ABSTRACT
Carnivorous plant species, such as Utricularia spp., capture and digest prey. This digestion can occur through the secretion of plant digestive enzymes and/or by bacterial digestive enzymes. To comprehend the physiological mechanisms of carnivorous plants, it is essential to understand the microbial diversity related to these plants. Therefore, in the present study, we isolated and classified bacteria from different organs of Utricularia breviscapa (stolons and utricles) and from different geographic locations (São Paulo and Mato Grosso). We were able to build the first bacterium collection for U. breviscapa and study the diversity of cultivable bacteria. The results show that U. breviscapa bacterial diversity varied according to the geographic isolation site (São Paulo and Mato Grosso) but not the analyzed organs (utricle and stolon). We reported that six genera were common to both sample sites (São Paulo and Mato Grosso). These genera have previously been reported to be beneficial to plants, as well as related to the bioremediation process, showing that these isolates present great biotechnological and agricultural potential. This is the first report of an Acidobacteria isolated from U. breviscapa. The role of these bacteria inside the plant must be further investigated in order to understand their population dynamics within the host.
Keywords:
Utricularia breviscapa; Microbial ecology; Aquatic microbiota; Microbial communities
Introduction
There are over 700 reported species of carnivorous plants distributed in 5 orders (Caryophyllales, Ericales, Lamiales, Oxalidales and Poales) and 10 families that occur in different habitats all over the world.11 Król E, Płachno BJ, Adamec L, Stolarz M, Dziubinska H, Trebacz K. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Ann Bot. 2011;109:47-64. This carnivorous syndrome has arisen independently at least six times in the evolutionary history of angiosperms over an extensive evolutionary timespan and involving different morphological adaptations to capture and digest the prey.22 Ellison AM, Gotelli NJ. Energetics and the evolution of carnivorous plants-Darwin's ‘most wonderful plants in the world’. J Exp Bot. 2009;60:19-42.,33 Reifenrath K, Theisen I, Schnitzler J, Porembski S, Barthlott W. Trap architecture in carnivorous Utricularia (Lentibulariaceae). Flora. 2006;201:597-605.
The plant must present certain features to be classified as carnivorous, such as actively attracting, capturing and digesting prey, absorbing nutrients from it and obtaining some advantages in growth or reproduction.44 Givnish TJ. New evidence on the origin of carnivorous plants. Proc Natl Acad Sci USA. 2015;112:10-11. The prey can be an additional source of N, P, S, K and Mg and complement these photoautotrophic plants.55 Adamec L. Mineral nutrition of carnivorous plants. Bot Rev. 1997;63:273-299.,66 Ellison AM, Gotelli NJ. Evolutionary ecology of carnivorous plants. Trends Ecol Evol. 2001;16:623-629.
The genus Utricularia (Lentibulariaceae), with over 220 species, is the most widespread of the carnivorous plants. One species of this genus is Utricularia breviscapa, which can be naturally found in the Antilles and South America. It is an aquatic plant, and its suspended structures contain air-filled parenchyma that allows it to float. Its stolons are threadlike, and bear segmented leaves and utricules (the traps).77 Taylor P. The genus Utricularia. In: A taxonomic monograph. Kew bulletin additional series XIV. Royal Botanic Gardens. Londres: Kew; 1989:668–671.Utricularia species present foliar structures called utricles, which are highly specialized traps that are capable of capturing prey by suction through the action of negative internal hydrostatic pressure.88 Poppinga S, Daber LE, Westermeier AS, et al. Biomechanical analysis of prey capture in the carnivorous Southern bladderwort (Utricularia australis). Sci Rep. 2017;7:1776.,99 Płachno BJ, Adamec LL, Lichtscheidl IK, Peroutka M, Adlassnig W, Vrba J. Fluorescence labelling of phosphatase activity in digestive glands of carnivorous plants. Plant Biol. 2006;8:813-820.
In many carnivorous plant species, digestion does not necessarily occur through the secretion of plant digestive enzymes but is performed by bacterial and/or fungal digestive enzymes.22 Ellison AM, Gotelli NJ. Energetics and the evolution of carnivorous plants-Darwin's ‘most wonderful plants in the world’. J Exp Bot. 2009;60:19-42.,1010 Chase MW, Christenhusz MJM, Sanders D, Fay MF. Murderous plants: Victorian Gothic, Darwin and modern insights into vegetable carnivory. Bot J Linn Soc. 2009;161:329-356. For example, in some species of carnivorous plants, such as Byblis (Byblidaceae), Brocchinia (Bromeliaceae), Darlingtonia, Heliamphora and some species of Sarracenia (Sarraceniaceae), bacterial enzymes are essential in the absence of plant enzyme secretion, and among the plants that have such glands, the bacterial community also plays an important role in the optimization process of prey degradation.44 Givnish TJ. New evidence on the origin of carnivorous plants. Proc Natl Acad Sci USA. 2015;112:10-11.,1111 Adlassnig W, Peroutka M, Lendl T. Traps of carnivorous pitcher plants as a habitat: composition of the fluid, biodiversity and mutualistic activities. Ann Bot. 2011;107:181-194.
These endophytes living inside the plant have innumerous advantages, since the internal tissues provide a stable environment without ultraviolet rays, temperature fluctuations or nutrient competition with other microorganisms as well as an increased availability of nutrients.1212 Bordiec S, Paquis S, Lacroix H, et al. Comparative analysis of defence responses induced by the endophytic plant growth-promoting rhizobacterium Burkholderia phytofirmans strain PsJN and the non-host bacterium Pseudomonas syringae pv. pisi in grapevine cell suspensions. J Exp Bot. 2011;62:595-603.–1414 Mcinroy JA, Kloepper JW. Population dynamics of endophytic bacteria in field-grown sweet corn and cotton. Can J Microbiol. 1995;41:895-901. This microorganism-plant interaction is important for the survival of the plant, since these microorganisms represent several benefits for the host plant, providing protection against pathogens, promoting growth, increasing the ability to capture trace elements and several other benefits that are extremely important for the maintenance of plant balance.1515 Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN. Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett. 2008;278:1-9.,1616 Sessitsch A, Kuffner M, Kidd P, et al. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem. 2013;60:182-194.
