Abstract
Autoimmune diseases constitute a heterogeneous group of conditions commonly treated with anti-inflammatory, immunosuppressant and immunomodulating drugs, with satisfactory results in most cases. Nevertheless, some patients become resistant to conventional therapy. The use of high doses of drugs in such cases results in the need for bone marrow reconstitution, a situation which has stimulated research into the use of hematopoietic stem cells in autoimmune disease therapy. Stem cell transplantation in such diseases aims to destroy the self-reacting immune cells and produce a new functional immune system, as well as substitute cells for tissue damaged in the course of the disease. Significant results, such as the reestablishment of tolerance and a decrease in the recurrence of autoimmune disease, have been reported following stem cell transplantation in patients with autoimmune disease in Brazil and throughout the world. These results suggest that stem cell transplantation has the potential to become an important therapeutic approach to the treatment of various autoimmune diseases including rheumatoid arthritis, juvenile idiopathic arthritis, systemic lupus erythematosus, multiple sclerosis, systemic sclerosis, Crohn's disease, autoimmune blood cytopenias, and type I diabetes mellitus.
Autoimmune disease; Stem cells; Transplantation; Lupus erythematosus; Arthritis; Type I diabetes mellitus
Braz J Med Biol Res, December 2007, Volume 40(12) 1579-1597 (Review)
The use of stem cells for the treatment of autoimmune diseases
S.B. Rosa1,2, J.C. Voltarelli5, J.A.B. Chies3 and  Correspondence and Footnotes
Correspondence and Footnotes
 P. Pranke1,4
P. Pranke1,4
1Laboratório de Hematologia, Faculdade de Farmácia, 2Programa de Pós-graduação em Biologia Celular e Molecular, 3Departamento de Genética, 4Programa de Pós-graduação em Medicina: Ciências Médicas, Faculdade de Medicina, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brasil
5Departamento de Clínica Médica, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brasil
 Text
Text
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Autoimmune diseases constitute a heterogeneous group of conditions commonly treated with anti-inflammatory, immunosuppressant and immunomodulating drugs, with satisfactory results in most cases. Nevertheless, some patients become resistant to conventional therapy. The use of high doses of drugs in such cases results in the need for bone marrow reconstitution, a situation which has stimulated research into the use of hematopoietic stem cells in autoimmune disease therapy. Stem cell transplantation in such diseases aims to destroy the self-reacting immune cells and produce a new functional immune system, as well as substitute cells for tissue damaged in the course of the disease. Significant results, such as the reestablishment of tolerance and a decrease in the recurrence of autoimmune disease, have been reported following stem cell transplantation in patients with autoimmune disease in Brazil and throughout the world. These results suggest that stem cell transplantation has the potential to become an important therapeutic approach to the treatment of various autoimmune diseases including rheumatoid arthritis, juvenile idiopathic arthritis, systemic lupus erythematosus, multiple sclerosis, systemic sclerosis, Crohn's disease, autoimmune blood cytopenias, and type I diabetes mellitus.
Key words: Autoimmune disease, Stem cells, Transplantation, Lupus erythematosus, Arthritis, Type I diabetes mellitus
Text
Autoimmune diseases (AD) are caused by immunologic imbalance and loss of tolerance, which lead the immune system to attack self tissues within the organism. AD can be mediated by immune complexes, circulating autoantibodies and autoreactive T lymphocytes. Conventional AD therapy is effective in most patients, but some patients are resistant to the anti-inflammatory and immunosuppressive agents used or are only capable of responding to high doses of such medicines, which are toxic. In such cases, bone marrow (BM) reconstitution is required. Thus, high doses of immunosuppressants, followed by hematopoietic stem cell transplantation (HSCT), have become an alternative treatment for many diseases involving the immune system. These include multiple sclerosis (MS), systemic sclerosis (SS), rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), and systemic lupus erythematosus (SLE) (1,2).
The application of HSCT to the treatment of AD has been studied since the 1970s. The success of this approach has been widely demonstrated in animal models as well as in BM transplant patients who were shown to also have concomitant AD. For example, an allogeneic BM transplantation (BMT), which was intended to cure aplastic anemia in 2 patients with concomitant RA, resulted in the complete remission of RA for at least 11 years (2).
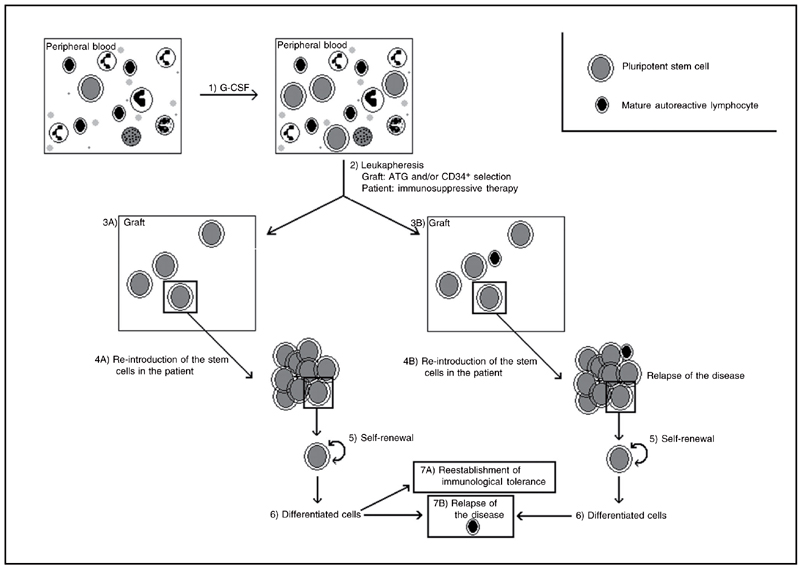
The use of stem cell therapy in diseases involving the immune system is increasingly common. By 2004, the European and North American data banks had accumulated information on about 800 patients treated with HSCT for a variety of diseases (3). Most of these procedures involved peripheral blood autologous HSCT (AHSCT). After the administration of growth factors to the patient, which mobilizes the stem cells from BM to peripheral blood, the patient's blood is collected and processed, and mature cells are excluded until a sufficient number of HSCs are obtained for transplantation. The patient then undergoes radioactive and/or cytotoxic drug therapy, which eliminate the cells in division, including immune system cells. The treated blood is returned to the patient via transfusion, and, by a mechanism known as homing, the HSCs migrate to the BM, where they begin a process of differentiation into mature functional cells. This treatment has been shown to result in the reestablishment of tolerance and a decrease in the recurrence of AD. Figure 1 shows a schematic representation of this procedure.
Stem cells
Stem cells are characterized by their capacity for self-renewal, multilineage differentiation, proliferation, mobilization, and homing (4). Stem cells can be divided into two main groups: embryonic stem cells (ESCs) and adult stem cells (ASCs).
ESCs, found in the embryo until approximately 3 days after fertilization, are totipotent, that is, they are capable of giving rise to more than 200 cell types, including extra-embryonic tissues such as the placenta and umbilical cord. ESCs obtained from the internal mass of cells that form the blastocyst, at 4 or 5 days following embryo fertilization of the embryo, but before implantation in the uterus, are pluripotent cells (5). That is, they are capable of giving rise to all cell types, with the exception of extra-embryonic tissues.
ASCs give rise to specific tissue progenitor cells and are, in most cases, multipotent, producing a more limited number of cell types. The stem cells most commonly used in clinical medicine are HSC, a type of multipotent ASC, which give rise to all types of blood and immune system cells (5). The main sources of HSCs are BM, umbilical cord blood (UCB) and, in smaller quantities, peripheral blood. Some ASCs appear to be capable of transdifferentiation. This process is characterized by the differentiation of a progenitor cell into a cell line other than that from which it originated. An example is the capacity of BM stem cells to give rise to neurons (6). Later, pluripotent stem cells, such as mesenchymal stem cells (MSCs) and multipotent adult progenitor cells, were discovered in several adult tissues.
In the next sections, we report on a number of AD for which stem cell transplantation is currently being considered a potential therapeutic approach. Additionally, we provide examples of the different therapeutic protocols used, and discuss the limitations and advantages of such procedures.
Rheumatoid arthritis and juvenile idiopathic arthritis
Autologous stem cell transplantation (ASCT) has been proposed as a possible treatment for severe AD such as RA, MS, SS, and SLE. Almost 1000 patients with various autoimmune disorders have undergone ASCT since 1996, among them a small number of children, most of them suffering from JIA (7).
RA is a systemic AD that, in the long term, can lead to irreversible destruction of the joints, loss of mobility, as well as a reduction in both the quality of life and life span. Cellular and humoral immune responses can contribute to the development of lesions. Rheumatoid factor, an autoantibody specific to the Fc region of human IgG, is found in 80% of patients with RA (8).
The cells involved in inflammatory reactions are derived from HSCs, which suggests that this disease may originate from problems in stem cells. It is not clear whether the inflammatory cells are derived from abnormal stem cells or whether they are normal progenitor cells that demonstrate pathogenicity within a microenvironment predominantly occupied by inflammatory cytokines. Proof that the defect lies in stem cells is the fact that RA can be transmitted or abolished by allogeneic stem cell transplantation (9).
