Abstract
The expansion of <FONT FACE=Symbol>gd </font>T cells in patients with active cutaneous leishmaniasis, with or without glucantime therapy, was investigated. Twenty patients with local cutaneous leishmaniasis including glucantime-treated (n=10) and untreated (n=10) patients were selected. The controls were healthy individuals (n=10) living in endemic areas. Whole blood was obtained and the T cell subpopulations were analyzed by flow cytometry. Significantly more <FONT FACE=Symbol>gd</FONT> CD3+ T cells were observed in untreated patients (15.9% ± 5.9), when compared with glucantime-treated patients (4.6% ± 1.4) and controls (5.3% ± 2.3). On the other hand, when the percentages of ab CD3+ T-cells were analyzed different results were obtained. A significant increase in <FONT FACE=Symbol>ab</FONT> T cells was seen in glucantime-treated patients (62.4% ± 7.6), when compared to the untreated patients (55.7% ± 5.5) and controls (55.1% ± 9.6). The percentage of total CD3+ T cells was statistically greater in both glucantime-treated (68.8% ± 7.4) and untreated patients (73.4% ± 5.9) when compared to the controls (61% ± 10.3). These results are consistent with previous results on the expansion of <FONT FACE=Symbol>gd</FONT>T cells during the course of cutaneous leishmaniasis. They also indicate that glucantime therapy can reverse the expansion of <FONT FACE=Symbol>gd</FONT>T cells and as a result increase the percentages of <FONT FACE=Symbol>ab</FONT> CD3+ T cells.
Cutaneous leishmaniasis; gamma-delta T cells; glucatime therapy
Expansion of gd T cells in patients infected with cutaneous leishmaniasis with and without glucantime therapy
Haideh Darabi; Mohsen Abolhassani; Amina Kariminia and Mohammad H. Alimohammadian
Department of Immunology, Pasteur Institute of Iran, Tehran 13164, IRAN
Address to correspondence Address to correspondence Dr. Haideh Darabi, Dept. Of Immunology, Pasteur Institute of Iran Tehran 13164, IRAN Phone/Fax: (98-21) 649-2596 E-mail: hdarabi@institute.pasteur.ac.ir
ABSTRACT
The expansion of gd T cells in patients with active cutaneous leishmaniasis, with or without glucantime therapy, was investigated. Twenty patients with local cutaneous leishmaniasis including glucantime-treated (n=10) and untreated (n=10) patients were selected. The controls were healthy individuals (n=10) living in endemic areas. Whole blood was obtained and the T cell subpopulations were analyzed by flow cytometry. Significantly more gd CD3+ T cells were observed in untreated patients (15.9% ± 5.9), when compared with glucantime-treated patients (4.6% ± 1.4) and controls (5.3% ± 2.3). On the other hand, when the percentages of ab CD3+ T-cells were analyzed different results were obtained. A significant increase in ab T cells was seen in glucantime-treated patients (62.4% ± 7.6), when compared to the untreated patients (55.7% ± 5.5) and controls (55.1% ± 9.6). The percentage of total CD3+ T cells was statistically greater in both glucantime-treated (68.8% ± 7.4) and untreated patients (73.4% ± 5.9) when compared to the controls (61% ± 10.3). These results are consistent with previous results on the expansion of gd T cells during the course of cutaneous leishmaniasis. They also indicate that glucantime therapy can reverse the expansion of gd T cells and as a result increase the percentages of ab CD3+ T cells.
Key Words: Cutaneous leishmaniasis, gamma-delta T cells, glucatime therapy.
Human leishmaniasis is caused by protozoan parasites of the genus Leishmania, which infects host macrophages. The clinical diseases may vary in form and severity from self-limiting granulomatous lesions of the skin to destructive mucosal involvement, and mild to fatal visceral infections. Both human and murine studies suggest that the progression to disease caused by leishmania infection depends on the types of T cells that are stimulated. The current hypothesis is that the activation of CD4+ Th1 cells leading to the production of IFN-g is critical for recovery from disease. Conversely, the stimulation of CD4+ Th2 cells, resulting in IL-4 and IL-10 production, likely contributes to disease progression [1, 2].