Therefore, it is important to understand the microbial diversity related to carnivorous plants, particularly bacterial diversity, since it can represent approximately 58% of the total viable microbial biomass,1717 Sirová D, Borovec J, Cerna B, Rejmánková E, Adamec L. Microbial community development in the traps of aquatic Utricularia species. Aquat Bot. 2009;90:129-136. playing a key role in the trap. Caravieri et al.1818 Caravieri FA, Ferreira AJ, Ferreira A, Clivati D, Miranda VFO, Araújo WL. Bacterial community associated with traps of the carnivoral plants Utricularia hydrocarpa and Genlisea filiformis. Aquat Bot. 2014;116:8-12. were the first to report the total bacterial diversity from Utricularia hydrocarpa and Genlisea filiformis traps using a non-cultivable approach, showing that only 1.2% of the observed operational taxonomic units (OTU) were shared by both analyzed plants (U. hydrocarpa and G. filiformis). Moreover, in addition to plant genotype, trap age can also influence the microbial communities inside the vesicles.1919 Płachno B, Łukaszek M, Wołowski K, Adamec L, Stolarczyk P. Aging of Utricularia traps and variability of microorganisms associated with that micro-habitat. Aquat Bot. 2012;97:44-48.
The present study aims to isolate the bacterial community from different organs (stolons and utricles) of U. breviscapa, which may have great biotechnological potential, in order to build the first bacterium collection for U. breviscapa and study the diversity of cultivable bacteria, aiming to understand their function in this unique environment.
Materials and methods
Plant sampling
The samples of U. breviscapa plants were collected from two different floodplains in Brazil: (1) Santo Antônio de Leverger municipality, Mato Grosso state (MT) and (2) Rio Tietê flood plain, Mogi das Cruzes municipality, São Paulo state (SP) (Table 1 and Fig. 1). The sample plants were stored in plastic bags duly identified with sample number and the point from which they were collected, maintained in freshwater at environmental temperature. After collection, the samples were transported to the Laboratory of Molecular Biology and Microbial Ecology where the isolation was immediately carried out. Vouchers are deposited in Herbarium HUMC.
Habit of Utricularia breviscapa with inflorescence (bar = 10 mm). The detail shows a stolon with utricles (arrow indicates an utricule; bar = 5 mm).
Bacterial isolation
The maceration method was adapted from Kuklinsky-Sobral et al.2020 Kuklinsky-Sobral J, Araújo WL, Mendes R, Pizzirani-Kleiner AA, Azevedo JL. Isolation and characterization of endophytic bacteria from soybean (Glycine max) grown in soil treated with glyphosate herbicide. Plant Soil. 2005;273:91-99. for the bacterial isolation from weighed stolons and utricles of U. breviscapa previously washed in sterilized water. Bacteria were isolated by soaking 5 stolon fragments (approximately 5 mm per fragment) or 10 utricles in phosphate-buffered saline (PBS) containing Na2HPO4 (1.44 g L-1), KH2PO4 (0.24 g L-1), KCl (0.20 g L-1), and NaCl (8.00 g L-1), adjusting the pH to 7.4, and macerating. After maceration, the sample volume was adjusted to 1 mL, appropriate dilutions were carried out and plated onto 10% trypticase soy agar (TSA) supplemented with 50 mg mL-1 of the fungicide Imazalil (Magnate 500 CE, Agricur), and the plates were incubated at 28 ºC for 2–15 days. The colonies were removed from the plates, inoculated onto 10% TSA agar medium, incubated at 28 ºC for 2–10 days, and then each isolate was suspended in a 20% glycerol solution and stored at -70 ºC.
Statistical analyses were carried out with biological triplicates for each treatment for bacterial quantification in the isolation experiments, which were performed in a completely random design. The significance of the observed differences was verified using a new analysis of variance (p = 0.05). Analyses were conducted using R software version 3.0.1.
Polymerase chain reaction (PCR) amplification and 16S rDNA sequencing
After cultivation, the bacterial DNA was extracted according to Araújo et al.2121 Araújo WL, Marcon J, Maccheroni W, van Elsas JD, van Vuurde JW, Azevedo JL. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl Environ Microbiol. 2002;68:4906-4914.. A partial sequence of the 16S rRNA gene was amplified using the pair of primers R13782222 Heuer H, Krsek M, Baker P, Smalla K, Wellington EMH. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol. 1997;63:3233-3241. and P027F.2323 Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, Pace NR. Rapid-determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci USA. 1985;82:6955-6959. PCRs were performed according to Dourado et al.2424 Dourado MN, Andreote FD, Dini-Andreote F, Conti R, Araújo JM, Araújo WL. Analysis of 16S rRNA and mxaF genes revealing insights into Methylobacterium niche-specific plant association. Genet Mol Biol. 2012;35:142-148. All PCR amplification was checked through electrophoresis on agarose gel (1.5%, w/v agarose) and UV visualization of the ethidium bromide-stained gels, after which the PCR products were purified using a GFX PCR DNA and gel band purification kit (Amersham Biosciences) and sequenced by Sanger Sequencing Technology2525 Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463-5467. using the primer 1378R.
