Abstract
Recombinant human thyroid-stimulating hormone (rhTSH) enhances 131I uptake, permitting a decrease in radiation for the treatment of multinodular goiter (MNG). Our objective was to evaluate the safety and efficacy of a single 0.1-mg dose of rhTSH, followed by 30 mCi 131I, in patients with MNG. Seventeen patients (15 females, 59.0 ± 13.1 years), who had never been submitted to 131I therapy, received a single 0.1-mg injection of rhTSH followed by 30 mCi 131I on the next day. Mean basal thyroid volume measured by computed tomography was 106.1 ± 64.4 mL. 131I 24-h uptake, TSH, free-T4, T3, thyroglobulin, anti-thyroid antibodies, and thyroid volume were evaluated at regular intervals of 12 months. Mean 131I 24-h uptake increased from 18.1 ± 9.7 to 49.6 ± 13.4% (P < 0.001), a median 2.6-fold increase (1.2 to 9.2). Peak hormonal levels were 10.86 ± 5.44 mU/L for TSH (a median 15.5-fold increase), 1.80 ± 0.48 ng/dL for free-T4, 204.61 ± 58.37 ng/dL for T3, and a median of 557.0 ng/mL for thyroglobulin. The adverse effects observed were hyperthyroidism (17.6%), painful thyroiditis (29.4%) and hypothyroidism (52.9%). Thyroid volume was reduced by 34.3 ± 14.3% after 6 months (P < 0.001) and by 46.0 ± 14.6% after 1 year (P < 0.001). Treatment of MNG with a single 0.1-mg dose of rhTSH, followed by a fixed amount of radioactivity of 131I, leads to an efficacious decrease in thyroid volume for the majority of the patients, with a moderate incidence of non-serious and readily treatable adverse effects.
Multinodular goiter; Thyroid; Recombinant TSH; Radioiodine; 131I
Braz J Med Biol Res, December 2007, Volume 40(12) 1661-1670
Effect of 30 mCi radioiodine on multinodular goiter previously treated with recombinant human thyroid-stimulating hormone
 Correspondence and Footnotes
Correspondence and Footnotes
 G.J. Paz-Filho1, C.O. Mesa-Junior1, M. Olandoski2, L.C. Woellner3, C.A. Goedert4, C.L. Boguszewski1, G.A. Carvalho1 and H. Graf 1
G.J. Paz-Filho1, C.O. Mesa-Junior1, M. Olandoski2, L.C. Woellner3, C.A. Goedert4, C.L. Boguszewski1, G.A. Carvalho1 and H. Graf 1
1Serviço de Endocrinologia e Metabologia, Hospital de Clínicas, Universidade Federal do Paraná, Curitiba, PR, Brasil
2Núcleo de Bioestatística, Pontifícia Universidade Católica do Paraná, Curitiba, PR, Brasil
3Centro de Medicina Nuclear, Curitiba, PR, Brasil
4Centro de Tomografia Computadorizada, Curitiba, PR, Brasil
 Patients and Methods
Patients and Methods
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Recombinant human thyroid-stimulating hormone (rhTSH) enhances 131I uptake, permitting a decrease in radiation for the treatment of multinodular goiter (MNG). Our objective was to evaluate the safety and efficacy of a single 0.1-mg dose of rhTSH, followed by 30 mCi 131I, in patients with MNG. Seventeen patients (15 females, 59.0 ± 13.1 years), who had never been submitted to 131I therapy, received a single 0.1-mg injection of rhTSH followed by 30 mCi 131I on the next day. Mean basal thyroid volume measured by computed tomography was 106.1 ± 64.4 mL. 131I 24-h uptake, TSH, free-T4, T3, thyroglobulin, anti-thyroid antibodies, and thyroid volume were evaluated at regular intervals of 12 months. Mean 131I 24-h uptake increased from 18.1 ± 9.7 to 49.6 ± 13.4% (P < 0.001), a median 2.6-fold increase (1.2 to 9.2). Peak hormonal levels were 10.86 ± 5.44 mU/L for TSH (a median 15.5-fold increase), 1.80 ± 0.48 ng/dL for free-T4, 204.61 ± 58.37 ng/dL for T3, and a median of 557.0 ng/mL for thyroglobulin. The adverse effects observed were hyperthyroidism (17.6%), painful thyroiditis (29.4%) and hypothyroidism (52.9%). Thyroid volume was reduced by 34.3 ± 14.3% after 6 months (P < 0.001) and by 46.0 ± 14.6% after 1 year (P < 0.001). Treatment of MNG with a single 0.1-mg dose of rhTSH, followed by a fixed amount of radioactivity of 131I, leads to an efficacious decrease in thyroid volume for the majority of the patients, with a moderate incidence of non-serious and readily treatable adverse effects.
Key words: Multinodular goiter, Thyroid, Recombinant TSH, Radioiodine, 131I
Introduction
Multinodular goiter (MNG) is a clinically recognized enlargement of the thyroid gland which is characterized by abnormal growth and structural and/or functional alterations. It is more frequently found among the elderly (particularly women), and its progression to hyperthyroidism by the development of autonomous activity is a common event (1).
There is no ideal treatment for MNG. Iodine supplementation or levothyroxine (LT-4)-suppressive therapy are disregarded as therapeutic options due to the risks of hyperthyroidism or subclinical hyperthyroidism, especially when higher doses of L-T4 are used (2) and due to the lack of evidence from prospective clinical trials evaluating L-T4 treatment for MNG (3-5). Surgery is the treatment of choice, with many positive aspects, particularly in patients with large MNG. However, complications such as hypoparathyroidism and vocal cord paralysis must be taken into account (6).
