Abstract
Eucalyptol is an essential oil that relaxes bronchial and vascular smooth muscle although its direct actions on isolated myocardium have not been reported. We investigated a putative negative inotropic effect of the oil on left ventricular papillary muscles from male Wistar rats weighing 250 to 300 g, as well as its effects on isometric force, rate of force development, time parameters, post-rest potentiation, positive inotropic interventions produced by Ca2+ and isoproterenol, and on tetanic tension. The effects of 0.3 mM eucalyptol on myosin ATPase activity were also investigated. Eucalyptol (0.003 to 0.3 mM) reduced isometric tension, the rate of force development and time parameters. The oil reduced the force developed by steady-state contractions (50% at 0.3 mM) but did not alter sarcoplasmic reticulum function or post-rest contractions and produced a progressive increase in relative potentiation. Increased extracellular Ca2+ concentration (0.62 to 5 mM) and isoproterenol (20 nM) administration counteracted the negative inotropic effects of the oil. The activity of the contractile machinery evaluated by tetanic force development was reduced by 30 to 50% but myosin ATPase activity was not affected by eucalyptol (0.3 mM), supporting the idea of a reduction of sarcolemmal Ca2+ influx. The present results suggest that eucalyptol depresses force development, probably acting as a calcium channel blocker.
Eucalyptol; Myocardial contractility; Calcium; Isoproterenol; Tetanic tension
Braz J Med Biol Res, March 2005, Volume 38(3) 453-461
Eucalyptol, an essential oil, reduces contractile activity in rat cardiac muscle
M.C.M.S. Soares1,3, C.E.N. Damiani1,4, C.M. Moreira1, I. Stefanon1,2 and D.V. Vassallo1,2
1Departamento de Ciências Fisiológicas, Universidade Federal do Espírito Santo, Vitória, ES, Brasil
2Escola de Medicina da Santa Casa de Misericórdia, Vitória, ES, Brasil
3Faculdade de Ciências da Saúde, FAESA, Vitória, ES, Brasil
4Departamento de Fisiologia, Universidade Federal do Paraná, Curitiba, PR, Brasil
 References
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Eucalyptol is an essential oil that relaxes bronchial and vascular smooth muscle although its direct actions on isolated myocardium have not been reported. We investigated a putative negative inotropic effect of the oil on left ventricular papillary muscles from male Wistar rats weighing 250 to 300 g, as well as its effects on isometric force, rate of force development, time parameters, post-rest potentiation, positive inotropic interventions produced by Ca2+ and isoproterenol, and on tetanic tension. The effects of 0.3 mM eucalyptol on myosin ATPase activity were also investigated. Eucalyptol (0.003 to 0.3 mM) reduced isometric tension, the rate of force development and time parameters. The oil reduced the force developed by steady-state contractions (50% at 0.3 mM) but did not alter sarcoplasmic reticulum function or post-rest contractions and produced a progressive increase in relative potentiation. Increased extracellular Ca2+ concentration (0.62 to 5 mM) and isoproterenol (20 nM) administration counteracted the negative inotropic effects of the oil. The activity of the contractile machinery evaluated by tetanic force development was reduced by 30 to 50% but myosin ATPase activity was not affected by eucalyptol (0.3 mM), supporting the idea of a reduction of sarcolemmal Ca2+ influx. The present results suggest that eucalyptol depresses force development, probably acting as a calcium channel blocker.
Key words: Eucalyptol, Myocardial contractility, Calcium, Isoproterenol, Tetanic tension
Introduction
Eucalyptol, 1,8 cineole, is an essential oil present in large amounts in a variety of plants which is frequently used in the manufacture of cosmetics, to increase percutaneous penetration of drugs, as a nasal decongestant and anticough agent, in aromatherapy, and in dentistry (1-4). Eucalyptol has been used to treat bronchitis, sinusitis and chronic rhinitis and also for the treatment of asthma (5). These actions seem to be related to an anti-inflammatory action (6) inhibiting the production of tumor necrosis factor alpha (7). The oil also inhibits the production of cytokines and prostaglandins by stimulated monocytes in vitro, explaining its bronchodilator effect (7). Gastric protection preventing ethanol-induced injury was also reported in rats (8). This compound also enhances blood circulation, leading to skin hyperemia after local application (9). Other reports have shown that prolonged exposure to eucalyptol (inhalation) increases cerebral blood flow correlated with eucalyptol concentration in blood (10), suggesting a vasodilator action. Cardiovascular effects were also recently reported. Lahlou et al. (11) showed that eucalyptol reduced heart rate by a parasympathetic-dependent action and induced hypotension by a direct vasorelaxation.