Analysis of the 16S rRNA gene sequence
Sequences were obtained from the bacteria isolated from U. breviscapa stolons and utricles from São Paulo and Mato Grosso state. Prior to the analysis, all chromatograms were trimmed with Phred-Phrap (http://www.phrap.com/phred/). The sequences were clustered as operational taxonomic units (OTU) using MOTHUR2626 Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537-7541. and a cut-off of 97% identity and further examined using rarefaction analysis and Libshuff, and dendrograms were constructed. Furthermore, richness and diversity were also calculated using MOTHUR based on non-parametric richness (Ace and Chao1) and diversity (Shannon-Weaver and Simpson) indexes with cut-offs for similarity at 97% (0.03), 95% (0.05) and 91% (0.09) identity. Taxonomic classifications were performed using RDP Query (https://rdp.cme.msu.edu) as database parameters. For phenetic analysis, the sequences were aligned using ClustalW,2727 Thompson JD, Higgins DG, Gibson TJ. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acid Res. 1994;22:4673-4680. and the distance was calculated using the Jukes and Cantor model2828 Jukes TH, Cantor CR. Evolution of protein molecules. In: Munro HN, ed. Mammalian protein metabolism. New York, USA: Academic Press; 1969:21–132. using Neighbor Joining method in MEGA 5 software.2929 Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731-2739. The branches were tested with bootstrap analyses (1000 replications), and the layout of trees was designed using the online application “Interactive Tree Of Life” (iTOL) (http://itol.embl.de/).3030 Letunic I, Bork P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics. 2007;23:127-128.
A total of 200 DNA sequences of partial 16S rRNA genes were deposited in the GenBank database under accession numbers KY453794 to KY453980.
Results and discussion
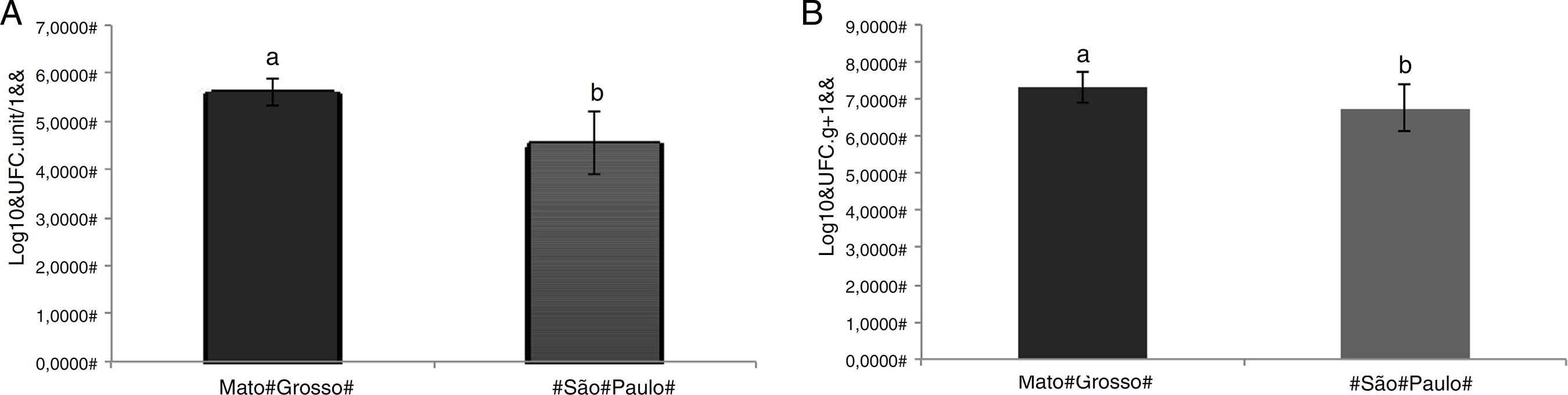
A total of 200 sequences were obtained from bacteria isolated from U. breviscapa. Higher isolated bacterial density was observed in association with plants from Mato Grosso (MT) (Fig. 2). The bacterial density averages from MT samples were 4.9 × 105 CFU utricle-1 and 3.6 × 107 CFU g of stolon-1, while the samples from São Paulo (SP) had bacterial densities of 6.0 × 104 CFU utricle-1 and 1.2 × 107 CFU g of stolon-1 (Fig. 2).
Bacterial density in (A) utricles and (B) stolons of U. breviscapa from Mato Grosso (MT) and São Paulo (SP). Statistically different at p < 0.05.
The richness estimator of OTUs performed with the Chao1 and Ace indexes at 97% (Table 2) showed a greater bacterial richness in SP plants than in MT plants. According to the Chao1 parameter, we can observe the highest richness in utricles followed by stolons in SP plants. However, based on the Ace estimator, the opposite was observed, in which the Ace index showed the highest richness in stolons followed by utricles. In terms of bacterial richness in MT plants, Chao1 estimates a statistically equal richness in both utricles and stolons (Table 2).
It is common knowledge that the performance of non-parametric estimators depends on the species abundance distribution in the sample, and the preference for one over another is a difficult issue. Basualdo3131 Basualdo CV. Choosing the best non-parametric richness estimator for benthic macroinvertebrates databases. Rev Soc Entomol Argent. 2011;70:27-38. observed in his work that the Ace index was better when the observed richness was low and Chao1 when the observed richness was high. In another study, Hortal et al.3232 Hortal J, Borges PA, Gaspar C. Evaluating the performance of species richness estimators: sensitivity to sample grain size. J Anim Ecol. 2006;75:274-287. mentioned that abundance-based non-parametric estimators (Ace and Chao1) are more precise compared with other richness estimators; however, their precision diminishes at lower sampling intensities, producing less consistent results. This suggests that the choice among richness estimator parameters can vary between sampling characteristics, even in the same study.
According to the Simpson index diversity at 97%, a higher diversity of OTUs was found in libraries constructed from SP plants, with the highest diversity in stolons (stolSP) followed by utricles (utricSP). Similar to São Paulo, the bacterial diversity index in MT plants was also higher in stolons (stolMT) followed by utricles (utricMT). The Shannon diversity index at 97% showed the same result.