The use of radioactive iodine (131I) for MNG treatment has been described in several studies, showing a goiter size reduction ranging from 34 to 49% in the first year (7-14). Most of this reduction occurs during the first three months after 131I therapy (12). However, retrospective studies suggest that the response is directly proportional to the administered 131I activity and inversely related to the initial thyroid volume (14). Also, 131I uptake is commonly low and heterogeneous. Hence, the effective radioactivity is often too high to be administered without hospitalization and isolation.
Recombinant human thyroid-stimulating hormone (rhTSH) has been shown to increase 131I uptake in normal subjects (15) and in patients with MNG (16), and also to provide a more homogenous distribution of radioactive iodine in the gland (17). Taken together, these observations suggest that pretreatment with rhTSH should allow the administration of lower activities of 131I in MNG (18). In a previous study, we showed that the combination of rhTSH given in two 0.1-mg injections prior to a fixed activity of 131I was safe and efficient in achieving thyroid volume (TV) reduction after 6 months (19).
The objective of the present study was to evaluate the efficacy (in terms of TV reduction) of a single dose of 0.1 mg rhTSH, associated with the maximum activity of 131I (30 mCi) allowed for outpatient 131I administration in Brazil. Since large goiters are highly prevalent in Brazil, a high activity was chosen because many patients may need higher radiation activities for an effective TV reduction. By adding rhTSH to the maximum outpatient activity of 131I, we would theoretically enhance TV reduction. We also discuss and compare the results obtained with those of our previous study in which a group with similar characteristics received two doses of rhTSH (19).
Patients and Methods

We evaluated 17 outpatients with MNG (15 females, 2 males, mean age 59.0 ± 13.1 years) who were being followed at the Hospital de Clínicas, Curitiba, PR, Brazil. None of the patients had surgical or TSH-suppressive therapy with LT-4, or 131I treatment. They either had contraindication for surgery or refused a surgical approach, and 131I was considered the treatment of choice. Patients with severe tracheal compression (observed by computed tomography) were excluded from the study, as well as patients with basal 24-h radioactive iodine uptake (RAIU) higher than 30% and patients with malignant nodules.
Prior to treatment, the presence of malignancy was excluded in all patients by ultrasound-guided fine-needle aspiration biopsy of suspicious and/or dominant nodules. TV was measured by a helical computed tomography (Secura, Philips Medical Systems, Andover, MA, USA) with 2.5-mm wide axial sections, followed by multiplane and 3-D reconstruction using the Advantage Work ADW 4.0 workstation (GE Medical Systems, Waukesha, WI, USA).
All patients underwent a 131I scintigraphy and a basal 24-h RAIU test using a rectilinear scanner with a 3-inch thick NaI crystal (Pho/Dot Scanner, Nuclear-Chicago, Des Plaines, IL, USA) and an uptake measurement device (model 8725, Nuclear-Chicago). These evaluations were performed not more than 3 months prior to treatment with rhTSH. Patients who were taking methimazole (MMI) were advised to stop it 2 weeks before scintigraphy and uptake determination.
Patients were consecutively assigned to the study. All were treated on the same day in October 2003 and followed-up for 1 year.
Basal TV was 106.1 ± 64.4 mL (range: 28 to 270 mL) and RAIU was 18.1 ± 9.7%. At the time of inclusion in the study, 6 patients had subclinical or clinical hyperthyroidism, identified by TSH levels below 0.1 mU/L and free-T4 in the normal or high-normal range. Five of these patients were treated with low doses of MMI (5 to 10 mg/day) for at least 3 months prior to the study, which was stopped 2 weeks prior to 131I therapy. The sixth patient with subclinical hyperthyroidism had never used MMI and was not started on it. Complete physical examination of all patients, including those with subclinical hyperthyroidism, focusing on the heart showed no evidence of cardiac disease.
All subjects were instructed to follow a low-iodine diet (removal of iodized salt and iodine-rich foods) for 2 weeks before the administration of rhTSH.
For treatment, a 0.9 mg vial of rhTSH (Thyrogen®, Genzyme Corp., Cambridge, MA, USA) was reconstituted with 1.2 mL sterile water (provided in the kit) and 1 mL of this solution was added to 9 mL sterile water for injection, providing a 10-mL solution at a 0.1-mg/mL concentration of rhTSH.
One milliliter rhTSH was administered intramuscularly in a single injection of 0.1 mg at day 1 (D1). A 131I activity of 50 µCi (1.85 MBq) was administered, followed by scintigraphy and RAIU on the subsequent day (D2). On that day, a standard therapeutic activity of 1110 MBq (30 mCi) 131I was administered to all patients. Blood samples were collected on days 1, 2, 3, 5, 10 in order to determine the immediate hormonal changes and at 1, 2, 3, 6, 9, and 12 months for TSH analysis by the micro-particle enzyme immunoassay using AxSYM 3rd generation TSH (Abbott Diagnostics, Abbott Park, IL, USA, reference values 0.49 to 4.67 mU/L, sensitivity 0.006 mU/L, CV £20%), free-T4 (micro-particle enzyme immunoassay, AxSYM Free-T4, Abbott Diagnostics, reference values 0.71 to 1.85 ng/dL, sensitivity 0.40 ng/dL, CV £10%), T3 (micro-particle enzyme immunoassay, AxSYM T3, Abbott Diagnostics, reference values 79.0 to 149.0 ng/dL, sensitivity 30.0 ng/dL, CV £16%), and thyroglobulin (Tg; fluoro-immunoassay, DELFIA Tg kit, Perkin-Elmer/Wallac, Waltham, MA, USA, reference range 2.0 to 70.0 ng/mL, CV £4.8%). Serum was collected on days 1, 30, 180, and 360 for the determination of anti-thyroglobulin antibody levels (TgAb; micro-particle enzyme immunoassay, AxSYM anti-Tg, Abbott Diagnostics, reference value <40.0 U/mL, sensitivity 2.0 U/mL, CV £15.1%), anti-thyroperoxidase antibody levels (TPOAb; micro-particle enzyme immunoassay, AxSYM anti-TPO, Abbott Diagnostics, reference value <35.0 U/mL, sensitivity 1.0 U/mL, CV £12.4%), and thyrotrophin receptor antibodies (TRAb; radioimmunoassay, Kronus, Dana Point, CA, USA, reference value <10.0 U/L). TV reduction was determined by a helical computed tomography scan performed 6 and 12 months after 131I.
