Abstract
Quinolones and fluoroquinolones are widely used to treat uropathogenic Escherichia coli infections. Bacterial resistance to these antimicrobials primarily involves mutations in gyrA and parC genes. To date, no studies have examined the potential relationship between biochemical characteristics and quinolone resistance in uropathogenic E. coli strains. The present work analyzed the quinolone sensitivity and biochemical activities of fifty-eight lactose-negative uropathogenic E. coli strains. A high percentage of the isolates (48.3%) was found to be resistant to at least one of the tested quinolones, and DNA sequencing revealed quinolone resistant determining region gyrA and parC mutations in the multi-resistant isolates. Statistical analyses suggested that the lack of ornithine decarboxylase (ODC) activity is correlated with quinolone resistance. Despite the low number of isolates examined, this is the first study correlating these characteristics in lactose-negative E. coli isolates.
ODC; gyrA ; parC ; uropathogenic
Introduction
Urinary tract infections (UTIs) are the second cause of antimicrobial prescriptions in South Brazil and are one of the major causes of office visits and hospitalization in the United States; these infections primarily affect women, pregnants and elderly people (Foxman, 2002Foxman B (2002) Epidemiology of Urinary Tract Infections: Incidence, Morbidity, and Economic Costs. Am J Med 113:5S–13S.; Tavares et al., 2008Tavares NUL, Bertoldi AD, Mucillo-Baisch AL (2008) Prescrição de antimicrobianos em unidades de saúde da família no Sul do Brasil. Caderneta de Saúde Pública 24:1791–1800.). Escherichia coli is the main agent of UTIs, especially in community-acquired UTIs, and quinolones and fluoroquinolones have been used extensively to treat these infections (Ronald, 2003Ronald A (2003) The etiology of urinary tract infection: traditional and emerging pathogens. Disease a Month 49:71–82.; Van Bambeke et al., 2005Van Bambeke F, Michot JM, Van Eldere J et al. (2005) Quinolones in 2005: an update. Clin Microbiol Infect 11:256–280.). Since these antimicrobials agents were introduced, resistant strains have emerged and spread around the world (Schito et al., 2009Schito GC, Naber KG, Botto H et al. (2009) The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents 34:407–413.). Many studies have sought to understand the mechanisms of resistance, to develop more efficient antibiotics, and more recently, to relate resistance to biochemical or genetic characteristics (Lemos et al., 2011Lemos TD, Tavares LGF, Arais LR et al. (2011) Avaliação da biotipagem na distinção de perfis de virulência de amostras de Escherichia coli uropatogênica isoladas no hospital universitário Antonio Pedro, Niterói, RJ XXVI Congresso Brasileiro de Microbiologia, Foz do Iguaçu, PR.; Rodriguez-Martínez et al., 2011Rodriguez-Martínez JM, Cano ME, Velasco C et al. (2011) Plasmid mediated quinolone resistance: an update. J Infect Chemother 17:149–182.). The main mechanism of quinolone resistance involves mutations in the quinolone resistance determining region (QRDR) of gyrA and parC genes. The most common mutations are within the Ser83 and Asp87 codons in gyrA and within the Gly78, Ser80 and Glu84 codons in parC. The gyrA and parC genes encode subunits of DNA gyrase and topoisomerase IV, two enzymes involved in DNA supercoiling and DNA decatenation, respectively (Hooper, 2000Hooper DC (2000) Mechanisms of Action and Resistance of Older and Newer Fluoroquinolones. Clin Infect Dis 31:24–28.). In E. coli, DNA gyrase is more susceptible to inhibition by quinolones than topoisomerase IV. In gram-negative bacteria, a single mutation in gyrA can reduce the susceptibility of DNA gyrase, furthermore additional mutations in gyrA or in gyrB and parC can increase resistance to the antibiotic (Jacoby, 2005Jacoby GA (2005) Mechanisms of Resistance to Quinolones. Clin Infect Dis 41:S120–S126.; Minarini et al., 2012Minarini LAR, Darini ALC (2012) Mutations in the quinolone resistance-determining regions of gyrA and parC in Enterobacteriaceae isolates from Brazil. Braz J Microbiol 43:1309–1314.).