Therefore, for all indexes used (Chao1, Ace, Simpson and Shannon), higher diversity rates were observed in plants from São Paulo. There is no apparent reason why São Paulo samples present a higher diversity when compared with Mato Grosso samples, but it is known that there are various characteristics that could be considered to contribute to this variation, of which the trap age has been the most cited in the literature, but the quality of water can also impact the microbial diversity. Plachno et al.1919 Płachno B, Łukaszek M, Wołowski K, Adamec L, Stolarczyk P. Aging of Utricularia traps and variability of microorganisms associated with that micro-habitat. Aquat Bot. 2012;97:44-48. found that old traps were colonized by attached bacteria, but they can also be found freely suspended in trap fluid, as shown in Sirová et al.1717 Sirová D, Borovec J, Cerna B, Rejmánková E, Adamec L. Microbial community development in the traps of aquatic Utricularia species. Aquat Bot. 2009;90:129-136. However, determining utricle dynamics is further complicated by the rapid aging of traps (or pitcher), as their life cycle is completed over approximately 30 days, and many environmental changes occur during this time.3333 Friday LE. Rapid turnover of traps in Utricularia vulgaris L. Oecologia. 1989;80:272-277. Although their life cycle is short, the interior of utricles presents low concentrations of oxygen, and it has been postulated that the organisms trapped by Utricularia die as a result of oxygen depletion.3434 Plachno BJ. The carnivorous plants and Charles Darwin – a story about beautiful fascination. In: Zarzycki K, Mirek Z, Korzeniak U, eds. Karol Darwin w oczach polskich botaników XIX–XXI w. PAN IB Kraków; 2010. This anoxic environment is reflected in its commensal communities, selecting for anaerobic and facultatively aerobic bacteria.3535 Alcaraz LD, Martínez-Sánchez S, Torres I, Ibarra-Laciette E, Herrera-Estrella L. The metagenome of Ultricularia gibba's traps: into the microbial input to a carnivorous plant. PLOS ONE. 2016;11:1-21. Pitsch et al.3636 Pitsch G, Adamec L, Dirren S, et al. The green Tetrahymena utriculariae n. sp. (Ciliophora, Oligohymenophorea) with its endosymbiotic algae (Micractinium sp.), living in traps of a carnivorous aquatic plant. J Eukaryot Microbiol. 2017;64:322-335. reported a ciliate commensal that uses algae to circumvent the low concentration of oxygen inside the utricles. Furthermore, some researchers have assumed that this high abundance of commensal organisms also occurs in empty traps; in the same way, these microorganisms are not specialized for living inside the traps, and they can therefore live either on the external surface or freely as plankton in the ambient water.3737 Adamec L. The smallest but fastest. Ecophysiological characteristics of traps of aquatic carnivorous Ultricularia. Plant Signal Behav. 2011;6:640-646.
Another factor that could influence the microbial community inside the traps is the composition of the water surrounding the trap that will be sucked in together with the prey or even attached to the prey. Thus, the bacterial community is selected according to the physical–chemical conditions inside the trap. It is important to emphasize that the two sampled sites were located in floodplain areas. River floodplain systems are known for their heterogeneity in habitats and hydrological pulses, presenting great temporal and spatial variation in their limnological conditions, which, in turn, should influence the bacterial community composition.3838 Laque T, Farjalla VF, Rosado AS, Esteves FA. Spatio temporal variation of bacterial community composition and positive controlling factors in tropical shallow lagoons. Microb Ecol. 2010;59:819-829.
The Libshuff significance test indicated that those libraries belonging to the same part of the plant (stolSP/stolMT and utricSP/utricMT) (Table 3) differ significantly for each sampled site (São Paulo and Mato Grosso). Moreover, although they are associated with the same site, the significance analysis for utricleMT and stolonMT shows a significant difference between these parts of the host plant. On the other hand, when comparing utricleSP and stolonSP, which present higher richness and diversity in their 16S rDNA libraries, do not vary, indicating that only in less diverse environments are there different colonization patterns between stolons and utricles.
A dendrogram of bacterial communities showed similarities between the same site libraries (Fig. 3). Agreeing with the richness and diversity indexes, the libraries were grouped according to the sampled site, grouping utricles and stolons from São Paulo (utricSP and stolSP) in the same branch (with presents higher diversity), while utricles and stolons from Mato Grosso (utricMT and stolMT) were grouped in another branch (which presents lower diversity). This indicates that there is greater bacterial community similarity between different parts of the same host plant collected from the same sampled site (São Paulo or Mato Grosso), corroborating the hypothesis that the place of origin plays the main role in bacteria-plant interactions, since the environment supplies the microbial diversity to the plant host.
In looking at the 200 isolates from SP and MT, respectively, the 16S rRNA analysis of plant isolates from MT showed 22 bacterial genera, among them Sphingomonas (36.71%), Aquitalea (18.99%) and Bacillus (6.33%), while in the plant isolates from São Paulo, we found 31 bacterial genera, of which the predominant genera were Microbacterium (20.66%), Sphingomonas (14.05%) and Pelomonas (9.09%) (Fig. 4). Caravieri et al.1818 Caravieri FA, Ferreira AJ, Ferreira A, Clivati D, Miranda VFO, Araújo WL. Bacterial community associated with traps of the carnivoral plants Utricularia hydrocarpa and Genlisea filiformis. Aquat Bot. 2014;116:8-12. also found Sphingomonas and Microbacterium genera in association with U. hydrocarpa, but the culture-independent methods used in their work found these OTUs among the less abundant genera.
Phenetic trees representing the cultivable bacterial found in utricles and stolons from U. breviscapa were built using Neighbor Joining method in MEGA 5 software. (A) Isolates from Mato Grosso and (B) from São Paulo. The solid gray circles next to the tree branches correspond to bootstrap values higher than 50%. Brachyspira innocens (S000437178); Brachyspira hyodysenteriae (S000437188), Brachyspira hyodysenteriae (S000437188) and Brachyspira hyodysenteriae (S000437188) was used as outgroup.
Six genera were common to both sampled sites (SP and MT): Aquitalea, Azospirillum, Bacillus, Chromobacterium, Novosphingobium and Sphingomonas (Figs. 4 and 5). As shown for bacterial density, it was also verified that the abundance of cultivable bacterial species varied according to the sampled sites, suggesting that the plant selects the bacterial community in its tissues, and this selection must occur from the diversity present in its surrounding environment. Moreover, Koopman et al.3939 Koopman MM, Fuselier DM, Hird S, Carstens BC. The carnivorous pale pitcher plant harbors diverse, distinct, and time-dependent bacterial communities. Appl Environ Microbiol. 2010;76:1851-1860. observed that the bacterial community within the traps of Sarracenia alata plants was significantly different from the soil surrounding the plant and varied over time, suggesting that the community associated with the plants should not occur randomly but should be dependent on the interaction between the plant genotype and the environmental conditions.