Eucalyptol is metabolized to 2-exo-hydroxy-1,8 cineole by microsomes from human and rat liver, but it is not clear whether this substance can be metabolized by humans in vivo (12). Eucalyptol diffuses faster by inhalation than by oral administration or through the skin (9,13). Its presence can be detected in blood 5 min after inhalation, with maximal concentration being reached within 18 min (14).
Non-fatal symptoms were observed in children following nasal administration of eucalyptol. Effects included mucous membrane irritation, tachycardia, dyspnea, nausea, vomiting, muscle weakness, somnolence, and coma (15). Accidental ingestion by a 3-year-old boy was reported, causing depression of the central nervous system (16). Fatal episodes have also been reported in humans (17) resulting from accidental exposure and also from large-volume mouthwash ingestion that contributed to severe anion-gap metabolic acidosis and osmolar gap, resulting in multiple organ system failure and death (18).
Since eucalyptol relaxes bronchial and vascular smooth muscle, it may have a putative direct action on the heart muscle. However, the actions of eucalyptol on cardiac contraction and contractile proteins have not been described. Hence, the present study was designed to investigate the actions of the oil on the contractile activity of cardiac muscle.
Material and Methods
Male Wistar rats weighing 250 to 300 g were used. Care and use of laboratory animals were in accordance with NIH guidelines. The rats received 500 units heparin ip and after 10 min they were anesthetized with 45 mg/kg sodium pentobarbital, ip. After thoracotomy, the hearts were rapidly removed and perfused through the aortic stump to permit proper selection and dissection of left ventricle papillary muscles. The preparations were mounted in a plexiglas chamber continuously superfused with gassed (95% O2 and 5% CO2) Krebs bicarbonate buffer solution of the following composition: 120 mM NaCl, 5.4 mM KCl, 1.2 mM MgCl26H2O, 1.25 mM CaCl22H2O, 2.0 mM NaH2PO4H2O, 1.2 mM Na2SO4, 27 mM NaHCO3, and 11 mM glucose, at 29 ± 1ºC. Muscles stretched to Lmax (muscle length at which active tension is maximal) were stimulated with isolated rectangular pulses (10 to 15 V, 12-ms duration) through a pair of platinum electrodes placed along the entire extension of the muscle. The standard stimulation rate was 0.5 Hz (steady state). Recording was started after 60 min to permit the beating preparation to adapt to the new environmental conditions.
The following parameters were analyzed: peak isometric force and its first time derivative, time to peak tension, relaxation time, taken as the time measured from the beginning to the peak force and from the peak force to the end of the contraction, respectively, relative potentiation obtained after a pause of 15 s, before and after eucalyptol treatment under steady-state stimulation.
Protocols
Muscles were stretched to Lmax and kept under steady-state conditions. Developed force was measured with an isometric force transducer (Nihon-Kohden, TB 612T, Tokyo, Japan), recorded on a chart recorder (Nihon-Kohden, RM-6200), and normalized to the muscle cross-sectional area (g/mm2). Considering the papillary muscle as a cylinder and tissue density as 1, the cross-sectional area was calculated by dividing the muscle length at Lmax by its weight. The first time derivative was obtained with a differentiator amplifier (Nihon-Kohden ED 601-G). Calibration was obtained by recording contraction at high velocity and drawing a straight line at the highest slope of the ascending portion of the contraction curve. This slope corresponds to the ratio between force (in g/mm2) and time (in s), which is the peak or maximum first time derivative.
To avoid the possibility of a hypoxic core we performed experiments at low temperature (29 ± 1ºC), as previously described (19). To investigate the actions of increasing eucalyptol concentrations on sarcoplasmic reticulum activity, a 15-s pause interval was used as previously described (20), and the results are presented as relative potentiation (the amplitude of post-rest contractions divided by steady-state contractions).
The following protocols were used:
1) The effects of eucalyptol (0.003, 0.007, 0.01, 0.03, 0.07, 0.1, and 0.3 mM) on cardiac contractions were tested in 8 preparations (cross-sectional area = 0.9 ± 0.06 mm2).