The frequency of lactose-negative (lac−) E. coliphenotype has shown to be very low, ranging from 5 to 10% (Winn et al., 2006Winn W, Allen S, Janda W et al. (2006) Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. Lippincott Williams & Wilkins, Philadelphia.; Oliveira et al., 2006Oliveira BG, Albini CA, Botão GMD et al. (2006) A identificação direta pelos meios cromogênicos é confiável a ponto de dispensar as provas bioquímicas? Newslab 75:130–142.). To characterize this group, the present work selected and evaluated uropathogenic E. coli isolates from UTIs regarding their quinolone resistance and biochemical activity profiles.
Materials and Methods
Bacterial sample
Fifty-eight lac− E. coli isolates from the urine of UTI patients from the Ponta Grossa, Brazil region were analyzed. These isolates were selected from inpatients and outpatients from 2008 to 2010. The urinary bacterial concentration used to diagnose a urine infection was > 105 colony-forming units per milliliter. The bacteria were stored until their use at −20 °C in BHI medium (Himedia, Mumbai, India) containing 15% glycerol. The lac− phenotype was confirmed in MacConkey agar (BD, Sparks, MD, EUA). E. coli ATCC 25922 was used as a control in the antibiotic susceptibility and biochemical tests.
Antibiotic susceptibility tests
The susceptibility of the E. coli isolates to the quinolones nalidixic acid (Nal), ciprofloxacin (Cip), norfloxacin (Nor) and ofloxacin (Ofx), and the beta-lactams cefotaxime (Ctx), ceftazidime (Ctz), aztreonam (Atm) and amoxicillin clavulanate (Amc) was determined using the disk diffusion method following the recommendations of the Clinical and Laboratory Standards Institute (2010)Clinical and Laboratory Standards Institute (2010). Performance standards for antimicrobial susceptibility testing; Twentieth informational supplement Document M100-S20. Wayne, Pa: Clinical and Laboratory Standards Institute.. Disks (Laborclin, Pinhais, PR, Brazil) were stored at −20 °C until their use.
Biochemical characterization
The E. coli biotype was determined using the Enterobacteriaceae identification kit (Newprov, Pinhais, PR, Brazil) according to the manufacturer’s instructions. This kit, approved by the Agência Nacional de Vigilância Sanitária (ANVISA), provides the following ten differential biochemical tests: L-tryptophan deamination; sulfidric acid, indole and gas production; glucose and rhamnose fermentation; lysine and citrate utilization; ornithine decarboxylation and motility.
Detection of gyrA and parC mutations
Multiplex PCR was used to amplify the gyrA and parCregions. Single E. coli colonies grown on MacConkey agar (BD, Sparks, MD, USA) were suspended in 15 μL of sterile deionized water and disrupted after 15 min of incubation at 95 °C. Then, the following reagents were added to a final volume of 30 μL: Taq DNA polymerase Invitrogen buffer (1), magnesium chloride (2.5 mM), deoxyribonucleotide triphosphates (dNTPs) (Invitrogen, Carlsbad, California, USA) (0.2 mM), primers (IDT, Coralville, Iowa, USA) (0.2 μM of GyrA primer and 0.4 μM of ParC primer) and Taq DNA polymerase (0.75 U). The primer sequences are listed in Table 1.
The genes were amplified using the following the thermal cycling profile: 2 min at 95 °C and 35 cycles of 30 s at 95 °C, 60 s at 55.4 °C and 60 s at 72 °C. The PCR products were separated on a 1 x TAE, 2% agarose gel and quantified using UVP Labwork Software (UVP Inc.).