The Ethics Committee of the Hospital de Clínicas, Universidade Federal do Paraná (HC-UFPR), approved the study and all patients signed an informed consent form.
Statistical analysis was performed using the SPSS software version 10.0. Data are reported as means ± SD or as median and range, when not normally distributed (according to the Kolmogorov-Smirnov test). The Mann-Whitney and paired Student t-tests were used to analyze differences between groups, as appropriate. Pearson's correlation coefficient was used to determine the correlation between variables. The time effect was analyzed by the paired Student t-test. Two-sided tests were used and P < 0.05 was considered to be significant.
Results
Six months after treatment, mean TV was reduced from 106.1 ± 64.4 to 73.0 ± 52.0 mL, a mean reduction of 34.3 ± 14.3% (P < 0.001). After 12 months, mean volume was 58.2 ± 41.2 mL, a mean decrease of 46.0 ± 14.6% compared to baseline volume (P < 0.001). There was an additional and significant reduction between the 6th and the 12th month (P = 0.01), but most of the total reduction was obtained during the first 6 months (74%). Before treatment, patients were divided into two groups, i.e., euthyroidism and subclinical/clinical hyperthyroidism. Although no statistically significant data could be obtained from this classification due to the low power of the test performed (power <0.800, with a = 0.050), the tendency to a lack of difference between groups can be observed in Table 1. Overall TV changes are shown in Table 2.
After rhTSH administration, RAIU increased from 18.1 ± 9.7 to 49.6 ± 13.4% (P < 0.001). The ratio between post- and pre-rhTSH RAIU (RR) was calculated to indicate the fold increase in uptake in response to rhTSH. Median RR was 2.6 (1.2 to 9.2). There was an inverse correlation between RR and the pre-rhTSH 131I 24-h uptake (r = -0.613, P = 0.009), indicating that patients with lower basal uptake values achieved higher RR values. Also, there was a positive correlation between RR and peak TSH (r = 0.538; P = 0.032). The scintigraphic pattern changed from heterogeneous to a diffuse homogenous uptake after rhTSH administration in all patients.
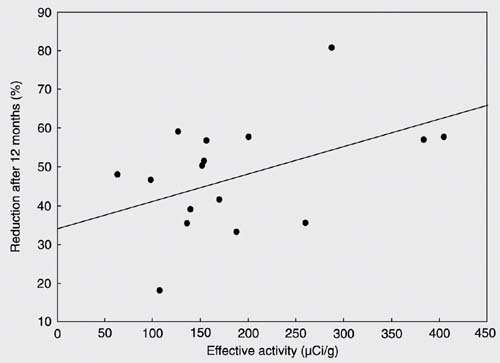
Effective activity of radiation was defined as the fixed 131I activity of 1110 MBq (30 mCi) divided by the basal thyroid mass and corrected for post-rhTSH uptake. Median effective activity was 154.5 µCi/g (63.2-404.7 µCi/g), and was not correlated with TV reduction after 12 months (r = 0.473; P = 0.055; Figure 1). Also, TV reduction after 12 months did not correlate with post-rhTSH uptake (r = 0.305; P = 0.233). Mean TSH levels increased from 0.62 ± 0.62 mU/L (median: 0.46; range: 0.01 to 2.56 mU/L) at D1 to 10.86 ± 5.44 mU/L (median: 9.97; range 0.08 to 23.52 mU/L) at D2 (P < 0.001). After peaking, TSH started to decline to levels below the normal range, reaching a nadir on day 30 (median 0.01; 0.01 to 1.76 mU/L). By the 2nd month, 52.9% of patients (N = 9) still had TSH levels below the normal range and 4 patients (23.5%) developed clinical or subclinical hypothyroidism. These patients were initiated on LT-4 treatment. The incidence of hypothyroidism was 41.2% by the 3rd month, 47.0% by the 6th month, and 52.9% by the 12th month.
Free-T4 increased from 1.16 ± 0.24 ng/dL at D1 to 1.80 ± 0.48 ng/dL at D3 (48 h after rhTSH; P < 0.001). Mean free-T4 levels remained within the normal range in all evaluations. Six patients had free-T4 levels higher than 1.9 ng/dL on D3 (mean 2.41 ± 0.23 ng/dL). Basal T3 levels were 110.09 ± 15.07 ng/dL, increasing to 204.61 ± 58.37 ng/dL at D2 (P < 0.001). After D5, mean T3 levels returned to normal. Values with concomitant positive TgAb titers were excluded from the analysis of Tg levels due to the possibility of interference in the assay (particularly when Tg levels were normal). Tg rose from median basal levels of 78.5 ng/mL (19.8 to 453.0 ng/mL) to a peak of 557.0 ng/mL (196.0 to 1450.0 ng/mL; P < 0.001) on day 5. By the 6th and 12th months, Tg levels returned to values similar to basal (median 165.0 and 55.5 ng/mL, respectively; P = 0.164).