One hypothesis concerning RA is that it involves the aging of stem cells. The HLA-DR4 haplotype appears to be linked to RA. Healthy HLA-DR4+ individuals present premature aging of HSCs. Aged T cells express distinct immunoregulatory receptors that potentiate their activity, independently of antigenic recognition. These cells present pro-inflammatory properties and trigger auto-reactivity. In RA, the abnormal functioning of stem cells is probably not limited to HSCs, but may also involve, for example, MSCs. Thus, the premature aging of stem cells in RA may lead to tissue destruction resulting from the action of T cells and failure of the tolerance mechanism (10).
Study of the application of stem cells to the treatment of RA started at the beginning of the 1980s in research using animal models. Clinical studies mainly involve autologous HSCs or, more rarely, syngeneic or allogeneic BMT (11). The transplantation protocol varies between studies. As in other AD, in order to perform peripheral blood AHSCT the cells are mobilized using granulocyte-colony stimulating factor (G-CSF) with or without immunosuppression, prior to leukapheresis. The graft may or may not be manipulated in vivo or ex vivo using antithymocyte globulin (ATG) and/or by selection of CD34+ cells, the classic marker of HSCs. The purpose of these procedures is to eliminate mature autoreactive lymphocytes, thus preventing them from being re-introduced into the organism. The patient is submitted to an intense immunosuppressive process followed by the reintroduction of stem cells into the receptor. Several clinical studies are discussed throughout this review and are listed in Table 1. The patients selected in the following studies presented severe RA which had proven to be resistant to conventional therapies.
Mobilization of HSCs with G-CSF is considered to be a suitable technique. However, the administration of cyclophosphamide (CY) prior to G-CSF appears to offer some advantages. Immunosuppression induced by CY may reduce the risk of intensifying the RA symptoms produced by G-CSF. CY seems to increase HSC levels, facilitating graft manipulation, and to reduce the number of potentially pathogenic T lymphocytes re-introduced into the patient. Moreover, it appears to stimulate renovation of immunocompetent cells, reestablishing the immunological balance, without the need for HSC re-infusion (12).
This conditioning regime should provide great efficacy with reduced toxicity. Thus, the dose of CY to be used has been established as 200 mg/kg since it produces an effective response in RA treatment and presents acceptable levels of toxicity (13). In a study involving 4 patients who received CY and ATG, 1 patient was submitted to total body irradiation (TBI), 2 patients, including the one that received TBI, presented disease reactivation post-transplantation, though the symptoms were mild and transitory. The other 2 patients reached improvement level ACR70. According to the American College of Rheumatology (ACR), ACR70 corresponds to an improvement level of 70% in the count of joint pain and edema, together with an improvement of 70% in at least 3 of the 5 parameters, including the level of reactive C protein, erythrocyte sedimentation, and functional incapacity. Similarly, ACR20 and ACR50 correspond to improvements of 20 and 50%, respectively (14).
Research into the use of high doses of CY in the absence of stem cell re-infusion is based on the HSC expression of high levels of aldehyde dehydrogenase, an enzyme responsible for the cellular resistance of CY (9), and 2 patients submitted to this procedure showed complete remission of the disease (15). Moore and colleagues (16) studied 33 patients who were randomly submitted to either the autologous transplantation of non-manipulated cells or the transplantation of selected CD34+ cells. The effects of using non-manipulated stem cells were better than those obtained with selected CD34+ cells. In a syngeneic transplantation of HSCs, in which the patient with RA received HSCs from his twin brother, no recurrence of the disease was seen for at least 24 months. There were some differences in the expression of some T cell receptor subtypes between the brothers. Following transplantation, the T cell receptor repertory of the host became practically the same as that of donor. Thus, the syngeneic lymphocytes from a healthy donor are capable of modifying the immunoregulation disorder, contributing to the production of graft-versus-autoimmunity (17).
Most studies on HSCT involve isolated cases or small sample groups. A wider study, based on the records of both the European Group for Blood and Marrow Transplantation (EBMT) and the Autologous Blood and Marrow Transplant Registry, involved the collection of results from around the world of cases of treatment of RA with stem cells, particularly severe and resistant cases. From 1996 to 2000, 76 patients were registered. The mortality rate related to the treatment was low, with the death of 1 patient, who had been submitted to graft purification and immunosuppression. Most of the patients responded well to the treatment, although with a high rate of relapse. AHSCT makes it possible to control the return of the disease with the use of conventional antirheumatic drugs. The good response to these post-transplantation drugs suggests that ASCT is associated in some way with immunomodulation (8,13,14,16).
Thus, HSCT in RA therapy is well tolerated by patients, with few cases of deaths related to transplantation. Currently, AHSCT is not considered to be a curative therapy for RA. However, it may represent a possible "back to the beginning of the disease mechanism", facilitating the control of symptom intensification through the use of maintenance therapy (9). This post-transplantation maintenance has been the focus of the EBMT/EULAR (European League Against Rheumatism) Autologous Stem Cell Transplantation International Rheumatoid Arthritis Trial (3). However, this trial was suspended because of insufficient patient accrual.
Systemic JIA is a heterogeneous form of arthritis in childhood and represents 10-20% of all cases of JIA in the Caucasian populations of Northern America and Europe. Up to 30% of patients will still have active disease after 10 years, and morbidity within this group is high (18). Despite modern therapies, JIA remains a significant cause of morbidity in children because it does not usually respond to anti-TNF agents.
Since 1997, HSCT has been applied as an experimental procedure to more than 50 children with refractory JIA. In a retrospective analysis of follow-up data on 34 children with JIA who were treated with ASCT at nine different European transplant centers, the results showed that 18 of the 34 patients (53%) achieved complete drug-free remission at follow-up times of 12 to 60 months. In 7 of these patients earlier treatment with anti-TNF had failed. Six of the 34 patients (18%) showed a partial response (ranging from 30 to 70% improvement) and 7 (21%) were resistant to ASCT. There were 3 cases of transplant-related mortality (9%) and 2 of disease-related mortality (6%). These results show that ASCT in severely ill patients with JIA induces a drug-free remission of the disease and a profound increase in general well-being in a substantial proportion of patients, but the procedure carries a significant risk of mortality (19). More recently, this risk has been reduced by pre-treating with steroids the patients in whom the disease is very active prior to transplantation. The European Bone Marrow Transplantation Group plans to continue using ASCT in the treatment of JIA, particularly the systemic onset form of the disease, with fludarabine in the conditioning regimen and probably, in the future, MSC instead of HSC. Only 1 patient with RA was transplanted during the Brazilian Cooperative Trial of Autologous Hematopoietic Stem Cell Transplantation for AD. A 20-year-old female patient with the polyarticular form of JIA received a conditioning regime of CY (200 mg/kg) and rabbit ATG (4.5 mg/kg) and autologous peripheral blood stem cells in September/2005 at the Hospital Sírio Libanês in São Paulo. Anti-CD20 monoclonal antibody (Rituximab) was given (375 mg/m2 every 3 months) to prevent relapse and the patient maintains complete remission after 1 year of follow-up (20 and Massumoto C, personal communication).
Systemic lupus erythematosus
There is evidence that patients with SLE, a systemic AD, present BM dysfunction, with a marked reduction in the numbers of CD34+ cells and a possibly reduced stem cell proliferation capability. The patients present elevated levels of apoptosis in the CD34+ cell subpopulation and a reduced frequency of colony-forming units when compared to control groups. These abnormalities appear to be related to a defect in the microenvironment of the BM, contradicting the hypothesis that they are related to some primary defect in the stem cells. During the post-transplantation period, a greatly reduced number of apoptotic CD34+ cells are seen when compared with the pre-treatment period (21).
The EBMT and the EULAR registered 53 cases of the use of HSCT in patients with SLE between 1995 and 2002. In the United States, a transplant center reported the use of HSCT in 50 cases from 1997 to 2005 (21). Currently, research protocols use a conditioning protocol consisting of CY and G-CSF for mobilization, and high doses of CY combined with ATG, and in vitro CD34+ cell selection (21,22).
Besides the SLE patients submitted to HSCT reported in the EBMT/EULAR (21) and the American (22) studies, 32 other isolated cases have been reported. There were 22 cases of clinical remission. About 30% of the patients who had remission of the disease presented a relapse (23).
With this disease, the longer the post-transplantation period, the greater the risk of relapse, which is associated with low level of anti-dsDNA antibodies prior to HSCT. According to the EBMT/EULAR studies, there was no difference in the frequency of relapse with selection of CD34+ cells or with a more intense conditioning regime. The adverse effects of the treatment are not severe and the activity of the disease can be controlled with therapies to which the patient was previously resistant (21,22).
There are many examples of clinical improvement in patients with SLE submitted to AHSCT or to treatment with high doses of CY (24). However, new inflammatory and autoimmune processes have occurred in patients submitted to HSCT (21) and in patients treated only with CY without infusion of stem cells (24). This probably occurs as the result of the deletion of regulatory T cells (21).
In Brazil, the first four cases of SLE submitted to AHSCT in a multicenter cooperative trial were reported in 2003 (1). The patients received high doses of CY and ATG, which were both administrated before and after cell infusion. The use of ATG immediately after infusion was intended to complement the in vivo T cell deletion since in the protocol the infused cells did not suffer in vitro selection. Three patients presented severe kidney failure and 1 patient died. The 3 surviving patients showed remission of kidney disease after transplantation, but 1 patient relapsed thereafter and showed a decline in renal function (Scheinberg M, personal communication). Four other patients were included in the clinical trial, 1 patient could not have his stem cells mobilized and returned to conventional immunosuppression and 3 patients died from transplant complications (acute renal failure and septicemia) (20).