The amplification products (20 μL) were treated with 10 U of exonuclease I (Biolabs, Ipswich, New England) and 1.0 U of alkaline phosphatase (USB, Cleveland, Ohio, USA) at 37 °C for 90 min. Then, the enzymes were inactivated at 80 °C for 30 min (Werle et al., 1994Werle E, Schneider C, Renner M et al. (1994). Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Res. 22:4354–4355.).
The treated PCR products (5 μL) were sequenced using 0.5 μL of primer (10 μM), 1 μL of Big Dye Terminator mix (Applied Biosystems, Carlsbad, California, USA), 3 μL of Big Dye Buffer (1X) and ultrapure H2O to a final volume of 10 μL using the following program: 2 min at 96 °C and 35 cycles of 45 s at 96 °C, 30 s at 55.4 °C and 4 min at 60 °C. The sequencing PCR products (10 μL) were precipitated using 2 μL of ammonium acetate (7.5 M), 60 μL of absolute ethanol and 10 μL of ultrapure water followed by 45 min of centrifugation. The supernatant was discharged and the precipitate was washed with 70% ethanol, dried and dissolved in deionized formamide. Sequencing was performed using a 24-capillary 3500xL System (Applied Biosystems, Carlsbad, California, USA). Reads were trimmed for the removal of low quality bases using the Phred program (Ewing et al., 1998Ewing B, Hillier L, Wendl M et al. (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8:175–185.). To detect nucleotide mutations, the DNA sequences were aligned using Clustal W (Thompson et al., 1994Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–80.) against the wild-type gyrA or parC gene nucleotide sequences from E. coli K12 substr. MG1655 (accession numbers 946614 and 947499, respectively).
Statistical analysis
Statistical analyses to correlate antibiotic resistance and biochemical characteristics were performed using Yates’ chi-squared test because it is recommended for small sample numbers (Graphpad Prism 6.0). P value < 0.01 were considered statistically significant.
Results and Discussion
Forty-eight percent of the lac− E. coli isolates (28/58) were able to grow in the presence of at least one quinolone. Among the resistant isolates, seventy-nine percent (22/28) were resistant to all quinolones tested, and 21% (6/28) were resistant only to nalidixic acid. In contrast, only one isolate displayed intermediate resistance to the beta-lactams cefotaxime and ceftazidime. Our data showed high quinolone resistance rates among the isolates. Other studies that analyzed uropathogenic E. coli isolates in the same area revealed a norfloxacin resistance rate of 11.6% in Ponta Grossa (Bail et al., 2006Bail L, Ito CA, Esmerino LA (2006) Infecção do trato urinário: comparação entre o perfil de susceptibilidade e a terapia empírica com antimicrobianos. Revista Brasileira de Análises Clínicas 38:51–56.) and 13.8% in Curitiba (Ito et al., 2008Ito CA, Gales AC, Tognim MC et al. (2008) Quinolone-resistant Escherichia coli. Braz J Infect Dis 12:5–9.). In Fortaleza in the northeast of Brazil, a lower resistance rate of 7.5% was described for norfloxacin (Araújo et al., 2011Araújo SM, Mourão TC, Oliveira JL et al. (2011) Antimicrobial resistance of uropathogens in women with acute uncomplicated cystitis from primary care settings. Int Urol Nephrol 43:461–466.). In Europe, a multicentric study showed a ciprofloxacin resistance rate from 1.4% to 12.9% (Schito et al., 2009Schito GC, Naber KG, Botto H et al. (2009) The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents 34:407–413.), whereas a higher rate (31%) was registered in the hospitalized population in Ribeirão Preto, Brazil (Santo et al., 2006Santo E, Salvador MM, Marin JM (2006) Antimicrobial resistance among urinary tract Escherichia coli isolates from inpatients and outpatients in a tertiary care center in Sao Paulo, Brazil. Int J Infect Dis 11:558–559.). Because our isolates were primarily of community origin, the elevated resistance was unexpected. The available literature analyzed all uropathogenic E. coli isolates without determining the lactose phenotype of the isolates; our work suggests that the quinolone resistance frequency is increased in lac− E. coli strains.