2) Under steady-state conditions, force was measured at increasing Ca2+ concentrations (0.62, 1.25, 2.5, 3.75, and 5 mM) in the absence (cross-sectional area = 1.10 ± 0.11 mm2, N = 12) and in the presence of 0.3 mM eucalyptol. In another group of papillary muscles (cross-sectional area = 0.96 ± 0.05 mm2, N = 12), under steady-state conditions, the positive inotropic effect produced by (-)isoproterenol (20 nM) was investigated with or without 0.3 mM eucalyptol.
3) The influence of eucalyptol (0.01, 0.05, 0.1, and 0.5 mM) on the contractile proteins was studied in another group of papillary muscles (cross-sectional area = 0.98 ± 0.06 mm2, N = 10) during tetanic stimulations. Tetanic tension was obtained after treatment with 5 mM caffeine for 20 min at a rate of 10 Hz for 15 s, as previously described (21). Tetanic contractions developed a fast upstroke, with the peak being measured as tetanic peak force, followed by a slow decay and a plateau, tetanic plateau force, measured at the middle of the plateau. Between tetanic stimuli the muscles remained under steady-state stimulation for at least 10 min. To insure that the effects of eucalyptol were not dependent on time, a time control was performed in another group of papillary muscles (cross-sectional area = 1.04 ± 0.03 mm2, N = 18) under the same conditions. Finally, to observe the recovery of muscle contraction, at the end of these experiments the preparations were washed twice with Krebs solution and kept under steady-state conditions, and tension was measured after 60 min.
4) The action of 0.3 mM eucalyptol on myosin ATPase activity was also studied.
Tissue preparation
Tissue samples (left ventricles) were quickly harvested, rinsed, blotted, and homogenized. Myosin was prepared from minced and homogenized left ventricles (N = 5), and extracted briefly with KCl-phosphate buffer (0.3 M KCl, 0.2 M phosphate buffer, pH 6.5) (22). After myosin precipitation by 15-fold dilution with water, the muscle residue was separated by filtration through a cheesecloth. This procedure precipitates fragments of cells including membranes. The filtrate containing myosin was centrifuged at 33,000 g for 30 min. After decanting the supernatant the precipitate was redissolved in 0.6 M KCl for elution of myosin under high ionic strength and 1 ml water was added to each g of tissue to produce a new precipitate. The material was again centrifuged at 33,000 g for 30 min and the muscle residue separated by filtration. The material was redissolved again in 14 ml of water per g of tissue, centrifuged and filtered as before. The precipitate was dissolved in 50 mM HEPES, pH 7.0, and 0.6 M KCl plus 50 glycerol (v/v) and stored at -20ºC. Before use, the stocked myosin was diluted in water (1:12) and centrifuged at 600 g for 15 min. The precipitate was resuspended in 50 mM HEPES, pH 7, and 0.6 M KCl, and centrifuged at 600 g again. The supernatant was used for enzyme assays.
Assay of myosin ATPase activity
Myosin ATPase activity was assayed (23,24) by measuring Pi liberation from 1 mM ATP in the presence of 50 mM HEPES, pH 7, 0.6 M KCl, 5 mM CaCl2 or 10 mM EGTA in a final volume of 200 µl. In the presence of this high ionic strength with no addition of Mg2+ to the incubation medium, only myosin activity is measured and there is no significant activity of Mg2+-ATPase, which is an actin-contaminated myosin. These assay conditions also excluded the activity of Ca2+-ATPase from the sarcoplasmic reticulum membranes that require high Mg2+ and low Ca2+ concentrations. ATP was added to the reaction mixture and pre-incubated for 5 min at 30ºC. The reaction was initiated by adding the enzyme fraction (3-5 µg protein) to the reaction mixture. Incubation times and protein concentration were chosen in order to ensure linearity of the reaction. Samples were assayed in duplicate or triplicate and corrected for non-enzymatic hydrolysis by using controls assayed under the same conditions, except that the protein sample was added after the reaction was stopped with 200 µl of 10% trichloroacetic acid. The reaction was initiated by adding the protein sample to avoid the inactivation at 30ºC caused by the lack of substrate. The enzyme activity was calculated as the difference between the activity observed in the presence of Ca2+ and the activity observed in the presence of 10 mM EGTA. Inorganic phosphate was determined by the method of Chan et al. (25). The specific activity was reported as nmol Pi released per min per mg protein. To determine whether eucalyptol affected myosin ATPase activity, protein samples were incubated with a 0.3 mM concentration of the oil.