Several studies in the international literature have shown that AHSCT is efficient in achieving remission of disease in patients with resistant SLE. Transplantation is able to change the behavior of severe diseases, making them more benign and responsive to therapy. It has been suggested that the reported higher rates of mortality in Brazil and other countries (25) can be controlled by appropriate patient selection, the choice of a less aggressive conditioning regime and the acquisition of more experience in managing specific complications associated with the procedure (21).
Multiple sclerosis
Multiple sclerosis is an organ-specific AD mediated by T cells triggered against structural components of myelin in the central nervous system (CNS). Subsequent to inflammation in the CNS, demyelinization and loss of axons may occur, resulting in interruption of the electrical signal. Most MS patients present episodic relapse and improvement, known as relapsing-remitting MS, followed by a phase called secondary progressive MS. There is yet another form of MS known as primary progressive MS, which is generally resistant to conventional therapies (26).
Available treatments for MS are not curative. They are able to reduce inflammation in the CNS and to delay the advance of the disease, but disease control is frequently unsatisfactory. The use of stem cells in the treatment of MS is based on the immunosuppressor and immunomodulatory effects of AHSCT, which may favor the immunological balance (26). Furthermore, the multi-focal nature of MS makes the injection of stem cells into each affected site impracticable, which means that the cells need to be attracted to the pathological areas. The intravenous administration of stem cells may be an alternative in MS and other neuroinflammatory conditions, in which there is permeability of the hematoencephalic blood brain barrier in the inflammatory areas. Moreover, the discovery that stem cells are capable of reaching the CNS and of transdifferentiating or acquiring oligodendrocyte and possibly neuronal properties, suggests that they may be able to act in re-myelinization and neuron repair.
The first reported AHSCT in patients with MS was performed in 1995 (26). By November 2002, MS was the AD with the largest number of reports of AHSCT. More than 200 patients were submitted to AHSCT specifically for the treatment of MS (3). Table 2 lists the clinical trials of HSCT for MS. Practically all the patients were in the progressive phase of MS, and most of them were in the secondary progressive MS phase. These patients had not responded to previous conventional treatments and presented severe functional incapacity. There is no variation in the results from different transplant centers (27). AHSCT significantly reduced the inflammation in the CNS, as shown by magnetic resonance imaging, and enhanced the results obtained with conventional therapies (26). The most common adverse effect was the occurrence of infection, which affected more than 50% of the patients in the clinical trial of Fassas and colleagues (27). The mortality rate associated with transplantation is high (5-10%) when compared with that of conventional treatment (0%), although it is believed that with a more precise patient selection procedure this rate could be reduced. The morbidity and mortality rates seem to be related to the intensity of T cell deletion and to the conditioning regime, as well as to advanced age and severe functional incapacity of the patients (26). There is a need to determine what degree of risk is acceptable in patients with a disease that, although severe and rarely curable, has a low death rate.
Some clinical studies have reported the persistence of cerebral atrophy after AHSCT for MS. However, it is still not possible to know whether or not AHSCT has any effect on the advance of atrophy because the patients involved in these studies presented a severe and rapidly advancing form of MS. In this situation, cerebral atrophy might be taking place at an accelerated and cumulative pace and, moreover, a more active phase of the disease may occur during pre-treatment, mainly during cell mobilization. Accordingly, the loss of brain tissue could be a long-term consequence of previous activity of the disease. Furthermore, there is the possibility of pseudoatrophy, which occurs in response to the intense suppression of the disease activity induced by AHSCT (28).
AHSCT appears not to yield good results in patients whose clinical progression involves axon degeneration. Thus, the use of AHSCT in the early stages of MS would appear to be more suitable for patients with a low level of functional incapacity (26).
Matrix metalloproteinase-9 (MMP-9), a marker of MS pathogenesis, is regulated by the tissue inhibitor of MMP-1. The serum and expression level of mRNA of MMP-9 and of tissue inhibitor of MMP-1 were analyzed in peripheral mononuclear cells from 14 patients with MS after AHSCT. The results indicated inhibition of MMP-9 activity, which is in accordance with clinical condition and reduction of disease activity (33).
The mechanism by which AHSCT affects the course of MS is not known. The immediate effect appears to be the eradication of auto-reactive clones. Patients treated with AHSCT show a low T CD4+ cell count (33), and, as a consequence, the inflammatory process is reduced and there is a possible clinical improvement. Besides this immediate effect, the infusion of stem cells after high doses of immunosuppression appears to give rise to a "new" tolerance system (34). Another hypothesis to explain the improvement of patients is the capacity of stem cells to transdifferentiate into glial cells and neuronal precursors, contributing to the repair of damaged nervous tissue. Thus, AHSCT can lead to prolonged periods of stabilization of MS or change the degree of severity in the course of the disease.
An initiative of the EBMT/EULAR, the Autologous Stem Cell Transplantation International Multiple Sclerosis Trial intends to compare AHSCT with conventional treatment, and so determine the efficacy and toxicity related to stem cell therapy in progressive MS (3). Another controlled clinical trial (MIST), created by R. Burt in Chicago with the cooperation of some Brazilian centers, compared AHSCT with conventional therapy in the earlier inflammatory phase of interferon-refractory diseases (relapsing-remitting).
Under the Brazilian cooperative trial of HSCT for AD, 41 patients with progressive (primary or secondary) MS with a score £7.0 on the Expanded Disability Status Scale were transplanted from June/2001 through August/2006 at several centers, mainly in the University Hospital of the Ribeirão Preto Medical School and the Hospital Israelita Albert Einstein in São Paulo. Initially, 21 patients were conditioned with 1,3-bis[2-chloroethyl]-1-nitrosourea, etoposide, aracytin, melphalan, and horse ATG, and 3 patients died from transplant-related complications. Beginning in 2004, following a change in the conditioning regime to CY plus ATG, there were no deaths among 20 patients transplanted and there was no difference in the rate of response (stopping progression) between the two groups (before and after the change in the conditioning regime). Eight patients transplanted out of protocol (excessively high Expanded Disability Status Scale or stronger immunosuppression) either died or showed neurological progression (Hamerschlak N and Voltarelli J, personal communication).
Systemic sclerosis
Systemic sclerosis or scleroderma is characterized by predominant T cell activation, autoantibody production (anti-scleroderma-70) and cytokine secretion, leading to excessive fibrosis throughout the body. Severe forms of the disease give rise to high levels of morbidity and mortality (estimated at 40-50% in 5 years), secondary to lung, cardiac and kidney involvement. Conventional therapies apparently are not efficient in preventing the advance of the disease and reversion of the fibrosis (35).
In November 2003, 83 cases of patients with SS submitted to stem cell transplantation were registered in Europe and the United States (35). The therapeutic regimes used for the treatment of SS with ASCT registered in EBMT/EULAR were similar. For cell mobilization, the use of CY and G-CSF is preferred to that of G-CSF only. The selection of CD34+ cells prior to re-infusion was performed in 87% of patients. For conditioning, CY was applied in 90% of cases, either as a monotherapy or in combination with ATG (36).
Since 1996, good results have been obtained in SS after HSCT. In a study published in 2006, which included 6 patients with SS, 1 of them with associated SLE, improvement was reported in dermatologic sclerosis in all patients (37).
In a study by Farge and colleagues (36), 57 patients with SS were treated with HSCT in the period from 1996 to 2002 and followed up for more than 6 months. Complete remission was observed in 14 patients, and partial remission in 32 patients, for a period of over 3 years. Though some patients exhibited active disease following HSCT, they were found to be in better clinical condition than prior to treatment.
However, not all the reported outcomes are favorable. In a French multicenter study involving 11 patients, while a complete or partial response was observed in 8 patients, 5 of these patients showed reactivation of the disease. Nevertheless, according to the authors, AHSCT is able to extend the short life expectancy of patients with severe SS (36).
Toxicity risk factors were not totally analyzed due to the small number of patients and to the variety of protocols applied. Therapy with high doses of immunosuppressors appears to be less tolerated by patients with lung and heart damage due to the cardiotoxicity of CY. A careful selection of patients, close monitoring during treatment and changes in the transplantation protocol should reduce the number of deaths (35). A conditioning regime using thiotepa and low doses of CY seems to minimize the cardiotoxicity induced by CY (38), and it is recommended that TBI in the absence of lung protection be avoided.
Supervised by the EBMT and EULAR, a controlled and randomized phase III study is being organized, called Autologous Stem Cell Transplantation International Sclerodermal Trial (3). In the United States, a similar randomized phase III study, called Scleroderma: Cyclophosphamide or Transplantation, is being planned. The aim of these studies is to investigate the effects of intensive immunosuppression followed by HSCT versus conventional chemotherapy with CY in patients with scleroderma in order to establish a safer and more effective protocol (35).
In the Brazilian cooperative trial of AHSCT for AD, seven patients with progressive and refractory SS were treated (20). Three patients were mobilized but not transplanted (2 improved and 1 died from infection). One patient with overlap syndrome (SLE plus SS) died from disease reactivation (pulmonary and cerebral vasculitis) before receiving HSCT. Three patients improved significantly after transplantation (softening of the skin and stabilization of pulmonary function), but 1 of them died of pulmonary infection more than 2 years after transplantation. After this report, 2 other patients were transplanted at the University of São Paulo Hospital in Ribeirão Preto without early complications and with rapid improvement of skin lesions.