Four patients (23.5%) had positive basal titers of TgAb (none had TSH below 0.1 mU/L, and 1 patient had TSH between 0.1 and 0.4 mU/L at D1). This number increased to 9 patients at 6 months but returned to 4 patients with TgAb-positive titers at the last evaluation (2 of them had negative basal TgAb titers). Basal TPOAb titers were positive in 3 patients (17.6%; 1 of them had TSH below 0.4 mU/L and the others had normal TSH). By the 6th month, another patient developed positive TPOAb titers, which remained positive by the 12th month. No patients had positive TRAb titers before treatment. After 12 months, 5 of them developed detectable TRAb (three of them had TSH between 0.1 and 0.4 mU/L and the others had normal TSH). One patient who developed positive TRAb titers had positive TPOAb titers at D1.
One patient (a 71-year-old female) had basal levels of TSH of 0.03 mU/L, basal levels of free-T4 of 1.52 ng/dL and basal levels of T3 of 118.8 ng/dL. She was started on 10 mg MMI 3 months prior to treatment, her basal RAIU was 7% (measured 2 weeks after interruption of MMI, without an iodine-restricted diet) and she had positive TRAb titers (56 U/L), but Graves' ophthalmopathy was absent (as per clinical assessment). At first, she was not considered to have autoimmune hyperthyroidism (positive TRAb and low TSH were present, but she presented a MNG with very low basal RAIU and further evidence of autoimmune hyperthyroidism was lacking), and she was included in the treatment. However, after 6 months, she showed suppressed levels of TSH (0.002 mU/L) and high levels of free-T4 (3.21 ng/dL). A diagnosis of autoimmune hyperthyroidism was made and a new activity of 30 mCi 131I was administered to her (without rhTSH), with resolution of her hyperthyroidism after 2 months. This patient was excluded from all analyses.
Three patients (17.6%) developed at least one sign or symptom of hyperthyroidism (such as palpitation, increased heart rate, insomnia, anxiety, and asthenia) between D5 and D10. Development of signs or symptoms of hyperthyroidism was not related to a previous history of subclinical hyperthyroidism since none of these patients had basal levels of TSH lower than 0.1 mU/L, although rhTSH may induce hyperthyroidism in patients with subclinical hyperthyroidism. Two of these patients were started on propranolol at doses ranging from 80 to 120 mg daily, with prompt improvement in response to therapy. The other patient was already taking atenolol, 50 mg/day, and the dosage was doubled. ß-blockers were discontinued in these first 2 patients and the dosage was halved back to its former dose in the patient already on atenolol at D10. Three patients who did not show signs or symptoms of hyperthyroidism were already taking ß-blockers as part of their anti-hypertensive treatment. Five patients (29.4%) showed symptoms of painful radiation-related thyroiditis starting at least 1 week after 131I administration, characterized by cervical pain, which resolved with non-steroid anti-inflammatory drugs. One patient did not respond and was started on prednisone, 20 mg/day for 5 days, with resolution of thyroiditis. None of our patients developed serious side effects such as respiratory or compressive symptoms.
Dissatisfaction regarding final TV was observed in 1 patient (final TV of 67 mL, with a 39.0% reduction). New therapeutic approaches were offered to this patient, including a new trial of rhTSH and 131I, but she refused them.
Correlation between thyroid volume reduction after 12 months and effective administered activity (r = 0.473; P = 0.055).
Comparative analysis of patients with clinical/subclinical hyperthyroidism and euthyroidism, before and 24-48 h after recombinant human thyroid-stimulating hormone (rhTSH).
Basal and post-treatment evaluation of 17 patients with multinodular goiter on an iodine-restricted diet treated with 0.1 mg recombinant human thyroid-stimulating hormone (rhTSH) plus 30 mCi 131I.
Discussion
The efficacy of treatment of MNG with 131I alone is usually hampered by the low and irregular 131I uptake, requiring the administration of higher 131I activities in order to enhance the therapeutic response (1). rhTSH is fairly safe and has the ability to increase 131I uptake, thus being useful in the follow-up of differentiated thyroid cancer (20,21). In patients with MNG, it has been shown that rhTSH increases 131I uptake, changing the 131I uptake pattern from heterogeneous to homogeneous (17,18). Also, rhTSH transiently increases thyroid hormone levels and TV (22). Another recent study suggested that rhTSH could influence the 131I kinetics, hindering the decrease in thyroid uptake and enhancing the absorbed activity (23).
In the present study, the combination of rhTSH and a fixed activity of 1110 MBq (30 mCi) of 131I resulted in a mean TV reduction of 34.3 ± 14.3% after 6 months and 46.0 ± 14.3% after 1 year. An earlier image evaluation could be valuable since there may be a transient increase of the gland within the 1st month (22). TV reduction was more efficient when compared to studies which used the same radiation activity without rhTSH (24), but was similar to that reported in studies in which higher 131I activities were administered, without rhTSH (7-14). In the present study, effectiveness regarding TV reduction after 6 months was comparable to that found in our previous study (19), in which two doses of 0.1 mg rhTSH were given (reduction of 39.3 ± 19.5% in the previous study, P = 0.390). Data for the 1-year follow-up have not been published yet, but still no statistical difference has been found (reduction of 47.5 ± 21.7% in the previous study, P = 0.890). It is important to say that the patient characteristics in the two studies were similar regarding age, basal TV, basal 131I uptake, and basal thyroid function, permitting a consistent comparison between studies.