Protein determination
Protein was measured by the Coomassie blue method according to Bradford (26) using bovine serum albumin as standard.
Drugs and reagents used
ATP, dithiothreitol, reduced glutathione, HgCl2 (mercuric chloride), EGTA (ethylene glycol-bis (ß-amino ethyl ether)-N,N,N',N'-tetra acetic acid), HEPES (N-[2-hydroxyethyl]piperazine-N'-[2-ethanesulfonic acid]), sodium salt, (-)isoproterenol hydrochloride, and anhydrous caffeine were purchased from Sigma (St. Louis, MO, USA), KCl, CaCl2, and KH2PO4 were purchased from Merck (Darmstadt, Germany); pentobarbital sodium, 30 mg, was from Cristalia, Produtos Químicos Farmacêuticos Ltda. (São Paulo, SP, Brazil); heparin, 5000 IU was from Roche Q.F.S.A. (Rio de Janeiro, RJ, Brazil); eucalyptol was purchased from S.S. White (Rio de Janeiro, RJ, Brazil). All other reagents were of analytical grade from Sigma, Merck or Reagen (Rio de Janeiro, RJ, Brazil).
Stock solutions were prepared with eucalyptol solubilized in a mixture of 5% Tween 80 (100 or 1 mM) plus distilled fresh water and, after vigorous shaking, were added to the bath solution in an appropriate volume, never greater than 1% Krebs solution, to obtain the desired concentration.
Statistical analysis
Data are reported as means ± SEM with N for number of observations. Comparisons were made by the Student t-test or one- or two-way ANOVA. When a significant ANOVA was obtained, the Tukey test was applied. The level of significance was set at P < 0.05. All analyses and data plots were performed using the GraphPad Prism (version 2.0, GraphPad Software, San Diego, CA, USA) and GB-STAT (version 4.0, Dynamic Microsystem Inc., Silver Spring, MD, USA) software.
Results
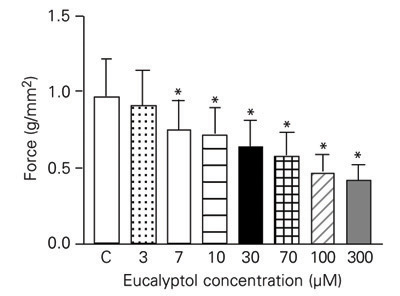
Figure 1 shows the effects of eucalyptol concentrations on the isometric force developed by papillary muscles. Eucalyptol reduced the force in a concentration-dependent manner (Figure 1), and 50% force reduction compared to control, that was taken as 100%, was attained at 0.3 mM. The first time derivative was also reduced, reproducing the same behavior as observed for force (results not shown). Eucalyptol also reduced time parameters, time to peak tension and relaxation time of the isometric contractions (Figure 2) in a concentration-dependent manner.
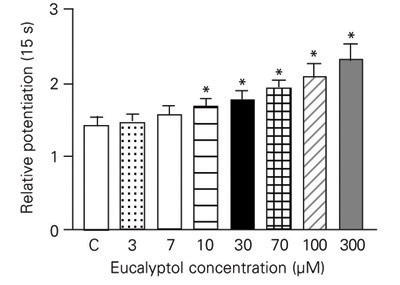
Post-rest potentiation was used to determine whether eucalyptol affects the function of the sarcoplasmic reticulum. Post-rest contractions (PRCs) obtained after 15-s pauses at different eucalyptol concentrations were recorded and analyzed as relative potentiation. Increasing eucalyptol concentrations produced a progressive increase in relative potentiation (Figure 3).
The physiological and pharmacological control of heart contractility can be exerted through changes in extracellular Ca2+ concentration. The dependence of force development upon changes in external Ca2+concentrations (0.62, 1.25, 2.5, 3.75, and 5 mM) in the absence and presence of eucalyptol (0.3 mM) is shown in Figure 4, upper panel. Although it reduced force, eucalyptol did not block the increase in force in response to increasing Ca2+ concentrations.
The influence of eucalyptol on the positive inotropic effect produced by isoproterenol was investigated. Figure 4, lower panel, shows that at low Ca2+ concentration, the negative inotropic effect elicited by eucalyptol was reversed by isoproterenol (20 nM).