Venn diagram represented by shared UTO's of stolons (stol) and utricules (utric) of U. breviscapa from Mato Grosso (MT) and São Paulo (SP) (dissimilarity of 0.09). Therefore: utricMT: utricules from Mato Grosso, stolSP: stolons from São Paulo, utricSP: utricules from São Paulo and stolMT: stolons from Mato Grosso.
The Utricularia samples were taken from muddy and dirty water, where the most abundant genera of both studied sites belonged to the Sphingomonas genus, which has been previously reported to colonize different polluted environments, both aquatic and terrestrial, being able to use polycyclic aromatic hydrocarbons as its sole source of carbon and energy.4040 Leys NMEJ, Ryngaert A, Bastiaens L, Verstraete W, Top EM, Springael D. Occurrence and phylogenetic diversity of Sphingomonas strains in soils contaminated with polycyclic aromatic hydrocarbons. Appl Environ Microbiol. 2004;70:1944-1955. Moreover, this genus has been reported to have a beneficial interaction with plants, producing phytohormones such as gibberellins and indole acetic acid, promoting the growth of Solanum lycopersicum,4141 Khan AL, Waqas M, Kang S, et al. Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J Microbiol. 2014;52:689-695. and producing exopolysaccharides,4242 Prajapati VD, Jani GK, Zala BS, Khutliwala TA. An insight into the emerging exopolysaccharide gellan gum as a novel polymer. Carbohyd Polym. 2013;93:670-678.,4343 Schultheis E, Dreger MA, Nimtz M, Wray V, Hempel DC, Nörtemann B. Structural characterization of the exopolysaccharide PS-EDIV from Sphingomonas pituitosas train DSM 13101. Appl Microbiol Biotechnol. 2008;78:1017-1024. which have also been reported to be a factor in plant–bacterium interactions for Paenibacillus polymyxa.4444 Yegorenkova IV, Tregubova KV, Ignatov VV. Paenibacillus polymyxa Rhizobacteria and their synthesized exoglycans in interaction with wheat roots colonization. Curr Microbiol. 2013;66:481-486.
In the present work, the genus Aquitalea was one of the most abundant genera associated with utricles from sampled SP and MT Utricularia. The genus Aquitalea was described in 2006 by Lau et al.,4545 Lau H, Faryna J, Triplett EW. Aquitale amagnusonii gen. nov., sp. nov., a novel Gram-negative bacterium isolated from a humic lake. Int J Syst Evol Microbiol. 2006;56:867-871. from a facultative anaerobic bacterium strain living in lakes. This genus is most commonly found in humic and oligotrophic lakes and in other water sources.4646 Sedlacek J, Kwon SW, Svec P, et al. Aquitalea pelogenes sp. nov., isolated from mineral peloid. Int J Syst Evol Microbiol. 2015;66:962-967. Woo et al.4747 Woo HL, Hazen TC, Simmons BA, DeAngelis KM. Enzyme activities of anaerobic lignocellulolytic bacteria isolated from wet tropical forest soils. Syst Appl Microbiol. 2010;37:60-67. showed that the genus Aquitalea was one of the most abundant genera isolated from wet tropical forest soils, an environment rich in organic matter to decompose, presenting high levels of enzyme activities. Although little is known about the mechanism of digestion in Utricularia, evidence has been raised for the presence of enzymatic activity inside the trap, and a considerable proportion of the enzymatic activity in the trap fluid is assumed to be derived from the bacterial community.4848 Sirová D, Adamec L, Vrba J. Enzymatic activities in traps of four aquatic species of the carnivorous genus Utricularia. New Phytol. 2003;159:669-675.
Other abundant genera associated with Utricularia from SP were Pelomonas and Microbacterium, which have also been reported as beneficial bacteria to host plants. Pelomonas spp. isolated from industrial water or from humic and oligotrophic lakes are able to fix nitrogen and reduce nitrate4545 Lau H, Faryna J, Triplett EW. Aquitale amagnusonii gen. nov., sp. nov., a novel Gram-negative bacterium isolated from a humic lake. Int J Syst Evol Microbiol. 2006;56:867-871.,4949 Gomila M, Bowien B, Falsen E, Moore ERB, Lalucat J. Description of Pelomonas aquatica sp. nov. and Pelomonas puraquae sp. nov., isolated from industrial and haemodialysis water. Int J Syst Evol Microbiol. 2007;57:2629-2635.; they can also be involved in the bioremediation process because they degrade aromatic compounds. Microbacterium isolated endophytically and from the plant rhizoplane are also able to fix nitrogen,5050 Zakhia F, Jeder H, Willems A, Gillis M, Dreyfus B, Lajudie P. Diverse bacteria associated with root nodules of spontaneous legumes in Tunisia and first report for nifH-like gene within the genera Microbacterium and Starkeya. Microb Ecol. 2006;51:375-393. solubilize phosphate, oxidize sulphur, reduce nitrate and produce both indole acetic acid (IAA) and ACC deaminase (associated with stress reduction in plants by the inhibition of ethylene synthesis), showing their potential to promote plant growth.5151 Madhaiyan M, Poonguzhali S, Lee J, Lee K, Saravanan VS, Santhanakrishnan P. Microbacterium azadirachtae sp. nov., a plant growth-promoting Actinobacterium isolated from the rhizoplane of neem seedlings. Int J Syst Evol Microbiol. 2010;60:1687-1692.