We chose to use the highest 131I activity allowed by Brazilian regulations due to the high basal TV and to the low pre-rhTSH RAIU, in order to achieve proper TV reduction in most of the patients. Also, we used a fixed instead of a calculated activity of 131I because some patients would have to receive a much higher activity and would have to be admitted to the hospital. Previous studies using lower 131I activities without rhTSH have shown effective TV reduction. However, treatments had to be repeated two or more times in some patients (12,13). In the present study, 94.1% of the patients achieved a TV reduction of at least 25%. The protocol was convenient and easy to perform. Its costs may be substantially lower since it decreases the likelihood of a second treatment being needed. Also, it can be done in an outpatient setting, avoiding the need for hospitalization.
In a prolonged follow-up of 4 years, additional volume reduction was similar for patients treated with rhTSH plus 131I or 131I alone (25), despite the fact that, in the first year, more pronounced reduction was seen in the group treated with rhTSH + 131I (26). In a recent study, a similar volume reduction was achieved with 30 mCi of 131I and a dose of rhTSH three times higher than used in the present study (27). In a randomized, placebo-controlled study, effective reduction was increased by rhTSH (28). These studies have provided important data about the treatment of MNG with rhTSH plus 131I. However, it is not possible to compare these studies due to the different amounts of rhTSH used and 131I administered, and also due to the different characteristics of the patients.
We could not find any correlation of TV reduction with post-rhTSH 131I uptake, area under the curve of TSH, basal TV, or effective activity of 131I. However, the level of significance was very close to 0.05 for the effective activity (P = 0.055). Even though no correlations could be shown, TV reduction was satisfactory. This probably happened because all variables are interdependent and influence TV reduction together. The effects of LT-4 on TV could be taken into consideration, but, as shown by previous studies (29), we do not believe that LT-4 could have influenced TV reduction.
After rhTSH, mean RAIU was 49.6 ± 13.4%, also comparable to the uptake found in our previous study (53.5 ± 11.0%; 19). However, this increase in uptake could be underestimated because tracer activity was given a few minutes after rhTSH, while sodium/iodine symporter was not yet completely stimulated (30). Moreover, the iodine-restricted diet could have contributed, in part, to the increased uptake after rhTSH. Anti-thyroid drug use could be another confounder because its discontinuation two weeks before radioiodine could be insufficient to obtain maximum 131I uptake. However, RAIU, TV and thyroid hormone levels could not be analyzed in two separate groups (patients with normal thyroid function and patients with subclinical hyperthyroidism) because the power of the test was too low.
There was a lower increase in TSH, free-T4 and T3 levels in the present study compared to our previous one (19). In fact, mean free-T4 levels increased 56.5 ± 29.3% and reached a normal-high peak, in contrast to the free-T4 levels of our previous study, which reached a 146% increase and remained high until the first-month evaluation (19). Peak levels of T3 were 87.0 ± 49.0% higher than basal in the present study, as opposed to a 202% increase in T3 when two doses of rhTSH were given (19). These differences are reflected in the occurrence of hyperthyroidism, which was higher (39%) in patients who received two doses of rhTSH (19), vs 17.6% in the present study. Even though Tg levels rose considerably, peak level was lower than the mean level of 1838.5 ng/dL found in our previous study (19). This could be attributed to less stimulation with rhTSH and a milder destruction of the parenchyma by 131I when a single dose of rhTSH is given. After 12 months, Tg reached levels similar to baseline, reflecting the absence of stimulation by rhTSH.
TgAb titers returned to baseline values by the 12th month, reflecting destruction of thyroid tissue. We could not define a pattern in the change in TPOAb titers. TRAb titers increased considerably, a fact related to the effect of radiation and more common among patients with positive TPOAb titers (31). Rubio et al. (32) have shown that positive TPOAb and TRAb titers may occur after 131I, regardless of rhTSH. Moreover, most TRAb have blocking effects on TSH receptors.
The incidence of adverse effects was considerably high, but all of them were mild to moderate and limited to the first 10 days of therapy. All patients with signs and symptoms of hyperthyroidism were treated with 80 mg (if mild) or 120 mg (if moderate) of propranolol. Treatment was limited to the 10th day, with complete resolution. Patients who were already taking ß-blockers as anti-hypertensive therapy did not develop signs or symptoms of hyperthyroidism, suggesting that ß-blockers could be administered prior to treatment to prevent patients from developing hyperthyroidism. Also, all patients who developed symptoms of painful thyroiditis (characterized by cervical pain) were treated with a non-steroidal anti-inflammatory drug (if the symptom was mild). One patient did not respond to the anti-inflammatory drug and was treated with 20 mg prednisone for 5 days, with important clinical improvement. The incidence of positive TgAb titers was higher among patients who developed symptoms of thyroiditis (60.0 vs 8.3%), suggesting that positive titers of TgAb may increase the risk for radiation thyroiditis. The incidence of painful thyroiditis in the present study (29.4%) was similar to that of the comparison study (33.3%), in which two doses of rhTSH were used (19).
The incidence of hypothyroidism was 47.0% after 6 months and 52.9% after 1 year. After 2 years, all patients in the present study were submitted to thyroid function tests, and the incidence of hypothyroidism remained 52.9%. This indicates that even though the incidence of hypothyroidism was high, it did not increase after one year. All patients who developed hypothyroidism had positive TPOAb titers. Also, patients who developed hypothyroidism had lower basal TV than patients who remained euthyroid at the end of the study (60 vs 155 mL, P < 0.003). These findings suggest that positive TPOAb titers and the presence of smaller goiters may increase the risk for the development of hypothyroidism. After one year, the incidence of hypothyroidism was 52.9% in patients who received one dose of rhTSH compared to 70.6% in patients who received two doses of rhTSH (19; Paz-Filho GJ, Mesa-Junior CO, Carvalho GA, Graf H, unpublished data).