Autoimmune cytopenias
Autoimmune thrombocytopenic purpura (AITP) is a disease in which platelets are sensitized by anti-platelet antibodies or circulating immune complexes, provoking the early removal of these cells from the circulation. This process results in thrombocytopenia and bleeding. About one third of patients with AITP do not respond well to conventional therapies. High doses of immunosuppressors may lead to remission of the disease; however, this involves risks related to myelosuppression. HSCT aims to accelerate reestablishment of the hematological parameters and concomitantly reduce the number of autoimmune cells in the organism (39). Several sporadic case reports of HSCT for autoimmune cytopenias have been published, but only two studies have been reported with moderate numbers of patients.
A study described the clinical history of patients registered in the EBMT who had received HSCT to treat severe refractory autoimmune cytopenia. In 12 patients with AITP submitted to autologous transplantation the responses were highly diverse, varying from a transient response to continuous remission or even death related to transplantation (40). In the US, of 14 patients with chronic refractory AITP submitted to high dose cyclophosphamide (200 mg/kg) followed by AHSCT, 6 achieved durable complete responses and 2 obtained durable partial responses (41). The study concluded that the infusion of HSCs had accelerated the hematological reestablishment. Since clinical improvement was not associated with quantification of anti-GPIb or anti-GPIIIa antibodies, it is likely that other platelet antigens were involved in the AITP. The responses were not associated with the number of CD3+ cells infused into the patients, although the deletion of T lymphocytes may have prevented the re-infusion of auto-reactive T cells (39).
Clinical trials involving larger numbers of patients could provide more reliable information on the importance of lymphocyte deletion and immunogenetic characterization of patients that are responsive or non-responsive to treatment. It would then be possible to compare different treatment protocols: a) therapy based only on high doses of CY; b) therapy using high doses of CY combined with stem cell transplantation, and c) the latter procedure used in association with agents inducing the maintenance of post-transplantation tolerance (39).
Autoimmune hemolytic anemia (AIHA) is characterized by the early destruction of erythrocytes due to the fixation of immunoglobulin or complement protein on the membrane surface. The initial symptoms can result from anemia caused by hemolysis, the secondary effects of the hemolytic condition, or the primary disease which caused the AIHA. There are a few reports in the scientific literature of cases in which stem cell transplantation has been used for the treatment of AIHA (40,42) and the data suggest that immunosuppressive therapies should be used post-transplantation in this disease.
Despite the incipient data, stem cell transplantation offers a new therapeutic possibility for patients with AIHA.
Diabetes mellitus
In recent years, there has been a rapid growth in the number of cases of diabetes throughout the world. This epidemic affects approximately 6-8% of the world population and the number of newly diagnosed patients increases yearly (43). Of these, approximately 10% are type I diabetes, insulin-dependent diabetes mellitus, an AD caused by the progressive destruction of the insulin-secreting pancreatic ß-cells in the islets of Langerhans (4,44) which regulate blood sugar levels by secretion of insulin. Recent clinical data suggest that the disease could be cured if an adequate supply of new ß-cells were made available. Hence, one goal of pancreatic developmental biology is to understand how endogenous ß-cells are made, with the hope of producing them exogenously (44).
Pancreatic islet cell transplantation is an attractive treatment of type I diabetes (45). Clinical islet transplantation trials based on cadaveric allogeneic islets have demonstrated that it is indeed possible to restore near-physiological insulin secretion capacity in type I diabetic patients through transplantation of insulin-producing cells (46). There are a number of difficulties associated with islet allotransplantation such as problems related to alloimmunity, autoimmunity, and the need for larger islet cell masses. Employment of animal islets and stem cells as alternative sources of insulin production will be considered in order to deal with the problem of human tissue shortage (45). Lifelong immunosuppressive therapy and inadequate sources of transplantable islets have limited the benefits of islet transplantation to less than 0.5% of type I diabetics. Whereas the potential risk of infection by endogenous animal viruses limits the uses of islet xenotransplantation, deriving islets from stem cells seems to be able to overcome the current problems of islet shortages and immune compatibility (4).
The fact that BM contains HSCs and MSCs, which are capable of differentiating into a number of different kinds of cells such as pancreatic ß-cells, has, in recent years, attracted the attention of researchers to the study of stem cells in the treatment of type I diabetes. However, there is some controversy regarding the results obtained with both ESCs and ASCs, in relation to their potential for in vitro secretion of insulin and in vivo normalization of hyperglycemia (4). Stem cells from the spleen have been demonstrated to home to the pancreas where they mature into fully functional islet cells responsible for restoring normoglycemia (47).
Transplantation of ESC-derived insulin-producing cells has reversed diabetes in animals, indicating that these cells synthesize and release insulin. Experiments on diabetic mice with ß-cells totally destroyed by streptozocin showed that after BMT blood glucose was normal and survival was longer (4).
A recent study demonstrated the potential use of primary cultures of adult human liver cells as pancreatic progenitors, showing the plasticity of immature liver cells. The possibility of using liver as a pancreatic progenitor tissue has been demonstrated both in vivo and in vitro in Xenopus mice and humans. The use of adult human liver cells for generating functional insulin-producing tissue may pave the way to autologous implantations, thus allowing the diabetic patient to be the donor of his or her own insulin-producing tissue (43).
Stem cells offer the best chance of achieving this goal. Controversial results have been presented concerning the existence and nature of pancreatic islet stem or precursor cells (46). There is also controversy regarding the results obtained with ESCs and ASCs during in vitro secretion of insulin and in vivo normalization of hyperglycemia. Research into ESCs is thought to have much greater developmental potential than research into ASCs; however it is still in the basic research phase. Existing ESC lines are not believed to be identical or ideal for generating islets or ß-cells and additional ESC lines have to be established (4).
HSC chimerism achieved through BMT may affect type I diabetes in two ways: first, by inducing tolerance to pancreas and islet cell transplants, and second by reversing the autoimmune process prior to the development of terminal complications (48).
These studies show the possibility of BMT as a therapeutic approach for ß-cell replacement (4). However, allogeneic BMT does have limitations including the morbidity associated with lethal conditioning, graft-versus-host disease, and failure of engraftment. Currently, the morbidity and mortality associated with lethal conditioning could not be justified for tolerance induction or interruption of the autoimmune state in type I diabetes (48). While immune destruction is a problem in BMT, transplanting splenic mesenchymal cells has yielded promising results in both pancreatic ß-cell regeneration and immune destruction prevention (4).
Significant advances have occurred in the treatment of type I diabetes during the past century, but BMT is the only method capable of interrupting and reversing the autoimmune process prior to the development of terminal complications. When limitations associated with conventional BMT are overcome, BMT may become a therapeutic option in the treatment of autoimmune diabetes and tolerance induction. Techniques that promise to facilitate the use of BMT are currently being studied, including the development of partial conditioning strategies, BM graft engineering to enrich HSCs and cell populations that facilitate engraftment and approaches to limit graft-versus-host disease. The use of various stem cell types as sources for ß-cell regeneration is also important, as recently reviewed (49). The end result will be safer BMT, thereby allowing its use in nonmalignant settings such as AD and tolerance induction in solid organ and cell transplantation (48).
From December 2003 through July 2006 15 patients with early onset type I diabetes mellitus (less than 6 weeks from diagnosis) received AHSCT at the University Hospital of the School of Medicine of Ribeirão Preto, using CY (200 mg/kg) and rabbit ATG (4.5 mg/kg) as a conditioning regime (50). Of 14 patients without previous ketoacidosis and steroids for conditioning, 12 became insulin-free shortly after transplantation, 2 after 1 year from transplantation and 1 patient relapsed after a viral infection.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a fatal disease characterized by the progressive death of central and peripheral motor neurons, which results in severe motor dysfunction (5,51). In a few cases, between 5 and 10%, the origin is hereditary, apparently caused by a mutation of the superoxide dismutase gene (52), but in most cases (sporadic ALS) the cause of the disease in unknown. Autoimmune and inflammatory abnormalities may influence the progression of the disease but ALS is not a typical AD. The therapeutic strategy used in cases of ALS involves attempting to prevent neuronal loss and to stimulate cell regeneration. At present, the combined therapies designed to halt the advance of the disease, which use neuroprotective agents and drugs, have a statistically significant but small influence on patient survival. However, these therapies are incapable of restoring the lost motor function. In ALS, the therapeutic target is to improve the interaction between motor neurons and non-neuronal cells, mainly glial and microglial cells. Stem cell therapy is a promising strategy for restoring neuromotor function and/or preventing motor-neuron degeneration in cases of ALS (5).
Until a few years ago, it was believed that neuronal tissue was unchangeable. However, now it is known that cell replacement occurs in adulthood in some specific regions of the brain. Difficulty in obtaining adult neural stem cells has lead to the use of UCB and BM as alternative sources of stem cells in the therapy for ALS. These cells can be expanded ex vivo, inducing differentiation into the desired cellular type and then transplanted (5).
Transdifferentiation of human HSCs from UCB to neurons has been tested in vitro. These cells are differentiated into neural cells and, sequentially, into astrocytes in a suitable microenvironment. There are reports showing differentiation of ESCs into motor neurons in mice. However, the capacity of transdifferentiation is controversial and other biological explanations have been suggested, particularly cell fusion. Cogle and colleagues (6) showed the differentiation of human HSCs into neurons, astrocytes and microglial cells in the absence of cell fusion.
In another study, after infusion of human ESCs into the cephalorachidian liquid (CRL) of SOD1 mice, stem cells migrated towards the damaged area of the spinal cord, and about 6% of these injected cells gave rise to new neurons and the treated mice showed reversal of paralysis (52). According to Svendsen and Langston (51), these results are mainly due to the differentiation into glial cells and to the production of neuroprotective trophic factors, and not to the direct replacement of motor neurons.