The Bacillus spp. found in both libraries (SP and MT) also have plant beneficial characteristics, such as the presence of the nitrate reductase enzyme and the potential to inhibit several phytopathogenic fungi (for example, Alternaria alternata, Cryphonectria parasitica, Fusarium graminearum, Phytophthora capsici and Rhizoctonia solani), demonstrating their important role in host plant defense.5252 Zhao Z, Wanga Q, Wang K, Brian K, Liu C, Gu Y. Study of the antifungal activity of Bacillus vallismortis ZZ185 in vitro and identification of its antifungal components. Bioresour Technol. 2010;101:292-297.
Furthermore, SP plants presented exclusive groups, such as the Actinobacteria that were isolated from stolons and utricles, confirming the specificity dependent on the geographic location from which the plants were collected. However, despite presenting similar libraries with no difference in the Libshuff significance test, plant organs can also select a bacterial group, such as the phyla Acidobacteria and Bacteroidetes, which were found only in utricles from plants collected in São Paulo and Mato Grosso, respectively (Fig. 4). These results support the hypothesis that plants select the bacterial communities associated with stolons and utricles based on the available community present in their surrounding environment.
Another important point we need to address is the utricle isolates that belong to the Acidobacteria group, identified as the genus Terriglobus albidus (99% similarity), a genus of fastidious growth (Fig. 6). Phylum Acidobacteria is one of the most abundant in soils, and its members have been found by cultivation independent methods in many types of environments. Acidobacteria is known to be abundant in trap fluid, and some species are known to live in acidic environments.5353 Takeuchi Y, Chaffron S, Salcher MM, et al. Bacterial diversity and composition in the luid of pitcher plants of the genus Nepenthes. Syst Appl Microbiol. 2015;38:330-339. Sirová et al.1717 Sirová D, Borovec J, Cerna B, Rejmánková E, Adamec L. Microbial community development in the traps of aquatic Utricularia species. Aquat Bot. 2009;90:129-136. showed that the trap fluid pH is low in U. vulgaris, ranging from pH 4.2 to 5.1 according to the trap age, and this could be correlated with the phosphatase activity inside the trap.
Phenetic tree representing the cultivable Acidobacteria found in association with utricles of U. breviscapa from SP using Neighbor Joining method in MEGA 5 software. Numbers above the branches indicates bootstrap values. Brachyspira species were used as outgroup.
However, members of the Acidobacteria group have proven difficult to isolate and cultivate under laboratory conditions, and there are still few species with valid names, and few are well defined; among them are members of the genera Acidobacterium, Acanthopleuribacter, Bryobacter, Edaphobacter, Geothrix, Granulicella, Holophaga and Terriglobus.5454 Eichorst SA, Kuske CR, Schmidt TM. Influence of plant polymers on the distribution and cultivation of bacteria in the phylum Acidobacteria. Appl Environ Microbiol. 2010;77:586-596.,5555 Okamura K, Kawai A, Yamada T, Hiraishi A. Acidipilarosea gen. nov., sp. nov., an acidophilic chemoorganotrophic bacterium belonging to the phylum Acidobacteria. FEMS Microbiol Lett. 2011;317:138-142.Terriglobus sp. was previously isolated from water5656 Baik KS, Choi JS, Kwon J, et al. Terriglobus aquaticus sp. nov., isolated from an artificial reservoir. Int J Syst Evol Microbiol. 2013;63:4744-4749. and from specific soil conditions, such as desert soils,5757 Abed RM, Al-Kindi S, Al-Kharusi S. Diversity of bacterial communities along a petroleum contamination gradient in desert soils. Microb Ecol. 2015;69:95-105. arctic tundra soils5858 Rawat SR, Männistö MK, Bromberg Y, Häggblom MM. Comparative genomic and physiological analysis provides insights into the role of Acidobacteria in organic carbon utilization in Arctic tundra soils. FEMS Microbiol Ecol. 2012;82:341-355. and the rhizosphere of a medicinal plant.5959 Whang KS, Lee JC, Lee HR, Han SI, Chung SH. Terriglobustenax sp. nov., an exopolysaccharide-producing Acidobacterium isolated from rhizosphere soil of a medicinal plant. Int J Syst Evol Microbiol. 2014;64:431-437.T. albidus was described in 20156060 Pascual J, Wust PK, Geppert A, Foesel BU, Huber KJ, Overmann J. Terriglobus albidus sp. nov., a member of the family Acidobacteriaceae isolated from Namibian semiarid savannah soil. Int J Syst Evol Microbiol. 2015;65:3297-3304. isolated from a semiarid savannah soil collected in northern Namibia, however the present work present the first report of cultivable bacteria belonging to this genus isolated in association with a carnivorous plant.
Caravieri et al.,1818 Caravieri FA, Ferreira AJ, Ferreira A, Clivati D, Miranda VFO, Araújo WL. Bacterial community associated with traps of the carnivoral plants Utricularia hydrocarpa and Genlisea filiformis. Aquat Bot. 2014;116:8-12. using culture-independent methods, reported that Acidobacterium was one of the dominant genera in association with U. hydrocarpa. There are advantages in retrieving an Acidobacterium isolate due to its biotechnological applications, such as the production of an extracellular matrix that apparently facilitates the flocculation of cells in liquid media.6161 Eichorst SA, Breznak JA, Schmidt TM. Isolation and characterization of soil bacteria that define Terriglobus gen. nov., in the phylum acidobacteria. Appl Environ Microbiol. 2007;73:2708-2717. Therefore, our research group is currently investigating the biotechnological potential of these Acidobacterium isolates.
Conclusions
The results show that the bacterial diversity of U. breviscapa varied according to the isolation location (São Paulo and Mato Grosso) rather than organs. This is also the first report of an Acidobacteria (Terriglobus genus) isolated in association with utricles of U. breviscapa. The role of these bacteria inside the utricles and stolons must be further investigated in order to understand their population dynamics within the host plant.
REFERENCES
-
1Król E, Płachno BJ, Adamec L, Stolarz M, Dziubinska H, Trebacz K. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Ann Bot 2011;109:47-64.
-
2Ellison AM, Gotelli NJ. Energetics and the evolution of carnivorous plants-Darwin's ‘most wonderful plants in the world’. J Exp Bot 2009;60:19-42.