We also investigated whether the depressant action of eucalyptol only occurred via Ca2+ influx or might affect contractile proteins. For this purpose, tetanic contractions were induced after treatment with 5 mM caffeine. As expected, isometric force reduction was observed after 20 min. Tetanic contractions developed a fast upstroke (tetanic peak force) followed by a slow and progressive decay. Figure 5 shows that eucalyptol depressed the tetanic contractions developed by papillary muscles in a concentration-dependent manner. This action was observed both at tetanic peak force and at the middle of the force decay (plateau force). However, tetanic contractions might change as a function of time under control conditions. When we investigated time-dependent effects (time controls) on tetanic contractions, we found no differences in the tetanic peak force or at the middle of force decay (results not shown).
To investigate whether tetanic tension reduction depends on eucalyptol actions, the contractile protein activity of the myosin ATPase (24) was measured. Eucalyptol (0.3 mM) did not affect the myosin Ca2+-ATPase activity (control: 487 ± 32.7 vs eucalyptol: 491 ± 33.1 nmol Pi min-1 mg-1 protein, P > 0.05).
At the end of experiments with tetanic contractions the reversibility of eucalyptol effects was determined. Papillary muscles were washed twice and force measured again after 60 min (control tetanic tension before eucalyptol treatment: 1.67 ± 0.15, tetanic tension in the presence of eucalyptol: 0.56 ± 0.08, and tension after washing and no eucalyptol: 1.26 ± 0.16 g/mm2, N = 10; P < 0.05 for comparison among tetanic tension and control or tension after washing, one-way ANOVA). With the concentrations of eucalyptol used, force reversibility was close to 70% of the control.
Effect of increasing concentrations of eucalyptol on the isometric force developed by rat papillary muscles. Data are reported as means ± SEM for N = 8. *P < 0.05 for eucalyptol vs control (C, one-way ANOVA).
Effect of increasing concentrations of eucalyptol on the parameters time to peak tension (TPT, upper panel) and relaxation time (lower panel) of the isometric force developed by rat papillary muscles. Data are reported as means ± SEM for N = 8. *P < 0.05 for eucalyptol vs control (C, one-way ANOVA).
Effect of increasing concentrations of eucalyptol on the relative potentiation obtained after pauses of 15 s duration. Results are reported as means ± SEM for N = 8. *P < 0.05 for eucalyptol vs control (C, one-way ANOVA).
Effect of 0.1 mM eucalyptol on the isometric force developed by rat papillary muscles under increasing Ca2+ concentrations (upper panel). Data are reported as means ± SEM for N = 12. Randomized two-way ANOVA was significant comparing the two groups. *P < 0.05 compared to control (0.62 mM Ca2+) both for control (squares) and eucalyptol-treated muscles (triangles). Lower panel, Effects of 0.1 mM eucalyptol on the isometric force developed after treatment with 20 ng/ml isoproterenol by rat papillary muscles. Data are reported as means ± SEM for N = 12. C = control; ISO = isoproterenol; EUC = eucalyptol. *P < 0.01 compared to control (0.62 mM Ca2+; one-way ANOVA).
Effect of increasing concentrations of eucalyptol on the tetanic force development measured at peak (upper panel) and half decay time (lower panel). Data are reported as means ± SEM for N = 10. *P < 0.01 for eucalyptol vs control (C, one-way ANOVA).
Discussion
The present results show that eucalyptol depresses the isometric contraction developed by rat papillary muscles in a dose-dependent manner without affecting the function of the sarcoplasmic reticulum. This effect seems to be the result, in part, of calcium channel blockade because eucalyptol reduced the development of tetanic tension.
Eucalyptol is a monoterpene, one of the main components of essential oils used as aromatic compounds. In dentistry it is used as a temporary dressing and as a lubricant for root canal filling material or as a gutta-percha solvent (3,4). Used as a drug, this compound has several actions including bronchodilatation (5,7) and vasodilatation of cerebral arteries (10). According to Juergens et al. (7), these actions may be related to inhibitory effects on the synthesis of cytokines and prostaglandins. Recently, cardiovascular actions were reported (11) examining whether the autonomic nervous system is involved, showing that eucalyptol induced hypotension and bradycardia in either conscious or anesthetized rats and dose-dependently reduced potassium-induced contractions in aortic rings. However, direct actions of eucalyptol on the cardiac muscle or contractile proteins were not described yet. Moreover, other compounds of essential oils, such as eugenol and isoeugenol, produce vasodilatation (27,28) and depress cardiac contractions acting via calcium channels (29). Our results show that eucalyptol reduced the isometric force developed by papillary muscles in a dose-dependent manner. The compound also affected the temporal parameters, with both time to peak tension and relaxation time being slightly reduced, in agreement with a calcium channel blocker action of the compound (30).