Given the limitation of cellular transdifferentiation, the tendency of neural stem cell grafts to differentiate into glial cell lines after implantation in the spinal cord and the difficulty in transdifferentiating human blood cells into neuroglial cells in vitro, the therapy based on stem cells in ALS is still a long way from becoming routine. Nevertheless, the therapeutic needs of these patients have led to the clinical studies, despite the absence of conclusive evidence. In a clinical study, CD34+ cells purified from peripheral blood were injected into 3 patients with ALS. After 12 months, while none of the patients showed any severe adverse effects, there was no apparent clinical benefit. However, other studies have shown more promising results. Expanded MSCs were injected into the CRL of 7 patients and 3 months after transplantation 4 of them showed a positive clinical response (5).
The aim of applying stem cells in cases of ALS is to achieve differentiation of these cells into motor neurons and the establishment of appropriate functional connections. Stem cells may also act through the delivery of trophic and growth factors, giving rise to a favorable environment for the survival of neural cells and acting as protective agents against neurotoxic substances (5).
Most clinical studies involving the use of cell therapy in cases of ALS have employed the injection of autologous stem cells or ESCs directly into the CNS. Janson and colleagues (53), after trying the technique in primates, injected selected HSCs (CD34+ cells) into the spinal intrathecal space of 3 patients. The authors observed partial or mild effects on neurological function and the absence of complications. In another study carried out at the University of Turin, Italy (54), 7 patients with severe impairment of the lower limbs but no respiratory symptoms, received ex vivo expansion of autologous MSCs from BM directly into the surgically exposed spinal cord (T7-T9 level). Most of the patients only presented slight and reversible side effects (intercostal pain or dysesthesia), a result confirmed after a 2-year follow-up (55). Deceleration of the loss of muscular strength in the legs was observed 3 months after transplantation in 4 patients together with a slight improvement in 2 patients (54). Additionally, there was also a deceleration in the decline of respiratory function in 4 patients after 2 years. These effects were correlated with the number of injected cells (55). At a scientific event (56), a report was presented about the use of stem cells extracted from 4- to 8-week embryos that were injected into 61 patients with ALS on 1 to 4 occasions. Improvement of neurological function was reported in 34% of the patients after 2 months and, subsequently, in those patients who received repeated injections. The findings showed that the outcome was better in cases where the disease was at an earlier stage, but there are no data regarding survival. A researcher from Montevideo, Uruguay, treated 12 ALS patients with HSCs collected from peripheral blood, suspended in CRL and directly injected into the spinal cord. The results were quite encouraging, since neurological function improved in 3 patients and was stabilized in 9 (57).
There are few reports of the use of HSCT, followed by high dose immunosuppression in ALS. A report was sent to the European Registry of HSCT for AD (EBMT/EULAR, based in Basle, Switzerland) of 2 patients submitted to AHSCT in France (Tyndall A, personal communication). Despite transitory improvement over a period of 3 months in 1 of the patients, both died about 7 months after transplantation. In the US, a group of scientists from Baylor College of Medicine, Houston, TX, carried out a pilot study involving 6 patients at intermediate stages of the disease, who received allogeneic BMT from their HLA-identical siblings (58). All the patients underwent donor cell grafting and 3 patients showed no benefits from the procedure, with 2 of them dying and the disease continuing to advance in the other. Nevertheless, the 3 remaining patients showed a reduction in the advance of the disease. Autopsy of 1 of the patients revealed large quantities of donor DNA in various regions of the spinal cord and brain, demonstrating that allogeneic BM cells were capable of crossing the blood brain barrier and incorporating themselves into the nervous tissue.
There is evidence that the inflammatory process is a key factor in the pathogenesis of ALS (59) and that high dose immunosuppression with stem cell transplantation might change the natural history of the disease (60). Based on this evidence, AHSCT for ALS started in Brazil in December 2004 at the University Hospital of the School of Medicine of Ribeirão Preto. Two patients were transplanted using a triple immunosuppressive regimen (CY, ATG, and fludarabine). The first one with the bulbar form died at D+103 from pulmonary embolism and the second is still alive 28 months after transplantation. A more extensive experience of AHSCT was obtained at the Salvador Hospital in Bahia where 15 patients were transplanted using the same conditioning regime. There were 3 transplant-related deaths, 1 progression-related death and a sudden death unrelated to transplantation or progression. Ten patients are alive after a mean follow-up of 133 days (1-540) (Pallotta R, personal communication). Both protocols were halted by the national institutional review board (CONEP/Ministry of Health) pending reformulation.
Conclusion
Stem cell transplantation has been recommended for use in the treatment of AD because it provides immunosuppressive and immunomodulatory effects. Stem cell transplantation can destroy the defective immune system, renew the lymphatic system, reduce the disease activity, and lead to long-term remission of AD (27). AD patients who were once resistant to conventional therapy, following transplantation, have become sensitive to the same conventional therapy, highlighting the immunomodulatory properties of stem cell transplantation.
Most clinical studies based on stem cell therapy in AD patients use AHSCT mobilized from BM to peripheral blood. There is no significant difference in the transplantation protocols used for different diseases. The results shown in this review range from cases of complete or partial remission, relapse or stabilization of disease to cases involving the advance of the disease and death. Patient selection appears to directly influence the results of transplantation. Choosing patients in the initial stages of the disease appears to be safer and more effective. However, this procedure involves the risk of exposing patients who may respond well to conventional therapy to new non-standard treatment methods (21). It should be pointed out that most of the procedures reviewed here were performed in patients that failed to respond to conventional treatment therapies. In such patients, the use of stem cells, although still being an experimental procedure, provides a new chance to enhance the quality of life.
There is some controversy regarding the use of high doses of chemotherapy in the absence of stem cell infusion, which, in some cases, has led to satisfactory results, suggesting that immunosuppression may be the real cause of the improvement seen in the patients, and not the stem cells (26). Also, the total number of patients assessed in some studies is quite small since they represent case reports and small uncontrolled case-series. In addition, the individuals represent highly diverse genetic backgrounds, and only randomized clinical trials following standard procedures will allow us to reach meaningful conclusions. Furthermore, there is the need for studies which will evaluate the use of maintenance therapies after transplantation, define patient selection protocols and assess stem cell mobilization in the treatment of AD.
Patient relapse is more common in autologous (30%) than in allogeneic (5%) transplantation, with the latter also producing longer-lasting results (26). Relapse could be caused by contamination of the graft with autoimmune cells, the incapacity of the conditioning regime to destroy the immune cells responsible for the disease or the presence of the defect in the stem cell expansion (14). The contamination of grafts by auto-reactive lymphocytes can be controlled using pure cell lines expanded in the laboratory, which would also increase the number of stem cells available for the patient (26). The efficacy of an allogeneic transplantation is mainly the result of the change in the genetic susceptibility of the host to the disease, and also involves the graft-versus-disease effect. Currently, this procedure is not frequently used due to its toxicity and the need for HLA compatibility (9,14).
It is known that ESCs have a greater capacity for differentiation and less immunogenetic potential than ASCs. Nevertheless, ESCs could be used as an alternative when there is a high risk of immunological rejection with ASC therapy, as in the case of allogeneic transplantation, or in cases where there is relapse as a consequence of the patient genotype, as occurs in autologous transplantation. However, the use of ESCs remains limited and far from being a therapeutic alternative for such patients due to the risk of the development of teratomas and the ethical issues involving the use of this procedure.
MSCs represent a promising therapy for AD. These cells present immunosuppressive properties and there are reports suggesting the low level of expression of HLA molecules in MSCs. It is believed that the therapy mediated by mesenchymal cells may permit the secretion of immunosuppressor factors and may repair the tissue destroyed by the chronic inflammatory process.
A mixed approach involving the combination of genetic transference with cellular therapy is able to increase the therapeutic potency of stem cell transplantation. It permits the correction of genetic anomalies and helps in the control of disease by increasing the expression of regulatory elements (21). In neurodegenerative diseases, neural stem cells may remain in the quiescent stage due to the unfavorable microenvironment caused by disease. It is believed that genetically modified stem cells can stimulate the proliferation of endogenous stem cells and so lead to repair of the damaged neural region (5).
An interesting strategy would be to label the infused stem cells for later evaluation of their proliferation, differentiation, migration, and homing. Cellular labeling, such as that achieved by Cogle and colleagues (6), makes it possible to expand the knowledge available on the activity and biology of stem cells, to evaluate the effectiveness of transplantation and to develop better protocols for the application of stem cells to each immunological disease.
Stem cell-based therapy offers the possibility of developing new treatments in cases of AD. Several reports have analyzed the potential benefits of stem cell transplantation in these diseases. It is hoped that new studies currently underway will show good results for the treatment of AD in the near future.
Schematic model of the protocol for obtaining autologous hematopoietic stem cells from peripheral blood for transplantation showing the different stages of transplantation, re-establishment of immunological tolerance and possible causes of relapse. Following granulocyte-colony stimulating factor (G-CSF) treatment which stimulates the mobilization of stem cells from bone marrow to peripheral blood (1), the peripheral blood stem cells are collected by means of leukapheresis and purified (2). Purification of peripheral blood via antithymocyte globulin (ATG) in vivo and/or in vitro selection of CD34+ cells aims to isolate stem cells from mature cells. After collection of peripheral blood, the patient is submitted to immunosuppressive therapy (2). The graft containing the stem cells is reintroduced into the patient (4). If mature lymphoid cells remain in the graft after the purification stage, the patient may suffer a relapse (3B and 4B). The stem cells multiply via the self-renewal process (5) and are capable of differentiation (6), forming a new immunological system and therefore re-establishing immunological tolerance, consequently diminishing the symptoms of the disease or leading to long-term remission (7A). However, if new auto-reactive cells develop during the differentiation stage or the purification stage fails to remove such cells from the graft, the patient may suffer a relapse (7B).