CD4+ T lymphocytes recognize antigens in the context of self major histocompatibility complex using a T cell receptor (TCR) composed of a and b chains in association with the CD3 protein complex. Another population of T cells uses a different TCR, composed of g and d chains. Most gd T cells do not express CD4 or CD8 markers and are mainly present in the lymph nodes, spleen and blood of mammals [3]. These cells also accumulate in the gut mucosa, pulmonary mucosa, reproductive organs and epidermis [4, 5]. gd T cells make up <5% of peripheral blood lymphocytes [6]. Although, little is known about the immunological function of the gd T cells, they have been shown to secrete cytokines such as IL-2, IL-3, IL-4, IL-5, IL-10, TNF-a, IFN-g and GM-CSF [7]. Furthermore, the presence of gd T cells in certain lesions has suggested that they play a role in bacterial [7, 8] as well as parasitic infection [9]. Expansion of gd T cells has been observed in genetically resistant mice following L. Major infection, indicating that gd T cells may be involved in host defense against this parasite [10].
Pentavalent antimonials such as meglumine antimoniate (glucantime) are a common drug for treatment of leishmania in human. We studied the expansion of gd T cells in patients infected with cutaneous leishmaniasis, with and without glucantime treatment.
Material and Methods
Patients. Because of the limited number of cases, 20 patients with local cutaneous leishmaniasis (LCL) (age 36 ± 19.2 years) were selected randomly from the endemic area of Kashan (a city located 230 kilometers south of Tehran, Iran). Diagnosis of cutaneous leishmaniasis was confirmed to be L. Major by parasite isolation, culture, positive skin test [11] and by clinical identification. Patients were divided into two groups (10 in each group) based on therapy with or without glucantime. Healthy uninfected individuals matched with the same sex and age living in the endemic area (n = 10) were used as controls.
Flow cytometry. Whole blood was obtained from patients and healthy individuals. Samples were collected in sterile tubes containing sodium heparin anticoagulant and processed within 6h. The following reagents were used: mouse anti-human CD3-FITC, TCR ab-PE, TCR-gdPE (Becton Dickinson, U.S.). Also, the appropriate immunoglobulins were used as isotype controls. The lymphocyte population was gated by CD45/14 (leucogate). Phenotypic analysis was done with two-color staining. Analysis of cells was done with an FACScan and LYSIS II software (Becton Dickinson Immunocytometry systems, USA). At least 10,000 cells were analyzed per sample.
Statistical analysis. Student T-test analysis was used for determining difference between the groups.
Results
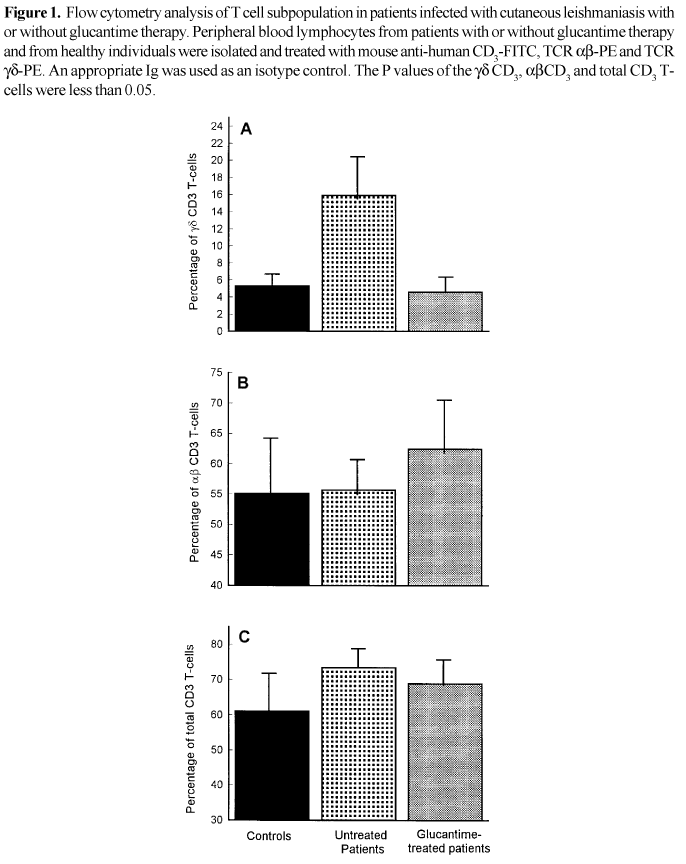
Expansion of gd CD3+ and ab CD3+ T-cells in glucantime-treated and untreated patients. Flow cytometry analysis of the blood samples of patients with active cutaneous leishmaniasis without glucantime therapy showed a significant increase of gd CD3 T cells (P<0.05) when compared with glucantime-treated patients and controls (Figure 1A). On the other hand, a significant increase of ab CD3 T cells (P<0.025) was shown in glucantime-treated patients (Figure 1B). No significant changes of ab CD3 T cells was obtained in the untreated patients, when compared with controls (Figure 1B). When the percentage of CD3 T cells was analyzed in all groups, a significant increase was obtained in patients, especially untreated individuals. The percentages of CD3 T cells correlated well with total gd and ab T cells (Figure 1C).