The force developed during cardiac muscle contraction is altered in response to changes in rate and rhythm (31). In cardiac muscle, the contractions occurring after short pauses are potentiated (20). In several species these PRCs usually become reduced in force as the rest periods increase (32). The rat cardiac muscle, different from other mammalian species, increases its force as the rest period increases (33). Mill et al. (20) reported that, despite these interspecies differences, PRCs depend on pause duration and on the amount of calcium stored at intracellular sites. This suggests that there is a significant difference between the activation processes of steady-state contraction (SSC) and PRC. The relative participation of the sarcoplasmic reticulum is more important for PRCs than for SSCs. Hence, if PRCs were more dependent on stored intracellular Ca2+ than SSCs, maneuvers which modify the transmembrane Ca2+ influx should affect SSCs more than PRCs. Regarding the actions of eucalyptol, although SSCs were progressively depressed, the PRCs were dose-dependently potentiated. This finding suggests that eucalyptol did not interfere with sarcoplasmic reticulum function and its effects were much more dependent on membrane Ca2+ influx through voltage-sensitive channels than on Ca2+ release from sarcoplasmic reticulum. Similar results were obtained in investigations of the effects of two Ca2+ channel blockers, verapamil and manganese (20). Therefore, if Ca2+ entry is partially blocked, the relative participation of the sarcoplasmic reticulum in tension development increases and the first contraction after a pause is potentiated.
ß-Adrenoceptor stimulation with isoproterenol increases the transmembrane Ca2+ influx and produces a faster Ca2+ uptake by the sarcoplasmic reticulum (34), which results in forceful and faster myocardial contraction. This positive inotropic effect of isoproterenol is small in isolated rat heart preparations at 1.25 and 2.5 mM Ca2+ concentrations (19). Since the rat myocardium saturates its positive inotropic response at extracellular Ca2+ concentrations lower than those for other species (19), protocols were performed at low extracellular Ca2+ (0.62 mM CaCl2) concentrations. We, therefore, investigated if increasing Ca2+ concentration (0.62 to 5 mM CaCl2) or isoproterenol administration reversed the negative inotropic response induced by eucalyptol. When muscles were treated with eucalyptol, force was reduced but the positive inotropic effects produced by increasing Ca2+ concentration or isoproterenol administration were not reversed.
In cardiac preparations, tetanic contractions are obtained after inhibition of sarcoplasmic reticulum activity with caffeine or ryanodine and this maneuver has been used to produce maximal activation of the contractile machinery in the intact myocardium (21,35-37). Caffeine acts by depleting the sarcoplasmic reticulum of its calcium content and also by inhibiting Ca2+ reuptake. The molecular site of action of caffeine also seems to be the ryanodine receptor (38).
Eucalyptol clearly affected contractile proteins, reducing tetanic tension. To be sure that the elicited tension decay was not a time-dependent cellular fatigue, time control experiments were performed. The results showed no difference in tension decay along the time course of the experiments. Thus, the effect of eucalyptol, rather than time, reduced the tension developed by papillary muscles during tetanic stimulation.
Since sarcoplasmic reticulum activity is abolished, this force reduction could be due to two mechanisms, i.e., reduction of the activity of the contractile machinery by eucalyptol by affecting myosin ATPase, or by reducing Ca2+ influx. To investigate if tetanic tension reduction was dependent on the actions of contractile proteins, we investigated the effect of eucalyptol on myosin ATPase activity. Eucalyptol (0.3 mM) was found to be unable to affect myosin Ca2+-ATPase activity, a fact supporting the suggestion of its Ca2+ channel blocker action.
To exclude possible irreversible toxic actions of eucalyptol produced by the concentrations used, normal Krebs solution without the oil was used at the end of the experiments with tetanic stimulation. Sixty minutes later it was possible to observe that muscle contractions recovered 70% of control force, suggesting that the concentrations used did not produce permanent myocyte damage.
This is the first report showing a new action of this monoterpene suggesting that: 1) eucalyptol depresses myocardial contractility in a concentration-dependent way and 2) increases the relative potentiation suggesting no interference with the function of the sarcoplasmic reticulum; 3) the negative inotropic effect of eucalyptol was reversed by Ca2+ and isoproterenol; 4) the depressant effect of eucalyptol on tetanic contractions without reduction of the activity of myosin ATPase also suggests a reduction of Ca2+ influx.