In this uncontrolled therapeutic study, we evaluated the efficacy and safety of treatment with one dose of rhTSH, and compared our data to those obtained in a previously published study (19), which had many similarities, except for the use of two doses of rhTSH. Although the present study did not have a placebo group, we conclude that treatment of MNG with a single 0.1-mg dose of rhTSH plus 1110 MBq (30 mCi) 131I is as efficient as two doses on consecutive days in reducing TV in MNG, with a lower incidence of hyperthyroidism.
References
1. Hegedus L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev 2003; 24: 102-132.
2. Roti E, Uberti ED. Iodine excess and hyperthyroidism. Thyroid 2001; 11: 493-500.
3. Leese GP, Jung RT, Guthrie C, Waugh N, Browning MC. Morbidity in patients on L-thyroxine: a comparison of those with a normal TSH to those with a suppressed TSH. Clin Endocrinol 1992; 37: 500-503.
4. Berghout A, Wiersinga WM, Drexhage HA, Smits NJ, Touber JL. Comparison of placebo with L-thyroxine alone or with carbimazole for treatment of sporadic non-toxic goitre. Lancet 1990; 336: 193-197.
5. Wesche MF, Tiel V, Lips P, Smits NJ, Wiersinga WM. A randomized trial comparing levothyroxine with radioactive iodine in the treatment of sporadic nontoxic goiter. J Clin Endocrinol Metab 2001; 86: 998-1005.
6. Thomusch O, Machens A, Sekulla C, Ukkat J, Lippert H, Gastinger I, et al. Multivariate analysis of risk factors for postoperative complications in benign goiter surgery: prospective multicenter study in Germany. World J Surg 2000; 24: 1335-1341.
7. Bonnema SJ, Bertelsen H, Mortensen J, Andersen PB, Knudsen DU, Bastholt L, et al. The feasibility of high dose iodine 131 treatment as an alternative to surgery in patients with a very large goiter: effect on thyroid function and size and pulmonary function. J Clin Endocrinol Metab 1999; 84: 3636-3641.
8. Kay TW, d'Emden MC, Andrews JT, Martin FI. Treatment of non-toxic multinodular goiter with radioactive iodine. Am J Med 1988; 84: 19-22.
9. Howarth DM, Epstein MT, Thomas PA, Allen LW, Akerman R, Lan L. Outpatient management of patients with large multinodular goitres treated with fractionated radioiodine. Eur J Nucl Med 1997; 24: 1465-1469.
10. Wesche MF, Buul MM, Smits NJ, Wiersinga WM. Reduction in goiter size by 131I therapy in patients with non-toxic multinodular goiter. Eur J Endocrinol 1995; 132: 86-87.
11. Huysmans DA, Hermus AR, Corstens FH, Barentsz JO, Kloppenborg PW. Large, compressive goiters treated with radioiodine. Ann Intern Med 1994; 121: 757-762.
12. Nygaard B, Hegedus L, Gervil M, Hjalgrim H, Soe-Jensen P, Hansen JM. Radioiodine treatment of multinodular non-toxic goitre. BMJ 1993; 307: 828-832.
13. Hegedus L, Hansen BM, Knudsen N, Hansen JM. Reduction of size of thyroid with radioactive iodine in multinodular non-toxic goitre. BMJ 1988; 297: 661-662.
14. Le Moli R, Wesche MF, Tiel-Van Buul MM, Wiersinga WM. Determinants of long term outcome of radioiodine therapy of sporadic non-toxic goitre. Clin Endocrinol 1999; 50: 783-789.
15. Torres MS, Ramirez L, Simkin PH, Braverman LE, Emerson CH. Effect of various doses of recombinant human thyrotropin on the thyroid radioactive iodine uptake and serum levels of thyroid hormones and thyroglobulin in normal subjects. J Clin Endocrinol Metab 2001; 86: 1660-1664.
16. Huysmans DA, Nieuwlaat WA, Erdtsieck RJ, Schellekens AP, Bus JW, Bravenboer B, et al. Administration of a single low dose of recombinant human thyrotropin significantly enhances thyroid radioiodide uptake in nontoxic nodular goiter. J Clin Endocrinol Metab 2000; 85: 3592-3596.
17. Nieuwlaat WA, Hermus AR, Sivro-Prndelj F, Corstens FH, Huysmans DA. Pretreatment with recombinant human TSH changes the regional distribution of radioiodine on thyroid scintigrams of nodular goiters. J Clin Endocrinol Metab 2001; 86: 5330-5336.
18. Nieuwlaat WA, Huysmans DA, van den Bosch HC, Sweep CG, Ross HA, Corstens FH, et al. Pretreatment with a single, low dose of recombinant human thyrotropin allows dose reduction of radioiodine therapy in patients with nodular goiter. J Clin Endocrinol Metab 2003; 88: 3121-3129.
19. Albino CC, Mesa CO Jr, Olandoski M, Ueda CE, Woellner LC, Goedert CA, et al. Recombinant human thyrotropin as adjuvant in the treatment of multinodular goiters with radioiodine. J Clin Endocrinol Metab 2005; 90: 2775-2780.
20. Pacini F, Molinaro E, Castagna MG, Agate L, Elisei R, Ceccarelli C, et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab 2003; 88: 3668-3673.
21. Haugen BR, Pacini F, Reiners C, Schlumberger M, Ladenson PW, Sherman SI, et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab 1999; 84: 3877-3885.
22. Nielsen VE, Bonnema SJ, Hegedus L. The effects of recombinant human thyrotropin in normal subjects and patients with goitre. Clin Endocrinol 2004; 61: 655-663.
23. Nielsen VE, Bonnema SJ, Boel-Jorgensen H, Veje A, Hegedus L. Recombinant human thyrotropin markedly changes the 131I kinetics during 131I therapy of patients with nodular goiter: an evaluation by a randomized double-blinded trial. J Clin Endocrinol Metab 2005; 90: 79-83.