Discussion
We report the expansion of peripheral blood gdT cells in patients infected with local cutaneous leishmaniasis without glucantime therapy (15.9% ± 5.9 vs. Control 5.3% ± 2.3). No expansion of gd T cells was found in glucantime-treated patients (4.6 % ± 1.4). The same results were obtained not only in patients suffering from visceral leishmaniasis [12] and in patients infected with L. Amazonensis, but also in the skin lesion of the patients infected with cutaneous leishmaniasis [13] and American leishmaniasis [4]. The expansion of gd T cells were also observed when normal human T-cell blasts were cultured in the presence of L. Donovani amastigotes [14].
Similar results were reported by many investigators in murine system. A significant increase in activated gd T cells was observed in lymphoid organs and the skin lesions of mice infected with L. Major. Also, an expansion was observed in mice treated with anti-IgD antibodies, or when infected with Nippostrongliss brasiliensis [5]. These agents seem to induce a strong Th2 response. These data are consistent with our results and suggest an implication of gd T cells in the immune response to leishmania parasites. Recent studies have shown that gd T cells are involved in the first line of defense against Leishmania major infection [4]. Anti-gd TCR mAb, following L. Major infection, significantly delayed the resolution of cutaneous lesions in genetically resistant CBA/J mice and resulted in the development of larger lesions containing an increased number of parasites in both BALB/c and CBA/J mice [15]. Also, it was shown that in most patients a subset of circulatory gd T cells co-expressed a CD8 marker [12]. This finding raises the possibility that some populations of gd T cells can interact with antigen in the context of class-I MHC proteins. Both gd CD8+ T cells and the null gd T cells were expanded in theLeishmania antigen response of human T cells lines [12].
Since the pentamonial therapy such as glucantime is widely used against leishmaniosis, we tried to study the expansion of gd T cells in patients with cutaneous leishmaniasis undergoing glucantime therapy. We observed that in glucantime-treated patients, the expansion of gd T cells was reversed (15.9% ± 5.9 for untreated vs. 4.6% ± 1.4 in treated patients). The same results were observed in mice treated with glucantime. As compared to untreated mice, the percentage of gdT cells was reduced by 50% in the spleens of glucantime-treated BALB/c mice [15]. These data may indicate that in the presence of glucantime the load of parasites will decrease and therefore there is no need for the expansion of gd T cells.
It seems the expansion of gd T cells in vivo depends on the activity of CD4+ ab T cells that secrete Th2 cytokines [5]. Our data indicate that untreated patients have the same percentages of ab T cells (55.7% ± 5.5) as the endemic controls (55.1% ± 9.6), however, the glucantime-treated patients had increased percentages of ab T cells (62.4% ± 7.6).
gd T cells play a protective role in infection with various pathogens and expand significantly on days 3 and 6 after infection by L. Monocytogenes [16]. Mice depleted of ab-T cells by mAb treatment, showed resistance to infection by L. Monocytogenes within the first few days after infection [17]. The precise role of gd T cells during infection with L. Major and the mechanism by which these cells could influence the outcome of the disease are important issues that remain to be elucidated.
