Abstract
We bacteriologically analyzed 156 species of Enterobacteriaceae, isolated from 138 patients with community-acquired diabetic foot ulcers, in a prospective study made at a diabetic center and at the Federal University of Ceará, Brazil, from March, 2000, to November, 2001.The samples were cultured using selective media, and identification, susceptibility tests and detection of plasmid-mediated-extended-spectrum-beta-lactamase (ESBL) producing strains were made with conventional and automated methods. The most frequently occurring pathogens were K. pneumoniae (21.2%), Morganella morganii (19.9%) and E. coli (15.4%). High resistance rates were noted for ampicillin, first generation cephalosporin, trimethoprim/sulfamethoxazole, tetracycline, amoxicillin-clavulanic acid and chloramphenicol. ESBL-producing strains were detected in 6% of the patients. Resistance among gram-negative bacteria has become increasingly common, even in community-acquired infections.
ESBL-producing strains; antimicrobial resistance; Enterobacteriaceae
Plasmid-mediated extended-spectrum b-lactamase-producing strains of Enterobacteriaceae isolated from diabetes foot infections in a Brazilian diabetic center
Motta R.N.; Oliveira M.M.; Magalhães P.S.F.; Dias A.M.; Aragão L.P.; Forti A.C.; Carvalho C.B.M.
Centre of Diabetes and Hypertension, Federal University of Ceará, Fortaleza/CE, Brazil
Correspondence Correspondence to Dr. Cibele B.M. Carvalho Departamento de Patologia e Medicina Legal (UFC) Rua Monsenhor Furtado s/n Cx Postal 3163. 60441-750 Fortaleza-CE/BR Fax: (85) 288 8316 E-mail: cbmc@secrel.com.br
ABSTRACT
We bacteriologically analyzed 156 species of Enterobacteriaceae, isolated from 138 patients with community-acquired diabetic foot ulcers, in a prospective study made at a diabetic center and at the Federal University of Ceará, Brazil, from March, 2000, to November, 2001.The samples were cultured using selective media, and identification, susceptibility tests and detection of plasmid-mediated-extended-spectrum-b-lactamase (ESBL) producing strains were made with conventional and automated methods. The most frequently occurring pathogens were K. pneumoniae (21.2%), Morganella morganii (19.9%) and E. coli (15.4%). High resistance rates were noted for ampicillin, first generation cephalosporin, trimethoprim/sulfamethoxazole, tetracycline, amoxicillin-clavulanic acid and chloramphenicol. ESBL-producing strains were detected in 6% of the patients. Resistance among gram-negative bacteria has become increasingly common, even in community-acquired infections.
Key Words: ESBL-producing strains, antimicrobial resistance, Enterobacteriaceae.
Nowadays, diabetes mellitus is one of the world's most important health problems. In Brazil, there are about 5 million diabetics, half of which are unaware that they have the disease. Among the population between 30 and 69 years old the prevalence of diabetics is 7.6%. Most diabetic patients who are attended at Brazilian University hospitals have foot ulcers. The prevalence of diabetes is 6.4% in the state of Ceará, Brazil, while the rate of cases that are undiagnosed is 64% [1,2].
It has been estimated that 15% of diabetic patients develop an ulcer on the foot or ankle at some time. Infection is a common sequel of diabetic foot ulceration and trauma, which, once established, progressively worsens and becomes more difficult to treat. The infections are normally polymicrobial. The organisms involved include aerobic gram-positive cocci, gram-negative bacilli (Escherichia coli, Klebsiella and Proteus species) and anaerobes (Bacteroides species and Peptostreptococcus) [3].
Enterobacteria are an important pathogenic group in community and hospital-acquired infections and resistance to antibiotics has become increasingly common. The most serious resistance patterns now emerging among Gram-negative organisms include resistance to extended-spectrum cephalosporins and penicillins [4].
In Latin America, resistant bacteria are emerging as a serious threat to patients with common infections in community and hospital settings. The time to change long-accepted prescription practices is coming, and every effort should be made to provide drug prescribers with pertinent information on changes in patterns of bacterial resistance to antibiotics [5].
In fact, local epidemiological studies of bacterial resistance are especially important in developing countries where auto medication (with antimicrobial agents) is common. Given the considerable importance of enterobacteria and their participation in diabetic foot ulcer infections, our objectives were:
a) to analyze, in a prospective study, the prevalence of enterobacteria and ESBL-producing strains isolated from diabetic patients with community-acquired foot infection.
b) to monitor the patterns of antibiotic susceptibilities of the bacterial species isolated in this investigation.
Materials and Methods
Patient Selection. The subjects of this study consisted of - 141 patients with diabetes who had an infected foot ulcer and who were attended at the CIDH (Centro Integrado de Diabetes e Hipertensão) located in Fortaleza, Ceará, Brazil, between March 1, 2000 and November 30, 2001. The data was collected prospectively. Each patient was included only once in the study. They were taken to a ambulatory clinic specialized in foot ulcers after they had been registered at the health station and after their diabetes was diagnosed.