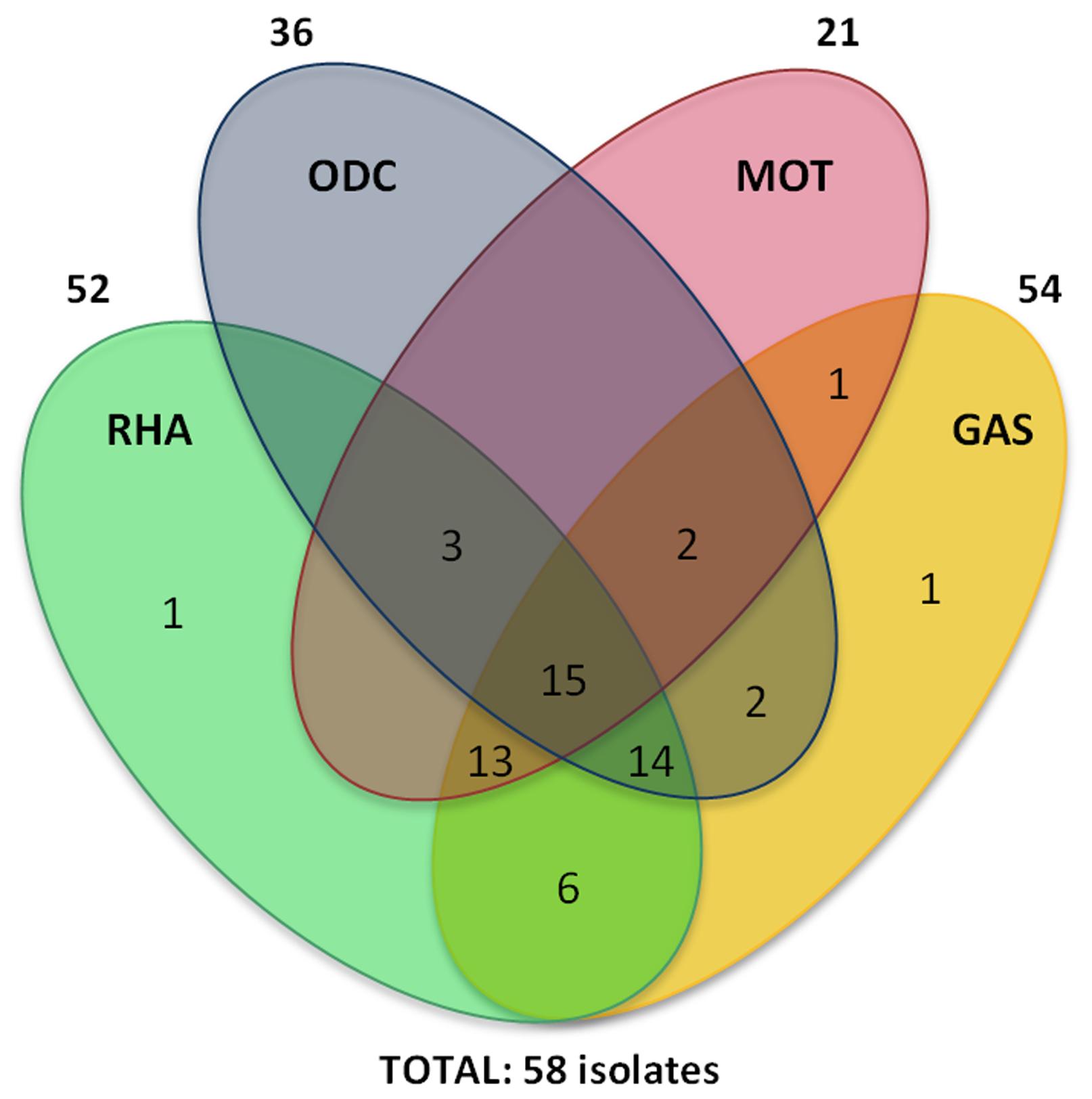
Biochemical tests identified nine E. coli biotypes. They presented a common behavior in the majority of the tests except Ornithine Decarboxylase (ODC), Motility (Mot), Gas production (Gas) and Rhamnose fermentation (Rha) (Figure 1). The most common biotypes (72%), 981, 991 and 971, were able to ferment glucose and rhamnose, to produce gas and indole and to metabolize lysine and citrate. On the other hand, they presented a distinguished profile in the ornithine decarboxylase and motility tests. Most of the ornithine decarboxylase positive (ODC+) E. coli isolates (26/36) showed to be sensitive to quinolone while most of the ODC− E. coli isolates (18/22) showed to be resistant to it. Statistical analyses revealed a relationship between ODC and quinolone resistance (p < 0.01); no relationship was identified between motility and the same resistance parameter (p > 0.05). Several studies relating biological and genetic characteristics with antibiotic resistance have been performed over the last years (Bashir et al., 2011Bashir S, Sarwar Y, Ali A et al. (2011) Multiple drug resistance patterns in various phylogenetic groups of uropathogenic E. coliisolated from Faisalabad region of Pakistan. Braz J Microbiol 42:1278–83.). Ferjani et al. (2011)Ferjani S, Saidani M, Ennigrou S et al. (2011) Virulence determinants, phylogenetic groups and fluoroquinolone resistance in Escherichia coli isolated from cystitis and pyelonephritis. Pathologie Biologie 60:270–274.showed a direct relationship among virulence determinants, phylogenetic groups and susceptibility to fluoroquinolones. A Brazilian study related