24. Zelmanovitz F, Zelmanovitz T, Zelmanovitz W. Radioactive iodine 131 treatment of nontoxic multinodular goiter with ambulatory doses. Thyroid 2000; 10: 144 (Abstract).
25. Cardia MS, Rubio IG, Medeiros-Neto G. Prolonged follow-up of multinodular goitre patients treated with radioiodine preceded or not by human recombinant TSH. Clin Endocrinol 2006; 64: 474.
26. Silva MN, Rubio IG, Romao R, Gebrin EM, Buchpiguel C, Tomimori E, et al. Administration of a single dose of recombinant human thyrotrophin enhances the efficacy of radioiodine treatment of large compressive multinodular goitres. Clin Endocrinol 2004; 60: 300-308.
27. Cohen O, Ilany J, Hoffman C, Olchovsky D, Dabhi S, Karasik A, et al. Low-dose recombinant human thyrotropin-aided radioiodine treatment of large, multinodular goiters in elderly patients. Eur J Endocrinol 2006; 154: 243-252.
28. Nielsen VE, Bonnema SJ, Boel-Jorgensen H, Grupe P, Hegedus L. Stimulation with 0.3-mg recombinant human thyrotropin prior to iodine 131 therapy to improve the size reduction of benign nontoxic nodular goiter: a prospective randomized double-blind trial. Arch Intern Med 2006; 166: 1476-1482.
29. Zelmanovitz F, Genro S, Gross JL. Suppressive therapy with levothyroxine for solitary thyroid nodules: a double-blind controlled clinical study and cumulative meta-analyses. J Clin Endocrinol Metab 1998; 83: 3881-3885.
30. Kogai T, Taki K, Brent GA. Enhancement of sodium/iodide symporter expression in thyroid and breast cancer. Endocr Relat Cancer 2006; 13: 797-826.
31. Nygaard B, Knudsen JH, Hegedus L, Scient AV, Hansen JE. Thyrotropin receptor antibodies and Graves' disease, a side-effect of 131I treatment in patients with nontoxic goiter. J Clin Endocrinol Metab 1997; 82: 2926-2930.
32. Rubio IG, Perone BH, Silva MN, Knobel M, Medeiros-Neto G. Human recombinant TSH preceding a therapeutic dose of radioiodine for multinodular goiters has no significant effect in the surge of TSH-receptor and TPO antibodies. Thyroid 2005; 15: 134-139.
Acknowledgments
We thank Geraldo Medeiros-Neto, MD, MACP (Universidade de São Paulo Medical School) for continuous assistance and support during the preparation of this manuscript.
 Address for correspondence: G.J. Paz-Filho, 1756 N Bayshore Dr, apt. 8F, Miami, FL 33132, USA. Fax: +1-305-243-9708. E-mail: gpazfilho@med.miami.edu
Address for correspondence: G.J. Paz-Filho, 1756 N Bayshore Dr, apt. 8F, Miami, FL 33132, USA. Fax: +1-305-243-9708. E-mail: gpazfilho@med.miami.edu
Received May 18, 2007. Accepted August 27, 2007.
- 1. Hegedus L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev 2003; 24: 102-132.
- 2. Roti E, Uberti ED. Iodine excess and hyperthyroidism. Thyroid 2001; 11: 493-500.
- 3. Leese GP, Jung RT, Guthrie C, Waugh N, Browning MC. Morbidity in patients on L-thyroxine: a comparison of those with a normal TSH to those with a suppressed TSH. Clin Endocrinol 1992; 37: 500-503.
- 4. Berghout A, Wiersinga WM, Drexhage HA, Smits NJ, Touber JL. Comparison of placebo with L-thyroxine alone or with carbimazole for treatment of sporadic non-toxic goitre. Lancet 1990; 336: 193-197.
- 5. Wesche MF, Tiel V, Lips P, Smits NJ, Wiersinga WM. A randomized trial comparing levothyroxine with radioactive iodine in the treatment of sporadic nontoxic goiter. J Clin Endocrinol Metab 2001; 86: 998-1005.
- 6. Thomusch O, Machens A, Sekulla C, Ukkat J, Lippert H, Gastinger I, et al. Multivariate analysis of risk factors for postoperative complications in benign goiter surgery: prospective multicenter study in Germany. World J Surg 2000; 24: 1335-1341.
- 7. Bonnema SJ, Bertelsen H, Mortensen J, Andersen PB, Knudsen DU, Bastholt L, et al. The feasibility of high dose iodine 131 treatment as an alternative to surgery in patients with a very large goiter: effect on thyroid function and size and pulmonary function. J Clin Endocrinol Metab 1999; 84: 3636-3641.
- 8. Kay TW, d'Emden MC, Andrews JT, Martin FI. Treatment of non-toxic multinodular goiter with radioactive iodine. Am J Med 1988; 84: 19-22.
- 9. Howarth DM, Epstein MT, Thomas PA, Allen LW, Akerman R, Lan L. Outpatient management of patients with large multinodular goitres treated with fractionated radioiodine. Eur J Nucl Med 1997; 24: 1465-1469.
- 10. Wesche MF, Buul MM, Smits NJ, Wiersinga WM. Reduction in goiter size by 131I therapy in patients with non-toxic multinodular goiter. Eur J Endocrinol 1995; 132: 86-87.
- 11. Huysmans DA, Hermus AR, Corstens FH, Barentsz JO, Kloppenborg PW. Large, compressive goiters treated with radioiodine. Ann Intern Med 1994; 121: 757-762.