Bacteriological Procedures. All the patients had signs of mild to moderate localized infection (cellulites, exudates, etc.). The surface of the wound was prepared with a vigorous saline scrub, followed by debridement of superficial exudates with sterile instruments. Specimens were obtained by scraping the ulcer base or the deepest part of the wound edge with a sterile swab. The specimens were collected with a swab and transported in Stuart transport medium [6,7]. Bacteriological studies were performed in the microbiology laboratory of the Federal University of Ceará. Total transfer time to the laboratory was no longer than two hours. The specimens were inoculated into blood agar (BAP), MacConkey agar and Brain Heart Infusion broth (BHI). The cultures were incubated at 35oC. Bacteria were identified by conventional biochemical tests [8] and/or by an automated system VITEK (bioMérieux Vitek, St. Louis).
Susceptibility Testing. Antimicrobial susceptibility testing of isolates was done by the reference agar diffusion method, as described by the National Committee for Clinical Laboratory Standards (NCCLS) [9] and by the VITEK system. Quality control was done by the Escherichia coli ATCC test (American Type Culture Collection) 25922. The antimicrobial agents tested were: ampicillin, amikacin, gentamicin, amoxicillin-clavulanic acid, aztreonam, cephalotin, cefoxitin, cefotaxime, ceftriaxone, ciprofloxacin, cefepime, chloramphenicol, imipenem, trimethoprim/sulfamethoxazole and tetracycline.
ESBL-Producing Strains. Those strains, that were phenotypically identified as ESBL-producing strains in the agar diffusion method and identified as ESBL-producing strains by the VITEK system, were subsequently tested with the double disk diffusion technique, as recommended by NCCLS.
Results
Information on Clinical Isolates. The Enterobacteria (97.8%) were the most frequently isolated bacteria, with 156 species from 138 patients. The five most frequently occurring pathogens were (number/%): Klebsiella pneumoniae (33/21.2%), Morganella morganii (31/19.9%), Escherichia coli (24/15.4%), Proteus mirabilis (23/14.7%) and Enterobacter aerogenes (10/6.4%). The six most frequent pathogens accounted for 77.6% of all isolates.
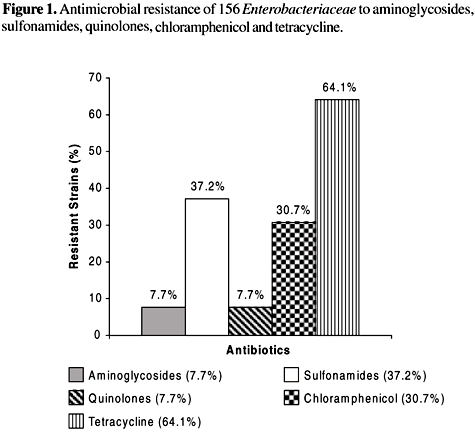
Susceptibilities. The resistance rates of the Enterobacteriaceae strains to ampicillin, tetracycline, cefalotin and sulfonamides were 68.6%, 64.1%, 51.3% and 37.2%, respectively (Figures 1 and 2). The most effective drugs against these enterobacteria were the carbapenems (100% were susceptible to imipenem), followed by ciprofloxacin and the fourth generation cephalosporins cefepime and gentamicin.
ESBL-Producing Strains. ESBL-producing strains were isolated from 10 patients. The species found were K. pneumoniae (seven isolates) and E. coli (three isolates). Four of the patients had been interned during the preceding 30 days. One patient had a recent lesion that had been developing for 10 days without any treatment with antibiotics. The remaining five patients had been treated with antibiotics during the preceding 30 days (cefalexin and ampicillin). None of the aminoglycosides showed good activity against these 10 isolates. The antimicrobial activities of the b-lactamase inhibitor compounds, quinolones, aztreonam, and another class of antibiotics, chloramphenicol and trimethoprim/sulfamethoxazole, were unsatisfactory. Only imipenem showed good activity; it inhibited 100% of the isolates.
Discussion
Enterobacteria are commonly isolated from infected ulcers in diabetic patients. In the seventies, Louie et al [10] isolated enterobacteria from 11 patients among a group of 20 patients with foot ulcers. Goldstein et al. (1996) [11] isolated enterobacteria from 12 of 25 patients, in a prospective study made to determine the relative frequency of bacterial isolates cultured from community-acquired foot infections. Patients who had previously received antibiotics were more likely to have MRSA, enteroccci and P. aeruginosa. In our study, enterobacteria were the most frequently isolated species (in 97.8% of the patients). Cefalexin was taken by 56% of these patients.