Protocols of autologous transplantation and results of clinical studies based on the use of stem cells in the treatment of rheumatoid arthritis.
Protocols of transplantation and results of clinical studies based on the use of autologous peripheral blood hematopoietic stem cell transplantation in multiple sclerosis.
References
1. Voltarelli JC, Stracieri ABPL, Oliveira MCB, Paton EJA, Dantas M. Transplante autólogo de células tronco hematopoéticas para nefrite lúpica: resultados brasileiros iniciais. J Bras Nefrol 2003; 25: 65-72.
2. Snowden JA, Kearney P, Kearney A, Cooley HM, Grigg A, Jacobs P, et al. Long-term outcome of autoimmune disease following allogeneic bone marrow transplantation. Arthritis Rheum 1998; 41: 453-459.
3. Hough RE, Snowden JA, Wulffraat NM. Haemopoietic stem cell transplantation in autoimmune diseases: a European perspective. Br J Haematol 2005; 128: 432-459.
4. Miszta-Lane H, Mirbolooki M, James Shapiro AM, Lakey JR. Stem cell sources for clinical islet transplantation in type 1 diabetes: embryonic and adult stem cells. Med Hypotheses 2006; 67: 909-913.
5. Silani V, Cova L, Corbo M, Ciammola A, Polli E. Stem-cell therapy for amyotrophic lateral sclerosis. Lancet 2004; 364: 200-202.
6. Cogle CR, Yachnis AT, Laywell ED, Zander DS, Wingard JR, Steindler DA, et al. Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet 2004; 363: 1432-1437.
7. Wulffraat M, de Kleer I, Brinkman D, ten Cate R, van der Net JJ, Rijkers GT, et al. Autologous stem cell transplantation for refractory juvenile idiopathic arthritis: current results and perspectives. Transplant Proc 2002; 34: 2925-2926.
8. Snowden JA, Passweg J, Moore JJ, Milliken S, Cannell P, Van Laar J, et al. Autologous hemopoietic stem cell transplantation in severe rheumatoid arthritis: a report from the EBMT and ABMTR. J Rheumatol 2004; 31: 482-488.
9. Bingham SJ, Moore JJ. Stem cell transplantation for autoimmune disorders. Rheumatoid arthritis. Best Pract Res Clin Haematol 2004; 17: 263-276.
10. Weyand CM, Goronzy JJ. Stem cell aging and autoimmunity in rheumatoid arthritis. Trends Mol Med 2004; 10: 426-433.
11. Burt RK, Oyama Y, Verda L, Quigley K, Brush M, Yaung K, et al. Induction of remission of severe and refractory rheumatoid arthritis by allogeneic mixed chimerism. Arthritis Rheum 2004; 50: 2466-2470.
12. Breban M, Dougados M, Picard F, Zompi S, Marolleau JP, Bocaccio C, et al. Intensified-dose (4 gm/m2) cyclophosphamide and granulocyte colony-stimulating factor administration for hematopoietic stem cell mobilization in refractory rheumatoid arthritis. Arthritis Rheum 1999; 42: 2275-2280.
13. Snowden JA, Biggs JC, Milliken ST, Fuller A, Brooks PM. A phase I/II dose escalation study of intensified cyclophosphamide and autologous blood stem cell rescue in severe, active rheumatoid arthritis. Arthritis Rheum 1999; 42: 2286-2292.
14. Burt RK, Georganas C, Schroeder J, Traynor A, Stefka J, Schuening F, et al. Autologous hematopoietic stem cell transplantation in refractory rheumatoid arthritis: sustained response in two of four patients. Arthritis Rheum 1999; 42: 2281-2285.
15. Brodsky RA, Petri M, Smith BD, Seifter EJ, Spivak JL, Styler M, et al. Immunoablative high-dose cyclophosphamide without stem-cell rescue for refractory, severe autoimmune disease. Ann Intern Med 1998; 129: 1031-1035.
16. Moore J, Brooks P, Milliken S, Biggs J, Ma D, Handel M, et al. A pilot randomized trial comparing CD34-selected versus unmanipulated hemopoietic stem cell transplantation for severe, refractory rheumatoid arthritis. Arthritis Rheum 2002; 46: 2301-2309.
17. McCool G, Hohsaka H, Wicks I. High-dose chemotherapy and syngeneic hemopoietic stem-cell transplantation for severe, seronegative rheumatoid arthritis. Ann Intern Med 1999; 131: 507-509.
18. Woo P. Systemic juvenile idiopathic arthritis: diagnosis, management, and outcome. Nat Clin Pract Rheumatol 2006; 2: 28-34.
19. de Kleer IM, Brinkman DM, Ferster A, Abinun M, Quartier P, Van Der Net J, et al. Autologous stem cell transplantation for refractory juvenile idiopathic arthritis: analysis of clinical effects, mortality, and transplant related morbidity. Ann Rheum Dis 2004; 63: 1318-1326.
20. Voltarelli JC, Stracieri ABPL, Oliveira MCB, Godoi DF, Moraes DA, Pieroni F. Transplante de células tronco hematopoéticas em doenças reumáticas. Parte 2: Experiência brasileira e perspectivas futuras. Rev Bras Reumatol 2005; 45: 301-312.
21. Jayne D, Passweg J, Marmont A, Farge D, Zhao X, Arnold R, et al. Autologous stem cell transplantation for systemic lupus erythematosus. Lupus 2004; 13: 168-176.
22. Burt RK, Traynor A, Statkute L, Barr WG, Rosa R, Schroeder J, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA 2006; 295: 527-535.
23. Pavletic SZ, Illei GG. The role of immune ablation and stem cell transplantation in severe SLE. Best Pract Res Clin Rheumatol 2005; 19: 839-858.
24. Petri M, Jones RJ, Brodsky RA. High-dose cyclophosphamide without stem cell transplantation in systemic lupus erythematosus. Arthritis Rheum 2003; 48: 166-173.
25. Lisukov IA, Sizikova SA, Kulagin AD, Kruchkova IV, Gilevich AV, Konenkova LP, et al. High-dose immunosuppression with autologous stem cell transplantation in severe refractory systemic lupus erythematosus. Lupus 2004; 13: 89-94.
26. Fassas A, Kimiskidis VK. Stem cell transplantation for multiple sclerosis: what is the evidence? Blood Rev 2003; 17: 233-240.
27. Fassas A, Passweg JR, Anagnostopoulos A, Kazis A, Kozak T, Havrdova E, et al. Hematopoietic stem cell transplantation for multiple sclerosis. A retrospective multicenter study. J Neurol 2002; 249: 1088-1097.
28. Inglese M, Mancardi GL, Pagani E, Rocca MA, Murialdo A, Saccardi R, et al. Brain tissue loss occurs after suppression of enhancement in patients with multiple sclerosis treated with autologous haematopoietic stem cell transplantation. J Neurol Neurosurg Psychiatry 2004; 75: 643-644.
29. Fassas A, Anagnostopoulos A, Kazis A, Kapinas K, Sakellari I, Kimiskidis V, et al. Autologous stem cell transplantation in progressive multiple sclerosis - an interim analysis of efficacy. J Clin Immunol 2000; 20: 24-30.
30. Carreras E, Saiz A, Marin P, Martinez C, Rovira M, Villamor N, et al. CD34+ selected autologous peripheral blood stem cell transplantation for multiple sclerosis: report of toxicity and treatment results at one year of follow-up in 15 patients. Haematologica 2003; 88: 306-314.
31. Nash RA, Bowen JD, McSweeney PA, Pavletic SZ, Maravilla KR, Park MS, et al. High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood 2003; 102: 2364-2372.
32. Saccardi R, Mancardi GL, Solari A, Bosi A, Bruzzi P, Di Bartolomeo P, et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood 2005; 105: 2601-2607.
33. Blanco Y, Saiz A, Carreras E, Graus F. Changes of matrix metalloproteinase-9 and its tissue inhibitor (TIMP-1) after autologous hematopoietic stem cell transplantation in multiple sclerosis. J Neuroimmunol 2004; 153: 190-194.
34. Muraro PA, Douek DC. Renewing the T cell repertoire to arrest autoimmune aggression. Trends Immunol 2006; 27: 61-67.
35. van Laar JM, McSweeney PA. High-dose immunosuppressive therapy and autologous progenitor cell transplantation for systemic sclerosis. Best Pract Res Clin Haematol 2004; 17: 233-245.
36. Farge D, Passweg J, van Laar JM, Marjanovic Z, Besenthal C, Finke J, et al. Autologous stem cell transplantation in the treatment of systemic sclerosis: report from the EBMT/EULAR Registry. Ann Rheum Dis 2004; 63: 974-981.
37. Tsukamoto H, Nagafuji K, Horiuchi T, Miyamoto T, Aoki K, Takase K, et al. A phase I-II trial of autologous peripheral blood stem cell transplantation in the treatment of refractory autoimmune disease. Ann Rheum Dis 2006; 65: 508-514.