Received on 10 June 2002; revised 18 September 2002.
- 1. Russo D.M., Chakrabarti P., and Burns GM., Jr. Native human T cells develope into Th1 or Th0 effectors and exhibit cytotoxicity early after stimulation with Leishmania-infected macrophages. J Infec Dis 1998;177:1345-51.
- 2. Kemp K., Kemp M., Kharazmi A., et al. Leishmania-specific T cells expressing interferon-gamma (IFN-g and IL-10 upon activation are expanded in individuals cured of visceral leishmaniasis. Clin Exp Immunol 1999;116:500-4.
- 3. Raulet D.H. The structure, function, and molecular genetics of the gd T cell receptor. Annu Rev Immunol 1989;26:175-207.
- 4. Satoskar A., Okano M., David J.R. gd T cells are not essential for control of cutaneous Leishmania major infection in genetically resistant C57BL/6 mice. J Infect Dis 1997;176:1649-52.
- 5. Rosat J.P., Conceicao-silva F., Waanders G.A., et al. Expansion of gd T cells in BALB/c mice infected with Leishmania major is dependent upon Th2-type CD4 T cells. Infec Immun 1995;63:3000-4.
- 6. Falcao R.P., Voltarelli J.C., Simoes B.P., et al. Malignant T gd lymphoproliferative disease with natural killer lytic activity. Am J Hematol 1992;41:128-31.
- 7. Has W., Pereira P., Tonegawa S. Gamma/delta cells. Ann Rev Immunol 1993;11:637-85.
- 8. Fu Y.X., Roak C.E., Kelly K., et al. Immune protection and control of inflammatory tissue necrosis by gd T cells. J Immunol 1994;244: 713-6.
- 9. Roberts S.J., Smith A.L., West A.B., et al. T cell ab and gd deficient mice display abnormal but distinct phenotypes towards a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA 1996;93:11774-9.
- 10. Rosat J.P., MacDonald H.R., Louis J.A. A role for gd T cells during experimental infection of mice withLeishmania major. J Immunol 1993;150:550-5.
- 11. Alimohammadian M.H., Almasi H., Khabiri A., Hatam G., et al. Identification of species and characteristics of an outbreak of cutaneous Leishmaniasis in a New Focus of Iran. Iran Biomed J 1999;3:31-9.
- 12. Russo D.M., Armitage R.J., Barral-Netto M., et al. Antigen-reactive gd T cells in human leishmaniasis. J Immunol 1993;151:3712-8.
- 13. Uyemura K., Klotz J., Pirmez C., et al. Microanatomic clonality of gd T cells in human leishmaniasis lesions. J Immunol 1992;148:1205-11.
- 14. Saha A., Chakrabarti G., Sen S., Bandyopadhyay S. Leishmania donovani parasites interact with gd human peripheral blood T cells and induce susceptibility to NK cell-mediated lysis. Scand J Immunol 1999;50: 588-95.
- 15. Rosat J.P., MacDonald H.R., Louis J.A. A role for gd T cells during experimental infection of mice with Leishmania major. J Immunol 1993;150:550-5.
- 16. Hiromatsu K., Yoshikai Y., Matsuzaki G., et al. Protective role of gd T cells in primary infection with Listeria monocytogenes in mice. J Exp Med 1992;175:49-56.
- 17. Naiki Y., Nishimura H., Itohara S., Yoshikai Y. gd T cells may dichotomously modulate infection with avirulent Salmonella cholerasuis via IFN-g and IL-13 in mice. Cell Immunol 2000;202:61-9.
Address to correspondence
Publication Dates
-
Publication in this collection
15 July 2003 -
Date of issue
Oct 2002
History
-
Reviewed
18 Sept 2002 -
Received
10 June 2002