E. colicarbohydrate fermentation to virulence factors. Dulcitol-positive and raffinose-negative isolates were found to be more virulent than other isolates (Lemos et al., 2011Lemos TD, Tavares LGF, Arais LR et al. (2011) Avaliação da biotipagem na distinção de perfis de virulência de amostras de Escherichia coli uropatogênica isoladas no hospital universitário Antonio Pedro, Niterói, RJ XXVI Congresso Brasileiro de Microbiologia, Foz do Iguaçu, PR.). Vila et al. (2002)Vila J, Simon K, Ruiz J et al. (2002) Are Quinolone-Resistant Uropathogenic Escherichia coli Less Virulent? J Infect Dis 186:1039–1042. related quinolone resistance and virulence factors, suggesting that quinolone-resistant strains are less virulent. The present study is the first to relate biochemical characteristics to quinolone resistance in lactose-negative E. coliisolates.
Biochemical activities of the studied uropathogenic E. coli isolates. The numbers indicate the number of isolates that presented the indicated activity or group of activities. ODC = ornithine decarboxylase; MOT = motility; RHA = rhamnose fermentation; GAS = gas production
Because the primary mechanism of quinolone resistance involves mutations in the QRDR of the gyrA and parC genes (Jacoby, 2005Jacoby GA (2005) Mechanisms of Resistance to Quinolones. Clin Infect Dis 41:S120–S126.), these regions of the isolates’ DNA were sequenced. One quinolone-sensitive lac− isolate was also included as a negative control. Of the twenty-eight quinolone resistant isolates analyzed, six could not be molecularly analyzed due to the low quality of their sequences. Their nucleotide substitutions are shown in Table 2.