38. Komatsuda A, Kawabata Y, Horiuchi T, Motegi M, Ozawa M, Fujishima N, et al. Successful autologous peripheral blood stem cell transplantation using thiotepa in a patient with systemic sclerosis and cardiac involvement. Tohoku J Exp Med 2006; 209: 61-67.
39. Lim SH, Kell J, al-Sabah A, Bashi W, Bailey-Wood R. Peripheral blood stem-cell transplantation for refractory autoimmune thrombocytopenic purpura. Lancet 1997; 349: 475.
40. Urban C, Lackner H, Sovinz P, Benesch M, Schwinger W, Dornbusch HJ, et al. Successful unrelated cord blood transplantation in a 7-year-old boy with Evans syndrome refractory to immunosuppression and double autologous stem cell transplantation. Eur J Haematol 2006; 76: 526-530.
41. Hunh RD, Fogarty PF, Nakamura R, Read EJ, Leitman SF, Rick ME. High-dose cyclophosphamide with autologous lymphocyte-depleted peripheral blood stem cell (PBSC) support for treatment of refractory chronic autoimmune thrombocytopenia. Blood 2003; 101: 71-77.
42. Seeliger S, Baumann M, Mohr M, Jurgens H, Frosch M, Vormoor J. Autologous peripheral blood stem cell transplantation and anti-B-cell directed immunotherapy for refractory auto-immune haemolytic anaemia. Eur J Pediatr 2001; 160: 492-496.
43. Meivar-Levy I, Ferber S. Regenerative medicine: using liver to generate pancreas for treating diabetes. Isr Med Assoc J 2006; 8: 430-434.
44. Murtaugh LC, Melton DA. Genes, signals, and lineages in pancreas development. Annu Rev Cell Dev Biol 2003; 19: 71-89.
45. Balamurugan AN, Bottino R, Giannoukakis N, Smetanka C. Prospective and challenges of islet transplantation for the therapy of autoimmune diabetes. Pancreas 2006; 32: 231-243.
46. Otonkoski T, Gao R, Lundin K. Stem cells in the treatment of diabetes. Ann Med 2005; 37: 513-520.
47. Melton DA. Reversal of type 1 diabetes in mice. N Engl J Med 2006; 355: 89-90.
48. Domenick MA, Ildstad ST. Impact of bone marrow transplantation on type I diabetes. World J Surg 2001; 25: 474-480.
49. Couri CE, Foss MC, Voltarelli JC. Secondary prevention of type 1 diabetes mellitus: stopping immune destruction and promoting beta-cell regeneration. Braz J Med Biol Res 2006; 39: 1271-1280.
50. Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2007; 297: 1568-1576.
51. Svendsen CN, Langston JW. Stem cells for Parkinson disease and ALS: replacement or protection? Nat Med 2004; 10: 224-225.
52. Vastag B. Stem cells step closer to the clinic: paralysis partially reversed in rats with ALS-like disease. JAMA 2001; 285: 1691-1693.
53. Janson CG, Ramesh TM, During MJ, Leone P, Heywood J. Human intrathecal transplantation of peripheral blood stem cells in amyotrophic lateral sclerosis. J Hematother Stem Cell Res 2001; 10: 913-915.
54. Mazzini L, Fagioli F, Boccaletti R, Mareschi K, Oliveri G, Olivieri C, et al. Stem cell therapy in amyotrophic lateral sclerosis: a methodological approach in humans. Amyotroph Lateral Scler Other Motor Neuron Disord 2003; 4: 158-161.
55. Mazzini L, Fagioli F, Boccaletti R. Stem-cell therapy in amyotrophic lateral sclerosis. Lancet 2004; 364: 1936-1937.
56. Smikodub AI. Experience of application of embryonic stem cells in ALS. Cytotherapy 2004; 6: 431.
57. De Bellis R, Cordoba A, Bello L. Treatment of 12 patients with ALS, grafting cells through neuroendoscopy. Bone Marrow Transplant 2006; 37 (Suppl 1): S133.
58. Appel SH, Engelhardt JI, Henkel JS, Luo Y, Brenner MK, Popat UR. Allogeneic hematopoietic stem cell transplantation. 2004. Amyotroph Lateral Scler Other Motor Neuron Disor 2004; 5 (Suppl 2): 54.
59. Sargsyan SA, Monk PN, Shaw PJ. Microglia as potential contributors to motor neuron injury in amyotrophic lateral sclerosis. Glia 2005; 51: 241-253.
60. Voltarelli JC. Perspectivas de terapia cellular na esclerose lateral amiotrófica. Rev Bras Hematol Hemoter 2004; 26: 155-156.
 Address for correspondence: P. Pranke, Laboratório de Hematologia, Av. Ipiranga, 2752, 3º andar, 90160-000 Porto Alegre, RS, Brasil. Fax: +55-51-3308-5437. E-mail: patriciapranke@ufrgs.br
Address for correspondence: P. Pranke, Laboratório de Hematologia, Av. Ipiranga, 2752, 3º andar, 90160-000 Porto Alegre, RS, Brasil. Fax: +55-51-3308-5437. E-mail: patriciapranke@ufrgs.br
Research supported by FINEP and CNPq. Publication supported by FAPESP. Received October 29, 2006. Accepted July 2, 2007.
- 1. Voltarelli JC, Stracieri ABPL, Oliveira MCB, Paton EJA, Dantas M. Transplante autólogo de células tronco hematopoéticas para nefrite lúpica: resultados brasileiros iniciais. J Bras Nefrol 2003; 25: 65-72.
- 2. Snowden JA, Kearney P, Kearney A, Cooley HM, Grigg A, Jacobs P, et al. Long-term outcome of autoimmune disease following allogeneic bone marrow transplantation. Arthritis Rheum 1998; 41: 453-459.
- 3. Hough RE, Snowden JA, Wulffraat NM. Haemopoietic stem cell transplantation in autoimmune diseases: a European perspective. Br J Haematol 2005; 128: 432-459.
- 4. Miszta-Lane H, Mirbolooki M, James Shapiro AM, Lakey JR. Stem cell sources for clinical islet transplantation in type 1 diabetes: embryonic and adult stem cells. Med Hypotheses 2006; 67: 909-913.
- 5. Silani V, Cova L, Corbo M, Ciammola A, Polli E. Stem-cell therapy for amyotrophic lateral sclerosis. Lancet 2004; 364: 200-202.
- 6. Cogle CR, Yachnis AT, Laywell ED, Zander DS, Wingard JR, Steindler DA, et al. Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet 2004; 363: 1432-1437.
- 7. Wulffraat M, de Kleer I, Brinkman D, ten Cate R, van der Net JJ, Rijkers GT, et al. Autologous stem cell transplantation for refractory juvenile idiopathic arthritis: current results and perspectives. Transplant Proc 2002; 34: 2925-2926.
- 8. Snowden JA, Passweg J, Moore JJ, Milliken S, Cannell P, Van Laar J, et al. Autologous hemopoietic stem cell transplantation in severe rheumatoid arthritis: a report from the EBMT and ABMTR. J Rheumatol 2004; 31: 482-488.
- 9. Bingham SJ, Moore JJ. Stem cell transplantation for autoimmune disorders. Rheumatoid arthritis. Best Pract Res Clin Haematol 2004; 17: 263-276.
- 10. Weyand CM, Goronzy JJ. Stem cell aging and autoimmunity in rheumatoid arthritis. Trends Mol Med 2004; 10: 426-433.
- 11. Burt RK, Oyama Y, Verda L, Quigley K, Brush M, Yaung K, et al. Induction of remission of severe and refractory rheumatoid arthritis by allogeneic mixed chimerism. Arthritis Rheum 2004; 50: 2466-2470.
- 12. Breban M, Dougados M, Picard F, Zompi S, Marolleau JP, Bocaccio C, et al. Intensified-dose (4 gm/m2) cyclophosphamide and granulocyte colony-stimulating factor administration for hematopoietic stem cell mobilization in refractory rheumatoid arthritis. Arthritis Rheum 1999; 42: 2275-2280.
- 13. Snowden JA, Biggs JC, Milliken ST, Fuller A, Brooks PM. A phase I/II dose escalation study of intensified cyclophosphamide and autologous blood stem cell rescue in severe, active rheumatoid arthritis. Arthritis Rheum 1999; 42: 2286-2292.
- 14. Burt RK, Georganas C, Schroeder J, Traynor A, Stefka J, Schuening F, et al. Autologous hematopoietic stem cell transplantation in refractory rheumatoid arthritis: sustained response in two of four patients. Arthritis Rheum 1999; 42: 2281-2285.
- 15. Brodsky RA, Petri M, Smith BD, Seifter EJ, Spivak JL, Styler M, et al. Immunoablative high-dose cyclophosphamide without stem-cell rescue for refractory, severe autoimmune disease. Ann Intern Med 1998; 129: 1031-1035.
- 16. Moore J, Brooks P, Milliken S, Biggs J, Ma D, Handel M, et al. A pilot randomized trial comparing CD34-selected versus unmanipulated hemopoietic stem cell transplantation for severe, refractory rheumatoid arthritis. Arthritis Rheum 2002; 46: 2301-2309.
- 17. McCool G, Hohsaka H, Wicks I. High-dose chemotherapy and syngeneic hemopoietic stem-cell transplantation for severe, seronegative rheumatoid arthritis. Ann Intern Med 1999; 131: 507-509.