Address for correspondence: D.V. Vassallo, Departamento de Ciências Fisiológicas, CBM, UFES, Av. Marechal Campos, 1468, 29040-090, Vitória, ES, Brasil. Fax: +55-27-3335-7330. E-mail: daltonv2@terra.com.br
Received January 10, 2004. Accepted December 16, 2004.
- 1. Weyers W & Brodbeck R (1989). Skin absorption of volatile oils. Pharmacokinetics Pharmazie in unserer Zeit, 18: 82-86.
- 2. Laude EA, Morice AH & Grattan TJ (1994). The antitussive effects of menthol, camphor and cineole in conscious guinea-pigs. Pulmonary Pharmacology, 7: 179-184.
- 3. Uemura M, Hata G, Toda T & Weine FS (1997). Effectiveness of EUC and d-limonene as gutta-percha solvents. Journal of Endodontics, 23: 739-741.
- 4. Dogan H, Tasman F & Cehreli ZC (2001). Effect of gutta-percha solvents at different temperatures on the calcium, phosphorus and magnesium levels of human root dentin. Journal of Oral Rehabilitation, 28: 792-796.
- 5. Juergens UR, Stöber M, Schmidt-Schilling L, Kleuver T & Vetter H (1998). Antiinflammatory effects of eucalyptol (1.8-cineole) in bronchial asthma: inhibition of arachidonic acid metabolism in human blood monocytes ex vivo European Journal of Medical Research, 3: 407-412.
- 6. Santos FA & Rao VSN (2000). Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytotherapy Research, 14: 240-244.
- 7. Juergens UR, Stöber M & Vetter H (1998). Inhibition of cytokine production and arachidonic acid metabolism by eucalyptol (1.8 cineole) in human blood monocytes in vitro European Journal of Medical Research, 3: 508-510.
- 8. Santos FA & Rao VS (2001). 1,8-Cineol, a food flavoring agent, prevents ethanol-induced gastric injury in rats. Digestive Diseases and Sciences, 46: 331-337.
- 9. Kovar KA, Gropper B, Friess D & Ammon HPT (1987). Blood levels of 1,8 cineol and locomotor activity of mice after inhalation and oral administration of rosemary oil. Planta Medica, 53: 315-318.
- 10. Stimpfl T, Nael B, Nael C, Binder R, Vycudilik W & Buchbauer G (1995). Concentration of 1,8 cineole in blood during prolonged inhalation. Chemical Senses, 20: 349-350.
- 11. Lahlou S, Figueiredo AF, Magalhães PJ & Leal-Cardoso JH (2002). Cardiovascular effects of 1,8-cineole, a terpenoid oxide present in many plant essential oils, in normotensive rats. Canadian Journal of Physiology and Pharmacology, 80: 1125-1131.
- 12. Miyazawa M, Shindo M & Shimada T (2001). Oxidation of 1,8 cineole, the monoterpene cyclic ether originated from Eucalyptus polybractea, by cytochrome P450 3A enzymes in rat and human liver microsomes. Drug Metabolism and Disposition, 29: 200-205.
- 13. Tisserand R & Balacs T (1995). Essential Oil Safety Churchill Livingstone, London, UK, 2-16.
- 14. Jäger W, Nael B, Nael C, Binder R, Stimpfl T, Vycudilik W & Buchbauer G (1996). Pharmacokinetic studies of the fragrance compound 1,8-cineol in humans during inhalation. Chemical Senses, 21: 477-480.
- 15. Santos FA & Rao VSN (1997). Mast cell involvement in the rat paw oedema response to 1,8-cineole, the main constituent of eucalyptus and rosemary oils. European Journal of Pharmacology, 331: 253-258.
- 16. Patel S & Wiggins J (1980). Eucalyptus oil poisoning. Archives of Disease in Childhood, 55: 405-406.
- 17. De Vincenzi M, Silano M, De Vincenzi A, Maialetti F & Scazzocchio B (2002). Constituents of aromatic plants: eucalyptol. Fitoterapia, 73: 269-275.
- 18. Hoo Soo GW, Hinds RL, Dinovo E & Renner SW (2003). Fatal large-volume mouthwash ingestion in an adult: a review and the possible role of phenolic compound toxicity. Journal of Intensive Care Medicine, 18: 150-155.
