFH, et al. Sequential cytomegalovirus antigenemia monitoring in kidney transplant patients treated with antilymphocyte antibodies. Transpl Infect Dis. 2004;6:63-8.
-
21Kliem V, Fricke L, Wollbrink T, et al. Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: results of a randomized clinical trial. Am J Transplant. 2008;8:975-83.
-
22Kesiraju S, Paritala P, Rao Ch, et al. Anti-thymocyte globulin versus basiliximab induction in renal transplant recipients: long-term outcome. Saudi J Kidney Dis Transplant. 2014;25:9-15.
-
23Sesso RC, Lopes AA, Thomé FS, et al. Brazilian Chronic Dialysis Census 2014. J Bras Nefrol Orgao. 2016;38:54-61.
-
24Caring for Australians with Renal Impairment (CARI). The CARI guidelines. CMV disease and kidney transplant: prophylaxis for cytomegalovirus infection in patients following renal transplantation. Nephrol Carlton Vic. 2004;9 Suppl. 3:S27-31.
-
25Nishida H, Ishida H, Tanaka T, et al. Cytomegalovirus infection following renal transplantation in patients administered low-dose rituximab induction therapy. Transpl Int. 2009;22:961-9.
-
26Wirgart BZ, Claesson K, Eriksson B-M, et al. Cytomegalovirus (CMV) DNA amplification from plasma compared with CMV pp65 antigen (ppUL83) detection in leukocytes for early diagnosis of symptomatic CMV infection in kidney transplant patients. Clin Diagn Virol. 1996;7:99-110.
-
27Kelly J, Hurley D, Raghu G. Comparison of the efficacy and cost effectiveness of pre-emptive therapy as directed by CMV antigenemia and prophylaxis with ganciclovir in lung transplant recipients. J Heart Lung Transplant. 2000;19:355-9.
-
28Florescu DF, Qiu F, Schmidt CM, et al. A direct and indirect comparison meta-analysis on the efficacy of cytomegalovirus preventive strategies in solid organ transplant. Clin Infect Dis. 2014;58:785-803.
-
29Greanya ED, Partovi N, Yoshida EM, et al. The role of the cytomegalovirus antigenemia assay in the detection and prevention of cytomegalovirus syndrome and disease in solid organ transplant recipients: a review of the British Columbia experience. Can J Infect Dis Med Microbiol. 2005;16:335-41.
-
30Schröeder R, Michelon T, Wurdig J, et al. The incidence of cytomegalovirus infection in lung transplant recipients under universal prophylaxis with intravenous ganciclovir. Braz J Infect Dis. 2007;11:212-4.
-
31Limaye AP, Bakthavatsalam R, Kim HW, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006;81:1645-52.
Publication Dates
-
Publication in this collection
Jan-Feb 2017
History
-
Received
8 June 2016 -
Accepted
19 Sept 2016