- 12. Nygaard B, Hegedus L, Gervil M, Hjalgrim H, Soe-Jensen P, Hansen JM. Radioiodine treatment of multinodular non-toxic goitre. BMJ 1993; 307: 828-832.
- 13. Hegedus L, Hansen BM, Knudsen N, Hansen JM. Reduction of size of thyroid with radioactive iodine in multinodular non-toxic goitre. BMJ 1988; 297: 661-662.
- 14. Le Moli R, Wesche MF, Tiel-Van Buul MM, Wiersinga WM. Determinants of long term outcome of radioiodine therapy of sporadic non-toxic goitre. Clin Endocrinol 1999; 50: 783-789.
- 15. Torres MS, Ramirez L, Simkin PH, Braverman LE, Emerson CH. Effect of various doses of recombinant human thyrotropin on the thyroid radioactive iodine uptake and serum levels of thyroid hormones and thyroglobulin in normal subjects. J Clin Endocrinol Metab 2001; 86: 1660-1664.
- 16. Huysmans DA, Nieuwlaat WA, Erdtsieck RJ, Schellekens AP, Bus JW, Bravenboer B, et al. Administration of a single low dose of recombinant human thyrotropin significantly enhances thyroid radioiodide uptake in nontoxic nodular goiter. J Clin Endocrinol Metab 2000; 85: 3592-3596.
- 17. Nieuwlaat WA, Hermus AR, Sivro-Prndelj F, Corstens FH, Huysmans DA. Pretreatment with recombinant human TSH changes the regional distribution of radioiodine on thyroid scintigrams of nodular goiters. J Clin Endocrinol Metab 2001; 86: 5330-5336.
- 18. Nieuwlaat WA, Huysmans DA, van den Bosch HC, Sweep CG, Ross HA, Corstens FH, et al. Pretreatment with a single, low dose of recombinant human thyrotropin allows dose reduction of radioiodine therapy in patients with nodular goiter. J Clin Endocrinol Metab 2003; 88: 3121-3129.
- 19. Albino CC, Mesa CO Jr, Olandoski M, Ueda CE, Woellner LC, Goedert CA, et al. Recombinant human thyrotropin as adjuvant in the treatment of multinodular goiters with radioiodine. J Clin Endocrinol Metab 2005; 90: 2775-2780.
- 20. Pacini F, Molinaro E, Castagna MG, Agate L, Elisei R, Ceccarelli C, et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab 2003; 88: 3668-3673.
- 21. Haugen BR, Pacini F, Reiners C, Schlumberger M, Ladenson PW, Sherman SI, et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab 1999; 84: 3877-3885.
- 22. Nielsen VE, Bonnema SJ, Hegedus L. The effects of recombinant human thyrotropin in normal subjects and patients with goitre. Clin Endocrinol 2004; 61: 655-663.
- 23. Nielsen VE, Bonnema SJ, Boel-Jorgensen H, Veje A, Hegedus L. Recombinant human thyrotropin markedly changes the 131I kinetics during 131I therapy of patients with nodular goiter: an evaluation by a randomized double-blinded trial. J Clin Endocrinol Metab 2005; 90: 79-83.
- 24. Zelmanovitz F, Zelmanovitz T, Zelmanovitz W. Radioactive iodine 131 treatment of nontoxic multinodular goiter with ambulatory doses. Thyroid 2000; 10: 144 (Abstract).
- 25. Cardia MS, Rubio IG, Medeiros-Neto G. Prolonged follow-up of multinodular goitre patients treated with radioiodine preceded or not by human recombinant TSH. Clin Endocrinol 2006; 64: 474.
- 26. Silva MN, Rubio IG, Romao R, Gebrin EM, Buchpiguel C, Tomimori E, et al. Administration of a single dose of recombinant human thyrotrophin enhances the efficacy of radioiodine treatment of large compressive multinodular goitres. Clin Endocrinol 2004; 60: 300-308.
- 27. Cohen O, Ilany J, Hoffman C, Olchovsky D, Dabhi S, Karasik A, et al. Low-dose recombinant human thyrotropin-aided radioiodine treatment of large, multinodular goiters in elderly patients. Eur J Endocrinol 2006; 154: 243-252.
- 28. Nielsen VE, Bonnema SJ, Boel-Jorgensen H, Grupe P, Hegedus L. Stimulation with 0.3-mg recombinant human thyrotropin prior to iodine 131 therapy to improve the size reduction of benign nontoxic nodular goiter: a prospective randomized double-blind trial. Arch Intern Med 2006; 166: 1476-1482.
- 29. Zelmanovitz F, Genro S, Gross JL. Suppressive therapy with levothyroxine for solitary thyroid nodules: a double-blind controlled clinical study and cumulative meta-analyses. J Clin Endocrinol Metab 1998; 83: 3881-3885.
- 30. Kogai T, Taki K, Brent GA. Enhancement of sodium/iodide symporter expression in thyroid and breast cancer. Endocr Relat Cancer 2006; 13: 797-826.
- 31. Nygaard B, Knudsen JH, Hegedus L, Scient AV, Hansen JE. Thyrotropin receptor antibodies and Graves' disease, a side-effect of 131I treatment in patients with nontoxic goiter. J Clin Endocrinol Metab 1997; 82: 2926-2930.
- 32. Rubio IG, Perone BH, Silva MN, Knobel M, Medeiros-Neto G. Human recombinant TSH preceding a therapeutic dose of radioiodine for multinodular goiters has no significant effect in the surge of TSH-receptor and TPO antibodies. Thyroid 2005; 15: 134-139.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
29 Oct 2007 -
Date of issue
Dec 2007
History
-
Accepted
27 Aug 2007 -
Received
18 May 2007