The quinolone-sensitive isolate presented the same QRDR gyrA and parC sequence as the wild-type strain. The five exclusive nalidixic acid-resistant (NalR) isolates presented either the codon 83 mutation Ser → Leu (2/5), the codon 87 mutation Asp → Tyr (1/5) or no mutation in the sequenced area (2/5). They did not contain any QRDR parC mutations. It was expected that these exclusive NalR bacteria would not have many mutations as it is known that single mutations in the gyrA gene of gram-negative bacteria are able to generate this phenotype (Komp-Lindgren et al., 2003Komp-Lindgren P, Karlsson A, Hughes D (2003) Mutation Rate and Evolution of Fluoroquinolone Resistance in Escherichia coli Isolates from Patients with Urinary Tract Infections. Antimicrob Agents Chemother 47:3222–3232.) and that parC acts as a second mutation target that amplifies this resistance (Everett et al., 1996Everett MJ, Jin YF, Ricci V et al. (1996) Contributions of individual mechanisms to fluoroquinolone resistance in 36 Escherichia colistrains isolated from humans and animals. Antimicrob Agents Chemoter 40:2380–2386.). Because no mutations were found in two of the NalR isolates, their resistance could be explained either by mutations outside of QRDR regions or other mechanisms that were not explored here, such as changes in permeability or efflux pump activity (Friedman et al., 2001Friedman SM, Lu T, Drlica K (2001) Mutation in the DNA gyrase A gene of Escherichia coli that expands the quinolone resistance-determining region. Antimicrob Agents Chemoter 45:2378–2380.).
The seventeen sequenced E. coli isolates that showed resistance to all of the tested quinolones presented GyrA substitution at codons 83 (Ser → Leu) and 87 (Asp → Asn) and ParC substitution at codon 80 (Ser → Ile) and 84 (Glu → Val or Glu → Lys). Other studies have detected these QRDR GyrA and ParC amino acid mutations (Chen et al., 2001Chen JY, Siu LK, Chen YH et al. (2001) Molecular epidemiology and mutations at gyrA and parC genes of ciprofloxacin-resistant Escherichia coli isolates from a Taiwan medical center. Microb Drug Resist 7:47–53.; Mavroidi et al., 2012Mavroidi A, Miriagou V, Liakopoulos A et al. (2012) Ciprofloxacin-resistant Escherichia coli in central Greece: mechanisms of resistance and molecular identification. BMC Infect Dis 12:371–377.). These changes were shown to reduce the affinity of the drug for their targets, thus resulting in bacterial growth even in the presence of quinolone (Bernard et al., 2001Bernard FM, Maxwell A (2001) Interaction between DNA gyrase and quinolones: effects of alanine mutations at GyrA subunit residues Ser83 and Asp87. Antimicrob Agents Chemoter 45:1994–2000.).
Silva and Mendonça (2012)Silva GJ, Mendonça N (2012) Association between antimicrobial resistance and virulence in Escherichia coli. Virulence 3:18–28. suggested that the GyrA codon 83 mutation generates supercoiling DNA alterations that could modify the expression of virulence factors. In addition, Weber et al.(2013)Webber MA, Ricci V, Whitehead R et al. (2013) Clinically relevant mutant DNA gyrase alters supercoiling, changes the transcriptome, and confers multidrug resistance. MBio 4:1–10. demonstrated that alterations in supercoiling affect fundamental cellular processes, including transcription. Based on these observations, it is possible that the GyrA codon 83 mutation that was detected in all of the analyzed multi-resistant isolates could be preventing the transcription of the lac operon genes and the speC gene, thus generating lac− and ODC− phenotypes, respectively. Because we cannot rule out the possibility that these genes are absent from the genome of the isolates, further studies are necessary.
A recent work showed that sublethal concentrations of fluoroquinolones were able to produce oxidative stress. To prevent DNA damage caused by reactive oxygen species (ROS) in the bacterial cell, ODC is upregulated, and the polyamine concentration increases (Umezawa et al., 1997Umezawa N, Arakane K, Ryu A et al. (1997) Participation of reactive oxygen species in phototoxicity induced by quinolone antibacterial agents. Arch Biochem Biophys 342:275–281.; Tkachenko et al., 2011Tkachenko AG, Akhova AV, Shumkov MS et al. (2011) Polyamines reduce oxidative stress in Escherichia coli cells exposed to bactericidal antibiotics. Res Microbiol 163:83–91.). Therefore, it might be interesting to measure ROS production in the studied isolates in future experiments.