-
3Reifenrath K, Theisen I, Schnitzler J, Porembski S, Barthlott W. Trap architecture in carnivorous Utricularia (Lentibulariaceae). Flora 2006;201:597-605.
-
4Givnish TJ. New evidence on the origin of carnivorous plants. Proc Natl Acad Sci USA 2015;112:10-11.
-
5Adamec L. Mineral nutrition of carnivorous plants. Bot Rev 1997;63:273-299.
-
6Ellison AM, Gotelli NJ. Evolutionary ecology of carnivorous plants. Trends Ecol Evol 2001;16:623-629.
-
7Taylor P. The genus Utricularia. In: A taxonomic monograph. Kew bulletin additional series XIV. Royal Botanic Gardens Londres: Kew; 1989:668–671.
-
8Poppinga S, Daber LE, Westermeier AS, et al. Biomechanical analysis of prey capture in the carnivorous Southern bladderwort (Utricularia australis). Sci Rep 2017;7:1776.
-
9Płachno BJ, Adamec LL, Lichtscheidl IK, Peroutka M, Adlassnig W, Vrba J. Fluorescence labelling of phosphatase activity in digestive glands of carnivorous plants. Plant Biol 2006;8:813-820.
-
10Chase MW, Christenhusz MJM, Sanders D, Fay MF. Murderous plants: Victorian Gothic, Darwin and modern insights into vegetable carnivory. Bot J Linn Soc 2009;161:329-356.
-
11Adlassnig W, Peroutka M, Lendl T. Traps of carnivorous pitcher plants as a habitat: composition of the fluid, biodiversity and mutualistic activities. Ann Bot 2011;107:181-194.
-
12Bordiec S, Paquis S, Lacroix H, et al. Comparative analysis of defence responses induced by the endophytic plant growth-promoting rhizobacterium Burkholderia phytofirmans strain PsJN and the non-host bacterium Pseudomonas syringae pv. pisi in grapevine cell suspensions. J Exp Bot 2011;62:595-603.
-
13Mcinroy JA, Kloepper JW. Survey of indigenous bacterial endophytes from cotton and sweet corn. Plant Soil 1995;173:337-342.
-
14Mcinroy JA, Kloepper JW. Population dynamics of endophytic bacteria in field-grown sweet corn and cotton. Can J Microbiol 1995;41:895-901.
-
15Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN. Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 2008;278:1-9.
-
16Sessitsch A, Kuffner M, Kidd P, et al. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem 2013;60:182-194.
-
17Sirová D, Borovec J, Cerna B, Rejmánková E, Adamec L. Microbial community development in the traps of aquatic Utricularia species. Aquat Bot 2009;90:129-136.
-
18Caravieri FA, Ferreira AJ, Ferreira A, Clivati D, Miranda VFO, Araújo WL. Bacterial community associated with traps of the carnivoral plants Utricularia hydrocarpa and Genlisea filiformis Aquat Bot 2014;116:8-12.
-
19Płachno B, Łukaszek M, Wołowski K, Adamec L, Stolarczyk P. Aging of Utricularia traps and variability of microorganisms associated with that micro-habitat. Aquat Bot 2012;97:44-48.
-
20Kuklinsky-Sobral J, Araújo WL, Mendes R, Pizzirani-Kleiner AA, Azevedo JL. Isolation and characterization of endophytic bacteria from soybean (Glycine max) grown in soil treated with glyphosate herbicide. Plant Soil 2005;273:91-99.
-
21Araújo WL, Marcon J, Maccheroni W, van Elsas JD, van Vuurde JW, Azevedo JL. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl Environ Microbiol 2002;68:4906-4914.
-
22Heuer H, Krsek M, Baker P, Smalla K, Wellington EMH. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol 1997;63:3233-3241.
-
23Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, Pace NR. Rapid-determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci USA 1985;82:6955-6959.
-
24Dourado MN, Andreote FD, Dini-Andreote F, Conti R, Araújo JM, Araújo WL. Analysis of 16S rRNA and mxaF genes revealing insights into Methylobacterium niche-specific plant association. Genet Mol Biol 2012;35:142-148.
-
25Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 1977;74:5463-5467.
-
26Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 2009;75:7537-7541.
-
27Thompson JD, Higgins DG, Gibson TJ. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acid Res 1994;22:4673-4680.
-
28Jukes TH, Cantor CR. Evolution of protein molecules. In: Munro HN, ed. Mammalian protein metabolism. New York, USA: Academic Press; 1969:21–132.
-
29Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011;28:2731-2739.
-
30Letunic I, Bork P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 2007;23:127-128.
-
31Basualdo CV. Choosing the best non-parametric richness estimator for benthic macroinvertebrates databases. Rev Soc Entomol Argent 2011;70:27-38.
-
32Hortal J, Borges PA, Gaspar C. Evaluating the performance of species richness estimators: sensitivity to sample grain size. J Anim Ecol 2006;75:274-287.
-
33Friday LE. Rapid turnover of traps in Utricularia vulgaris L. Oecologia 1989;80:272-277.
-
34Plachno BJ. The carnivorous plants and Charles Darwin – a story about beautiful fascination. In: Zarzycki K, Mirek Z, Korzeniak U, eds. Karol Darwin w oczach polskich botaników XIX–XXI w PAN IB Kraków; 2010.
-
35Alcaraz LD, Martínez-Sánchez S, Torres I, Ibarra-Laciette E, Herrera-Estrella L. The metagenome of Ultricularia gibba's traps: into the microbial input to a carnivorous plant. PLOS ONE 2016;11:1-21.
-
36Pitsch G, Adamec L, Dirren S, et al. The green Tetrahymena utriculariae n. sp. (Ciliophora, Oligohymenophorea) with its endosymbiotic algae (Micractinium sp.), living in traps of a carnivorous aquatic plant. J Eukaryot Microbiol 2017;64:322-335.