There is a strong association between heavy antimicrobial consumption within a population and the frequency of recovery of resistant bacteria [12]. Cars et al. [13], compared the non-hospital use of antibiotics in the 15 member states of the European Union in 1997 and found that in 11 of these countries, the most commonly used antibiotic was broad-spectrum penicillin. Data on antibiotic sales are not publicly available in many countries, including Brazil. A high percentage of the Enterobacteria that we isolated in our study were resistant to ampicillin, tetracycline, first generation cephalosporin, trimethoprim/sulfamethoxazole, amoxicillin + clavulanic acid and chloramphenicol. It is unclear why the Gram-negative isolates should manifest such high rates of resistance. The reasons may include differences in the use of antimicrobial substances, differences in infection control practices, different climate, and other unrecognized factors [14].
Enterobacteria are an important group in community and hospital-acquired infections. They are common precipitants of sepsis by virtue of the inflammatory response, activated by endotoxins present in the Gram-negative cell wall. Patients with diabetes mellitus and dialysis patients are at high risk for enterobacteria infection. Unfortunately, resistance has become increasingly common among gram-negative bacteria, making empirical therapy decisions more difficult. The most serious resistance patterns now emerging among Gram-negative organisms include resistance to extended-spectrum cephalosporins and penicillins [4]. This resistance is commonly mediated by ESBLs in Escherichia coli and Klebsiella species, or by the hyper production of chromosomally mediated cephalosporinases (Bush group I amp C enzymes) in Citrobacter, Serratia and Citrobacter species [13]. The ESBL genes generally result from point mutations in the genes of broad-spectrum b-lactamase Ambler class A enzymes, such as TEM-1, TEM-2 or SHV-1. They are usually located in conjugative megaplasmids, which often carry genes responsible for resistance to other antibacterial drugs, making it extremely difficult to treat infections caused by bacteria that produce these enzymes [15].
Along with ESBLs, plasmid-mediated Ambler class C cephalosporinases (or Bush group 1 cephalosporinases) have been found in clinical isolates of the Enterobacteriaceae. These enzymes can produce resistance to cephamycins, extended spectrum cephalosporins and aztreonam, and unlike class A ESBLs, b-lactamase inhibitors do not inhibit these bacteria [16].
The emergence of plasmid-mediated extended-spectrum b-lactamases (ESBLs) among members of the Enterobacteriaceae has increased worldwide [17-19]. In France, 14.1% of the K. pneumoniae isolated from 12 university hospitals up to 1990 were cefotaxime resistant, and, 51% of the K. pneumoniae isolated in a training hospital were ESBL-producing [19]. In the United States, National Nosocomial Infection Surveillance notifications up to 1991 indicated 5% resistance to ceftazidime. Although these strains were initially only found on the east and west coasts, and in the Chicago region, K. pneumoniae ESBL producing strains are now widespread all over the United States (20,21). One thousand two hundred and ten clinical isolates of Escherichia coli, collected from a University hospital in Southern Taiwan in 1999, were screened for ESBL-producing strains; the rate of prevalence in that study was 1.6%, lower than that reported in Japan (2.9 to 8.%) [17].
Until 1987, there were no reports of ESBLs from the southern cone of South America (5). In 1997 the SENTRY Antimicrobial Surveillance Program analyzed ESBL producing strains (E. coli and K. pneumoniae), isolated from the bloodstream, and found that ESBL producing strains causing blood infections varied from 4.5% (Uruguay) to 12% (Chile and Mexico) among E. coli, and from 31% (Mexico) to 56.6% (Brazil) among K. pneumoniae [4].
In 1998 a Brazilian multicenter study was conducted by the Sentry Antimicrobial Surveillance Program in 20 clinical laboratories and 36 hospitals located in different regions of the country; 855 isolates were evaluated, including 591 enterobacteria. The two most frequently occurring pathogens were E. coli and K. pneumoniae. Among the Brazilian isolates collected by the SENTRY program, 13.6% of the E. coli and 42.1% of the K. pneumoniae were ESBL-producing strains [22,23]. In our study, the frequency of ESBL-producing strains was 6%, possibly reflecting community spread of these strains. The resistance profile was similar to that of other studies described in the SENTRY surveillance program, and it indicates a combination of other beta-lactamases, hyper production of ESBL, or 2br group ESBL, according to the Bush et al. classification [16].
Conclusions
Unfortunately, resistance among gram-negative bacteria has become increasingly common, making empirical therapy decisions more difficult. Sometimes empirical therapy is necessary, especially in therapeutic centers that have no microbiology laboratories and limited resources. Government assistance is often needed in order for the patients to obtain medication. Improvements need to be made to microbiological laboratories in hospitals and medical centers. There is also a need for periodic antibiotic resistance surveys to help orient physicians and the local population on the best treatment strategies. Without firmly established diagnosis and antibiotic use strategies, we are treating blinding, with inevitable negative consequences.