- 19. Vassallo DV, Lima EQ, Campagnaro P, Stefanon I, Leite CM & Mill JG (1994). Effects of isoproterenol on the mechanical activity of isolated papillary muscles and perfused rat hearts in different calcium concentrations. Pharmacological Research, 29: 251-260.
- 20. Mill JG, Vassallo DV & Leite CM (1992). Mechanisms underlying the genesis of post-rest contractions in cardiac muscle. Brazilian Journal of Medical and Biological Research, 25: 399-408.
- 21. Leite CM, Mill JG & Vassallo DV (1995). Characteristics of tetanic contractions in the caffeine treated rat myocardium. Canadian Journal of Physiology and Pharmacology, 73: 638-643.
- 22. Bremel RD & Weber A (1975). Calcium binding to rabbit skeletal myosin under physiological conditions. Biochimica et Biophysica Acta, 376: 366-374.
- 23. Claude D & Swynghedauw B (1975). A comparative study of heart myosin ATPase and light subunits from different species. Pflügers Archiv. European Journal of Physiology, 355: 39-47.
- 24. Cappelli V, Bottinelli R, Poggesi C, Moggio R & Reggiani C (1989). Shortening velocity and myosin and myofibrillar ATPase activity related to myosin isoenzyme composition during postnatal development in rat myocardium. Circulation Research, 65: 446-457.
- 25. Chan K, Delfert D & Junger KD (1986). A direct colorimetric assay for Ca2+-ATPase activity. Analytical Biochemistry, 157: 375-380.
- 26. Bradford MM (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72: 218-254.
- 27. Nishijima H, Uchida RK, Kawakami N, Shimamura K & Kitamura K (1998). Role of endothelium and adventitia on eugenol-induced relaxation of rabbit ear artery precontracted by histamine. Journal of Smooth Muscle Research, 34: 123-137.
- 28. Nishijima H, Uchida RK, Kameyama K, Kawakami N, Onkubo T & Kitamura K (1999). Mechanisms mediating the vasorelaxing action of eugenol, a pungent oil, on rabbit arterial tissue. Japanese Journal of Pharmacology, 79: 327-334.
- 29. Damiani CEN, Moreira CM & Vassallo DV (2002). Calcium-antagonist like-effects of eugenol in rat cardiac muscle. Journal of Hypertension, 20 (Suppl 4): S352. (Abstract R070).
- 30. Mattiazzi AR & Garay A (1983). Negative inotropic effect of verapamil, nifedipine and prenylamine and its reversal by calcium or isoproterenol. Archives of International Physiology and Biochemistry, 91: 133-144.
- 31. Mill JG, Vassallo DV, Leite CM & Campagnaro P (1994). Influence of the sarcoplasmic reticulum on the inotropic responses of the rat myocardium resulting from changes in rate and rhythm. Brazilian Journal of Medical and Biological Research, 27: 1455-1465.
- 32. Vassallo DV & Mill JG (1988). Mechanical behaviour of rest contractions in cardiac muscle. Acta Physiologica et Pharmacologica Latinoamericana, 38: 87-97.
- 33. Vassallo DV, Mill JG & Abreu GR (1990). Control of rest potentiation in ventricular myocardium by the Na-Ca exchange mechanism. Acta Physiologica et Pharmacologica Latinoamericana, 40: 129-136.
- 34. Holmer SR & Homcy CJ (1991). G Proteins in the heart. A redundant and diverse transmembrane signaling network. Circulation, 84: 1891-1892.
- 35. Henderson AH, Brutsaert DL, Forman R & Sonnenblick EH (1974). Influence of caffeine on force-development and force-frequency relations in cat and rat heart muscle. Cardiovascular Research, 8: 162-172.
- 36. Yue DT, Marban E & Wier WG (1986). Relationship between force and intracellular Ca2+ in tetanized mammalian heart muscle. Journal of General Physiology, 87: 223-242.
- 37. Gwathmey JK & Hajjar RJ (1990). Relation between steady-state force and intracellular [Ca2+] in intact human myocardium. Circulation, 82: 1269-1278.
- 38. Opie L (1998). The Heart: Physiology, from Cell to Circulation 3rd edn. Chapter 20. Lippincott-Raven Publishers, Philadelphia, PA, USA, 589-608.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
08 Mar 2005 -
Date of issue
Mar 2005
History
-
Received
10 Jan 2004 -
Accepted
16 Dec 2004