In conclusion, the studied uropathogenic lactose-negative E. coli isolates showed a high quinolone resistance rate and indicated that there is a relationship between the absence of ornithine decarboxylase activity and quinolone resistance. The present work could serve as the basis for more comprehensive studies including a greater number of isolates from different localities to confirm our results.
Acknowledgments
We thank CNPq, CAPES, INCT-FBN/CNPq-MCT and Fundação Araucária for providing financial support for this study. We would like to thank the researcher groups from UEPG, UNIFESP and UFPR for excellent technical support.
References
- Araújo SM, Mourão TC, Oliveira JL et al. (2011) Antimicrobial resistance of uropathogens in women with acute uncomplicated cystitis from primary care settings. Int Urol Nephrol 43:461–466.
- Bail L, Ito CA, Esmerino LA (2006) Infecção do trato urinário: comparação entre o perfil de susceptibilidade e a terapia empírica com antimicrobianos. Revista Brasileira de Análises Clínicas 38:51–56.
- Bashir S, Sarwar Y, Ali A et al. (2011) Multiple drug resistance patterns in various phylogenetic groups of uropathogenic E. coliisolated from Faisalabad region of Pakistan. Braz J Microbiol 42:1278–83.
- Bernard FM, Maxwell A (2001) Interaction between DNA gyrase and quinolones: effects of alanine mutations at GyrA subunit residues Ser83 and Asp87. Antimicrob Agents Chemoter 45:1994–2000.
- Cattoir V, Lesprit P, Lascols C et al. (2006) In vivo selection during ofloxacin therapy of Escherichia coli with combined topoisomerase mutations that confer high resistance to ofloxacin but susceptibility to nalidixic acid. J Antimicrob Chemother 58:1054–1057.
- Chen JY, Siu LK, Chen YH et al. (2001) Molecular epidemiology and mutations at gyrA and parC genes of ciprofloxacin-resistant Escherichia coli isolates from a Taiwan medical center. Microb Drug Resist 7:47–53.
- Clinical and Laboratory Standards Institute (2010). Performance standards for antimicrobial susceptibility testing; Twentieth informational supplement Document M100-S20. Wayne, Pa: Clinical and Laboratory Standards Institute.
- Everett MJ, Jin YF, Ricci V et al. (1996) Contributions of individual mechanisms to fluoroquinolone resistance in 36 Escherichia colistrains isolated from humans and animals. Antimicrob Agents Chemoter 40:2380–2386.
- Ewing B, Hillier L, Wendl M et al. (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8:175–185.
- Ferjani S, Saidani M, Ennigrou S et al. (2011) Virulence determinants, phylogenetic groups and fluoroquinolone resistance in Escherichia coli isolated from cystitis and pyelonephritis. Pathologie Biologie 60:270–274.
- Foxman B (2002) Epidemiology of Urinary Tract Infections: Incidence, Morbidity, and Economic Costs. Am J Med 113:5S–13S.
- Friedman SM, Lu T, Drlica K (2001) Mutation in the DNA gyrase A gene of Escherichia coli that expands the quinolone resistance-determining region. Antimicrob Agents Chemoter 45:2378–2380.
- Hooper DC (2000) Mechanisms of Action and Resistance of Older and Newer Fluoroquinolones. Clin Infect Dis 31:24–28.
- Ito CA, Gales AC, Tognim MC et al. (2008) Quinolone-resistant Escherichia coli Braz J Infect Dis 12:5–9.
- Jacoby GA (2005) Mechanisms of Resistance to Quinolones. Clin Infect Dis 41:S120–S126.