Acknowledgements
The authors wish to express their gratitude to Dr. Carlos Alberto Cunha Nascimento, Graziela Nogueira da Silva and Terezinha de Jesus dos Santos Rodrigues for technical help.
Received on 23 July 2002
revised 10 September 2002
-
1Sociedade Brasileira de Diabetes. Diagnóstico e Classificação de DM e tratamento de DM tipo 2. Available from: http://www.diabetes.org.br
- 2. Fundação Nacional de Saúde. Guia de Vigilância Epidemiológica. Available from: http://www.funasa.gov.br
- 3. Slovenkai M.P. Foot problems in diabetes. Medical Clinics of North America 1998;82: 9948-970.
- 4. Diekema D.J., Pfaller M.A., Jones R.N., et al. Survey of bloodstream infections due to Gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin Infect Dis 1999; 29:595-607.
- 5. Guzmán-Blanco M., Casellas J.M., Sader H.S. Bacterial resistance to antimicrobial agents in Latin America. The giant is awakening. Infect Dis Clin North Am 2000;14:67-81
- 6. Sapico S.L., Witte J.L., Canawati H.N., et al. The infected foot of the diabetic patient: quantitative microbiology and analysis of clinical features. Reviews of Infectious Diseases 1994;6:171-6.
- 7. Johnson S., Lebahn F., Peterson L.R., Gerding D.N. Use of anaerobic collection and transport swab device to recover anaerobic bacteria from infected foot ulcers in diabetics.Clin Infect Dis 1995;20:289-90.
- 8. Murray P.R., Baron E.J., Pfaller M.A., et al. Manual of Clinical Microbiology. 7th ed. Washington DC: ASM Press, 1999
-
9National Committee for Clinical laboratory Standards. Performance Standards for antimicrobial disk susceptibility tests; approved standard. NCCLS, 6th ed. 1999
- 10. Louie T.J.,Bartlett J.G., Tally F.P., Gorbach S.L. Aerobic and anaerobic bacteria in diabetic foot ulcers. Ann Intern Med 1976;85:461-3.
- 11. Goldstein E.J.C., Citron D.M., Nesbitt C.A. Diabetic foot infection. Diabetes Care 1996;19:638-41.
- 12. Enne V.I., Livermore D.M., Stephensa P., Hall L.M. Persistence of sulphonamide resistance in Escherichia coli in the UK despite national prescribing restriction. Lancet 2001;357:1325-8.
- 13. Cars O., Mölstad S., Melander A. Variation in antibiotic use in the European Union. Lancet 2001;357:1851-3.
- 14. Hart CA, Kariuki S. Antimicrobial resistance in developing countries. BMJ 1998;317:647-50.
- 15. Livermore, D. M. b-Lactamases in laboratory and Clinical Resistance. Clin Microbiol Rev 1995; 8:557-84.
- 16. Bush K., Jacoby G.A., Medeiros A.A. A functional classification scheme for b-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother 1995;39:1211-33.
- 17. Yan J.J., Ko W.C., Tsai S.H., et al. Dissemination of CTX-M-3 and CMY-2 b-Lactamases among clinical isolates of Escherichia coli in Southern Taiwan. J Clin Microbiol 2000;38:4320-5.
- 18. Piroth L., Aubé H., Doile J.M., Martin M.V. Spread of extended-spectrum b-lactamase producing Klebsiella pneumoniae: are b-lactamase inhibitors of therapeutic value? Clin Infect Dis 1998;27:76-80.
- 19. Sirot D.L., Goldstein F.W., Soussy C.J., et al. Resistance to cefotaxime and seven other b-lactams in members of the family Enterobacteriaceae: a 3 years survey in France. Antimicrob Agents Chemother 1992;36:1677-81.
- 20. Tenover F.C., Hughes J.M. The challenges of emerging infectious diseases: development and spread of multiply resistant bacterial pathogens. JAMA 1996;275(4):300-4.
- 21. Tenover F.C., Mohammed M.J., Gorton T.S., Dembek Z.F. Detecting and reporting of organisms producing extended-spectrum b-lactamases: survey of laboratories in Connecticut. J Clin Microbiol 1999;37:4065-70.
- 22. Sader H.S. Antimicrobial resistance in Brazil: comparison of results from two multicenter studies. Braz J Infect Dis 2000;4(2):91-9.
- 23. Gales A.C., Bolmströn A., Sampaio J., et al. Antimicrobial susceptibility of Klebsiella pneumoniae producing extended-spectrum beta-lactamases (ESBL) isolated in Brazilian Hospitals. Braz J Infect Dis 1997;1:196-203.
Publication Dates
-
Publication in this collection
21 Nov 2003 -
Date of issue
Apr 2003
History
-
Reviewed
10 Sept 2002 -
Received
23 July 2002