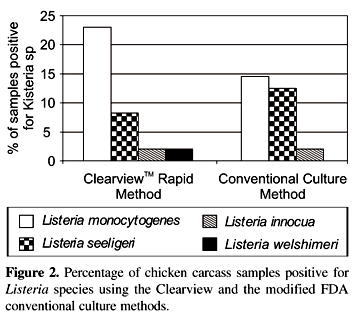
The occurence of Listeria species in refrigerated chicken carcasses was evaluated, comparing the conventional culture methodology of FDA, modified by the introduction of a secondary enrichment step prior plating, and the ClearviewTM rapid method (Oxoid, UK Ltd). Forty-eight refrigerated whole chicken carcasses purchased from supermarkets in Florianópolis, SC, Brazil, were analysed. Listeria species occured in 21 (43.7%) samples. Using the Clearview method, 17 (35.4%) samples were positive for Listeria species. Of these isolates, 11 (23%) were L. monocytogenes, 4 (8.3%) L. innocua, 1 (2.1%) L. welshimeri and 1 (2.1%) L. seeligeri. Using the conventional culture methodology of FDA (with modifications), 14 (29.2%) samples were positive for Listeria species. Among these 7 (14.6%) were L. monocytogenes, 6 (12.5%) L. innocua and 1 (2.1%) L. seeligeri. With the Clearview rapid method plus API Listeria for identification, results were confirmed to Listeria species level within 115-139 h. Using the conventional culture method of FDA (with modifications) plus API Listeria, results were confirmed within 120-160 h. However, the Clearview method could indicate the presence of Listeria organisms in only 43 h. Results given by the methods were in moderate concordance and the differences between them were not significant (C.I. = 95%).
Listeria; chicken carcasses; ClearviewTM