- Komp-Lindgren P, Karlsson A, Hughes D (2003) Mutation Rate and Evolution of Fluoroquinolone Resistance in Escherichia coli Isolates from Patients with Urinary Tract Infections. Antimicrob Agents Chemother 47:3222–3232.
- Lemos TD, Tavares LGF, Arais LR et al. (2011) Avaliação da biotipagem na distinção de perfis de virulência de amostras de Escherichia coli uropatogênica isoladas no hospital universitário Antonio Pedro, Niterói, RJ XXVI Congresso Brasileiro de Microbiologia, Foz do Iguaçu, PR.
- Mavroidi A, Miriagou V, Liakopoulos A et al. (2012) Ciprofloxacin-resistant Escherichia coli in central Greece: mechanisms of resistance and molecular identification. BMC Infect Dis 12:371–377.
- Minarini LAR, Darini ALC (2012) Mutations in the quinolone resistance-determining regions of gyrA and parC in Enterobacteriaceae isolates from Brazil. Braz J Microbiol 43:1309–1314.
- Oliveira BG, Albini CA, Botão GMD et al. (2006) A identificação direta pelos meios cromogênicos é confiável a ponto de dispensar as provas bioquímicas? Newslab 75:130–142.
- Rodríguez-Martínez JM, Velasco C, Pascual A et al. (2006) Correlation of quinolone resistance levels and differences in basal and quinolone-induced expression from three qnrA-containing plasmids. Clin Microbiol Infect 12:440–445.
- Rodriguez-Martínez JM, Cano ME, Velasco C et al. (2011) Plasmid mediated quinolone resistance: an update. J Infect Chemother 17:149–182.
- Ronald A (2003) The etiology of urinary tract infection: traditional and emerging pathogens. Disease a Month 49:71–82.
- Santo E, Salvador MM, Marin JM (2006) Antimicrobial resistance among urinary tract Escherichia coli isolates from inpatients and outpatients in a tertiary care center in Sao Paulo, Brazil. Int J Infect Dis 11:558–559.
- Schito GC, Naber KG, Botto H et al. (2009) The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents 34:407–413.
- Silva GJ, Mendonça N (2012) Association between antimicrobial resistance and virulence in Escherichia coli Virulence 3:18–28.
- Tavares NUL, Bertoldi AD, Mucillo-Baisch AL (2008) Prescrição de antimicrobianos em unidades de saúde da família no Sul do Brasil. Caderneta de Saúde Pública 24:1791–1800.
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–80.
- Tkachenko AG, Akhova AV, Shumkov MS et al. (2011) Polyamines reduce oxidative stress in Escherichia coli cells exposed to bactericidal antibiotics. Res Microbiol 163:83–91.
- Umezawa N, Arakane K, Ryu A et al. (1997) Participation of reactive oxygen species in phototoxicity induced by quinolone antibacterial agents. Arch Biochem Biophys 342:275–281.
- Van Bambeke F, Michot JM, Van Eldere J et al. (2005) Quinolones in 2005: an update. Clin Microbiol Infect 11:256–280.
- Vila J, Simon K, Ruiz J et al. (2002) Are Quinolone-Resistant Uropathogenic Escherichia coli Less Virulent? J Infect Dis 186:1039–1042.
- Webber MA, Ricci V, Whitehead R et al. (2013) Clinically relevant mutant DNA gyrase alters supercoiling, changes the transcriptome, and confers multidrug resistance. MBio 4:1–10.
- Werle E, Schneider C, Renner M et al. (1994). Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Res. 22:4354–4355.
- Winn W, Allen S, Janda W et al. (2006) Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. Lippincott Williams & Wilkins, Philadelphia.
Publication Dates
-
Publication in this collection
21 July 2015 -
Date of issue
Jul-Sep 2015
History
-
Received
06 Dec 2013 -
Accepted
30 Oct 2014