- 18. Woo P. Systemic juvenile idiopathic arthritis: diagnosis, management, and outcome. Nat Clin Pract Rheumatol 2006; 2: 28-34.
- 19. de Kleer IM, Brinkman DM, Ferster A, Abinun M, Quartier P, Van Der Net J, et al. Autologous stem cell transplantation for refractory juvenile idiopathic arthritis: analysis of clinical effects, mortality, and transplant related morbidity. Ann Rheum Dis 2004; 63: 1318-1326.
- 20. Voltarelli JC, Stracieri ABPL, Oliveira MCB, Godoi DF, Moraes DA, Pieroni F. Transplante de células tronco hematopoéticas em doenças reumáticas. Parte 2: Experiência brasileira e perspectivas futuras. Rev Bras Reumatol 2005; 45: 301-312.
- 21. Jayne D, Passweg J, Marmont A, Farge D, Zhao X, Arnold R, et al. Autologous stem cell transplantation for systemic lupus erythematosus. Lupus 2004; 13: 168-176.
- 22. Burt RK, Traynor A, Statkute L, Barr WG, Rosa R, Schroeder J, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA 2006; 295: 527-535.
- 23. Pavletic SZ, Illei GG. The role of immune ablation and stem cell transplantation in severe SLE. Best Pract Res Clin Rheumatol 2005; 19: 839-858.
- 24. Petri M, Jones RJ, Brodsky RA. High-dose cyclophosphamide without stem cell transplantation in systemic lupus erythematosus. Arthritis Rheum 2003; 48: 166-173.
- 25. Lisukov IA, Sizikova SA, Kulagin AD, Kruchkova IV, Gilevich AV, Konenkova LP, et al. High-dose immunosuppression with autologous stem cell transplantation in severe refractory systemic lupus erythematosus. Lupus 2004; 13: 89-94.
- 26. Fassas A, Kimiskidis VK. Stem cell transplantation for multiple sclerosis: what is the evidence? Blood Rev 2003; 17: 233-240.
- 27. Fassas A, Passweg JR, Anagnostopoulos A, Kazis A, Kozak T, Havrdova E, et al. Hematopoietic stem cell transplantation for multiple sclerosis. A retrospective multicenter study. J Neurol 2002; 249: 1088-1097.
- 28. Inglese M, Mancardi GL, Pagani E, Rocca MA, Murialdo A, Saccardi R, et al. Brain tissue loss occurs after suppression of enhancement in patients with multiple sclerosis treated with autologous haematopoietic stem cell transplantation. J Neurol Neurosurg Psychiatry 2004; 75: 643-644.
- 29. Fassas A, Anagnostopoulos A, Kazis A, Kapinas K, Sakellari I, Kimiskidis V, et al. Autologous stem cell transplantation in progressive multiple sclerosis - an interim analysis of efficacy. J Clin Immunol 2000; 20: 24-30.
- 30. Carreras E, Saiz A, Marin P, Martinez C, Rovira M, Villamor N, et al. CD34+ selected autologous peripheral blood stem cell transplantation for multiple sclerosis: report of toxicity and treatment results at one year of follow-up in 15 patients. Haematologica 2003; 88: 306-314.
- 31. Nash RA, Bowen JD, McSweeney PA, Pavletic SZ, Maravilla KR, Park MS, et al. High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood 2003; 102: 2364-2372.
- 32. Saccardi R, Mancardi GL, Solari A, Bosi A, Bruzzi P, Di Bartolomeo P, et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood 2005; 105: 2601-2607.
- 33. Blanco Y, Saiz A, Carreras E, Graus F. Changes of matrix metalloproteinase-9 and its tissue inhibitor (TIMP-1) after autologous hematopoietic stem cell transplantation in multiple sclerosis. J Neuroimmunol 2004; 153: 190-194.
- 34. Muraro PA, Douek DC. Renewing the T cell repertoire to arrest autoimmune aggression. Trends Immunol 2006; 27: 61-67.
- 35. van Laar JM, McSweeney PA. High-dose immunosuppressive therapy and autologous progenitor cell transplantation for systemic sclerosis. Best Pract Res Clin Haematol 2004; 17: 233-245.
- 36. Farge D, Passweg J, van Laar JM, Marjanovic Z, Besenthal C, Finke J, et al. Autologous stem cell transplantation in the treatment of systemic sclerosis: report from the EBMT/EULAR Registry. Ann Rheum Dis 2004; 63: 974-981.
- 37. Tsukamoto H, Nagafuji K, Horiuchi T, Miyamoto T, Aoki K, Takase K, et al. A phase I-II trial of autologous peripheral blood stem cell transplantation in the treatment of refractory autoimmune disease. Ann Rheum Dis 2006; 65: 508-514.
- 38. Komatsuda A, Kawabata Y, Horiuchi T, Motegi M, Ozawa M, Fujishima N, et al. Successful autologous peripheral blood stem cell transplantation using thiotepa in a patient with systemic sclerosis and cardiac involvement. Tohoku J Exp Med 2006; 209: 61-67.
- 39. Lim SH, Kell J, al-Sabah A, Bashi W, Bailey-Wood R. Peripheral blood stem-cell transplantation for refractory autoimmune thrombocytopenic purpura. Lancet 1997; 349: 475.
- 40. Urban C, Lackner H, Sovinz P, Benesch M, Schwinger W, Dornbusch HJ, et al. Successful unrelated cord blood transplantation in a 7-year-old boy with Evans syndrome refractory to immunosuppression and double autologous stem cell transplantation. Eur J Haematol 2006; 76: 526-530.
- 41. Hunh RD, Fogarty PF, Nakamura R, Read EJ, Leitman SF, Rick ME. High-dose cyclophosphamide with autologous lymphocyte-depleted peripheral blood stem cell (PBSC) support for treatment of refractory chronic autoimmune thrombocytopenia. Blood 2003; 101: 71-77.
- 42. Seeliger S, Baumann M, Mohr M, Jurgens H, Frosch M, Vormoor J. Autologous peripheral blood stem cell transplantation and anti-B-cell directed immunotherapy for refractory auto-immune haemolytic anaemia. Eur J Pediatr 2001; 160: 492-496.
- 43. Meivar-Levy I, Ferber S. Regenerative medicine: using liver to generate pancreas for treating diabetes. Isr Med Assoc J 2006; 8: 430-434.
- 44. Murtaugh LC, Melton DA. Genes, signals, and lineages in pancreas development. Annu Rev Cell Dev Biol 2003; 19: 71-89.
- 45. Balamurugan AN, Bottino R, Giannoukakis N, Smetanka C. Prospective and challenges of islet transplantation for the therapy of autoimmune diabetes. Pancreas 2006; 32: 231-243.
- 46. Otonkoski T, Gao R, Lundin K. Stem cells in the treatment of diabetes. Ann Med 2005; 37: 513-520.
- 47. Melton DA. Reversal of type 1 diabetes in mice. N Engl J Med 2006; 355: 89-90.
- 48. Domenick MA, Ildstad ST. Impact of bone marrow transplantation on type I diabetes. World J Surg 2001; 25: 474-480.
- 49. Couri CE, Foss MC, Voltarelli JC. Secondary prevention of type 1 diabetes mellitus: stopping immune destruction and promoting beta-cell regeneration. Braz J Med Biol Res 2006; 39: 1271-1280.
- 50. Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2007; 297: 1568-1576.
- 51. Svendsen CN, Langston JW. Stem cells for Parkinson disease and ALS: replacement or protection? Nat Med 2004; 10: 224-225.
- 52. Vastag B. Stem cells step closer to the clinic: paralysis partially reversed in rats with ALS-like disease. JAMA 2001; 285: 1691-1693.
- 53. Janson CG, Ramesh TM, During MJ, Leone P, Heywood J. Human intrathecal transplantation of peripheral blood stem cells in amyotrophic lateral sclerosis. J Hematother Stem Cell Res 2001; 10: 913-915.
- 54. Mazzini L, Fagioli F, Boccaletti R, Mareschi K, Oliveri G, Olivieri C, et al. Stem cell therapy in amyotrophic lateral sclerosis: a methodological approach in humans. Amyotroph Lateral Scler Other Motor Neuron Disord 2003; 4: 158-161.
- 55. Mazzini L, Fagioli F, Boccaletti R. Stem-cell therapy in amyotrophic lateral sclerosis. Lancet 2004; 364: 1936-1937.
- 56. Smikodub AI. Experience of application of embryonic stem cells in ALS. Cytotherapy 2004; 6: 431.
- 57. De Bellis R, Cordoba A, Bello L. Treatment of 12 patients with ALS, grafting cells through neuroendoscopy. Bone Marrow Transplant 2006; 37 (Suppl 1): S133.
- 58. Appel SH, Engelhardt JI, Henkel JS, Luo Y, Brenner MK, Popat UR. Allogeneic hematopoietic stem cell transplantation. 2004. Amyotroph Lateral Scler Other Motor Neuron Disor 2004; 5 (Suppl 2): 54.
- 59. Sargsyan SA, Monk PN, Shaw PJ. Microglia as potential contributors to motor neuron injury in amyotrophic lateral sclerosis. Glia 2005; 51: 241-253.
- 60. Voltarelli JC. Perspectivas de terapia cellular na esclerose lateral amiotrófica. Rev Bras Hematol Hemoter 2004; 26: 155-156.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
29 Oct 2007 -
Date of issue
Dec 2007
History
-
Accepted
02 July 2007 -
Received
29 Oct 2006