-
37Adamec L. The smallest but fastest. Ecophysiological characteristics of traps of aquatic carnivorous Ultricularia. Plant Signal Behav 2011;6:640-646.
-
38Laque T, Farjalla VF, Rosado AS, Esteves FA. Spatio temporal variation of bacterial community composition and positive controlling factors in tropical shallow lagoons. Microb Ecol 2010;59:819-829.
-
39Koopman MM, Fuselier DM, Hird S, Carstens BC. The carnivorous pale pitcher plant harbors diverse, distinct, and time-dependent bacterial communities. Appl Environ Microbiol 2010;76:1851-1860.
-
40Leys NMEJ, Ryngaert A, Bastiaens L, Verstraete W, Top EM, Springael D. Occurrence and phylogenetic diversity of Sphingomonas strains in soils contaminated with polycyclic aromatic hydrocarbons. Appl Environ Microbiol 2004;70:1944-1955.
-
41Khan AL, Waqas M, Kang S, et al. Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J Microbiol 2014;52:689-695.
-
42Prajapati VD, Jani GK, Zala BS, Khutliwala TA. An insight into the emerging exopolysaccharide gellan gum as a novel polymer. Carbohyd Polym 2013;93:670-678.
-
43Schultheis E, Dreger MA, Nimtz M, Wray V, Hempel DC, Nörtemann B. Structural characterization of the exopolysaccharide PS-EDIV from Sphingomonas pituitosas train DSM 13101. Appl Microbiol Biotechnol 2008;78:1017-1024.
-
44Yegorenkova IV, Tregubova KV, Ignatov VV. Paenibacillus polymyxa Rhizobacteria and their synthesized exoglycans in interaction with wheat roots colonization. Curr Microbiol 2013;66:481-486.
-
45Lau H, Faryna J, Triplett EW. Aquitale amagnusonii gen. nov., sp. nov., a novel Gram-negative bacterium isolated from a humic lake. Int J Syst Evol Microbiol 2006;56:867-871.
-
46Sedlacek J, Kwon SW, Svec P, et al. Aquitalea pelogenes sp. nov., isolated from mineral peloid. Int J Syst Evol Microbiol 2015;66:962-967.
-
47Woo HL, Hazen TC, Simmons BA, DeAngelis KM. Enzyme activities of anaerobic lignocellulolytic bacteria isolated from wet tropical forest soils. Syst Appl Microbiol 2010;37:60-67.
-
48Sirová D, Adamec L, Vrba J. Enzymatic activities in traps of four aquatic species of the carnivorous genus Utricularia New Phytol 2003;159:669-675.
-
49Gomila M, Bowien B, Falsen E, Moore ERB, Lalucat J. Description of Pelomonas aquatica sp. nov. and Pelomonas puraquae sp. nov., isolated from industrial and haemodialysis water. Int J Syst Evol Microbiol 2007;57:2629-2635.
-
50Zakhia F, Jeder H, Willems A, Gillis M, Dreyfus B, Lajudie P. Diverse bacteria associated with root nodules of spontaneous legumes in Tunisia and first report for nifH-like gene within the genera Microbacterium and Starkeya Microb Ecol 2006;51:375-393.
-
51Madhaiyan M, Poonguzhali S, Lee J, Lee K, Saravanan VS, Santhanakrishnan P. Microbacterium azadirachtae sp. nov., a plant growth-promoting Actinobacterium isolated from the rhizoplane of neem seedlings. Int J Syst Evol Microbiol 2010;60:1687-1692.
-
52Zhao Z, Wanga Q, Wang K, Brian K, Liu C, Gu Y. Study of the antifungal activity of Bacillus vallismortis ZZ185 in vitro and identification of its antifungal components. Bioresour Technol 2010;101:292-297.
-
53Takeuchi Y, Chaffron S, Salcher MM, et al. Bacterial diversity and composition in the luid of pitcher plants of the genus Nepenthes Syst Appl Microbiol 2015;38:330-339.
-
54Eichorst SA, Kuske CR, Schmidt TM. Influence of plant polymers on the distribution and cultivation of bacteria in the phylum Acidobacteria Appl Environ Microbiol 2010;77:586-596.
-
55Okamura K, Kawai A, Yamada T, Hiraishi A. Acidipilarosea gen. nov., sp. nov., an acidophilic chemoorganotrophic bacterium belonging to the phylum Acidobacteria. FEMS Microbiol Lett 2011;317:138-142.
-
56Baik KS, Choi JS, Kwon J, et al. Terriglobus aquaticus sp. nov., isolated from an artificial reservoir. Int J Syst Evol Microbiol 2013;63:4744-4749.
-
57Abed RM, Al-Kindi S, Al-Kharusi S. Diversity of bacterial communities along a petroleum contamination gradient in desert soils. Microb Ecol 2015;69:95-105.
-
58Rawat SR, Männistö MK, Bromberg Y, Häggblom MM. Comparative genomic and physiological analysis provides insights into the role of Acidobacteria in organic carbon utilization in Arctic tundra soils. FEMS Microbiol Ecol 2012;82:341-355.
-
59Whang KS, Lee JC, Lee HR, Han SI, Chung SH. Terriglobustenax sp. nov., an exopolysaccharide-producing Acidobacterium isolated from rhizosphere soil of a medicinal plant. Int J Syst Evol Microbiol 2014;64:431-437.
-
60Pascual J, Wust PK, Geppert A, Foesel BU, Huber KJ, Overmann J. Terriglobus albidus sp. nov., a member of the family Acidobacteriaceae isolated from Namibian semiarid savannah soil. Int J Syst Evol Microbiol 2015;65:3297-3304.
-
61Eichorst SA, Breznak JA, Schmidt TM. Isolation and characterization of soil bacteria that define Terriglobus gen. nov., in the phylum acidobacteria. Appl Environ Microbiol 2007;73:2708-2717.
Edited by
Publication Dates
-
Publication in this collection
Oct-Dec 2018
History
-
Received
18 Sept 2017 -
Accepted
24 Dec 2017 -
Published
31 Mar 2018